Immunocompetent and Immunodeficient Mouse Models for Enterovirus 71 Pathogenesis and Therapy
Abstract
:1. Introduction
2. In Vitro Propagation Systems
3. In Vivo Animal Models
3.1. Mouse Models Dependent on Mouse-Adapted EV71 Strains
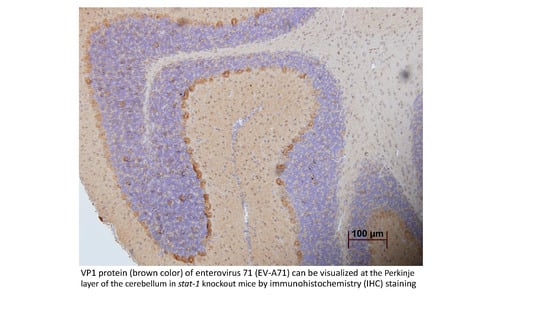
3.2. Immunodeficient Mouse Models
3.3. Transgenic Mouse Models
3.4. A Hybrid Mouse Model
4. Pathogenesis Mechanism
4.1. Viral Determinants of Virulence
4.2. Host Factors
5. Therapy
6. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Schmidt, N.J.; Lennette, E.H.; Ho, H.H. An apparently new enterovirus isolated from patients with disease of the central nervous system. J. Infect. Dis. 1974, 129, 304–309. [Google Scholar] [CrossRef] [PubMed]
- Brown, B.A.; Pallansch, M.A. Complete nucleotide sequence of enterovirus 71 is distinct from poliovirus. Virus Res. 1995, 39, 195–205. [Google Scholar] [CrossRef]
- Cox, J.A.; Hiscox, J.A.; Solomon, T.; Ooi, M.H.; Ng, L.F.P. Immunopathogenesis and virus-host interactions of enterovirus 71 in patients with hand, foot and mouth disease. Front. Microbiol. 2017, 8, 2249. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.C.; Liou, A.T.; Chang, Y.S.; Wu, S.Y.; Chang, C.S.; Lee, C.K.; Kung, J.T.; Tu, P.H.; Yu, Y.Y.; Lin, C.Y.; et al. Immunodeficient mouse models with different disease profiles by in vivo infection with the same clinical isolate of enterovirus 71. J. Virol. 2014, 88, 12485–12499. [Google Scholar] [CrossRef] [PubMed]
- Liou, A.-T.; Wu, S.-Y.; Liao, C.-C.; Chang, Y.-S.; Chang, C.-S.; Shih, C. A new animal model containing human SCARB2 and lacking stat-1 is highly susceptible to EV71. Sci. Rep. 2016, 6, 31151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ho, M.; Chen, E.R.; Hsu, K.H.; Twu, S.J.; Chen, K.T.; Tsai, S.F.; Wang, J.R.; Shih, S.R. An epidemic of enterovirus 71 infection in Taiwan. N. Engl. J. Med. 1999, 341, 929–935. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.-Y.; Huang, L.-M.; Gau, S.S.-F.; Wu, Y.-Y.; Hsia, S.-H.; Fan, T.-Y.; Lin, K.-L.; Huang, Y.-C.; Lu, C.-Y.; Lin, T.-Y. Neurodevelopment and cognition in children after enterovirus 71 infection. N. Engl. J. Med. 2007, 356, 1226–1231. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.-M.; Wang, J.-N.; Tsai, Y.-C.; Liu, C.-C.; Huang, C.-C.; Chen, Y.-J.; Yeh, T.-F. Cardiopulmonary manifestations of fulminant enterovirus 71 infection. Pediatrics 2002, 109, e26. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, H.; Utama, A.; Onnimala, N.; Li, C.; Zhang, L.-B.; Ma, Y.-J.; Pongsuwanna, Y.; Miyamura, T. Molecular epidemiology of enterovirus 71 infection in the western pacific region. Pediatr. Int. 2004, 46, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Mizuta, K.; Aoki, Y.; Matoba, Y.; Yahagi, K.; Itagaki, T.; Katsushima, F.; Katsushima, Y.; Ito, S.; Hongo, S.; Matsuzaki, Y. Molecular epidemiology of enterovirus 71 strains isolated from children in Yamagata, Japan, between 1990 and 2013. J. Med. Microbiol. 2014, 63, 1356–1362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- AbuBakar, S.; Chee, H.Y.; Al-Kobaisi, M.F.; Xiaoshan, J.; Chua, K.B.; Lam, S.K. Identification of enterovirus 71 isolates from an outbreak of hand, foot and mouth disease (HFMD) with fatal cases of encephalomyelitis in malaysia. Virus Res. 1999, 61, 1–9. [Google Scholar] [CrossRef]
- Wang, Y.; Feng, Z.; Yang, Y.; Self, S.; Gao, Y.; Longini, I.M.; Wakefield, J.; Zhang, J.; Wang, L.; Chen, X.; et al. Hand, foot, and mouth disease in china: Patterns of spread and transmissibility. Epidemiology 2011, 22, 781–792. [Google Scholar] [CrossRef] [PubMed]
- Fischer, T.K.; Nielsen, A.Y.; Sydenham, T.V.; Andersen, P.H.; Andersen, B.; Midgley, S.E. Emergence of enterovirus 71 C4a in Denmark, 2009 to 2013. Eurosurveillance 2014, 19, 20911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Medici, M.C.; Tummolo, F.; Arcangeletti, M.C.; De Conto, F.; Chezzi, C.; Dodi, I.; Calderaro, A. A cluster of enterovirus 71 subgenogroup C2 in a nursery school, Italy, 2014. New Microbiol. 2016, 39, 295–298. [Google Scholar] [PubMed]
- Akhmadishina, L.V.; Eremeeva, T.P.; Trotsenko, O.E.; Ivanova, O.E.; Mikhailov, M.I.; Lukashev, A.N. Seroepidemiology and molecular epidemiology of enterovirus 71 in Russia. PLoS ONE 2014, 9, e97404. [Google Scholar] [CrossRef] [PubMed]
- Zander, A.; Britton, P.N.; Navin, T.; Horsley, E.; Tobin, S.; McAnulty, J.M. An outbreak of enterovirus 71 in metropolitan Sydney: Enhanced surveillance and lessons learnt. Med. J. Aust. 2014, 201, 663–666. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.M.; Lei, H.Y.; Huang, M.C.; Su, L.Y.; Lin, H.C.; Yu, C.K.; Wang, J.L.; Liu, C.C. Modulation of cytokine production by intravenous immunoglobulin in patients with enterovirus 71-associated brainstem encephalitis. J. Clin. Virol. 2006, 37, 47–52. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.Y.; Dong, D.Y.; Liu, R.J.; Han, J.F.; Wang, G.C.; Zhao, H.; Li, X.F.; Deng, Y.Q.; Zhu, S.Y.; Wang, X.Y.; et al. Human igg subclasses against enterovirus type 71: Neutralization versus antibody dependent enhancement of infection. PLoS ONE 2013, 8, e64024. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.M.; Chen, I.C.; Su, L.Y.; Huang, K.J.; Lei, H.Y.; Liu, C.C. Enterovirus 71 infection of monocytes with antibody-dependent enhancement. Clin. Vaccine Immunol. 2010, 17, 1517–1523. [Google Scholar] [CrossRef] [PubMed]
- Han, J.F.; Cao, R.Y.; Deng, Y.Q.; Tian, X.; Jiang, T.; Qin, E.D.; Qin, C.F. Antibody dependent enhancement infection of enterovirus 71 in vitro and in vivo. Virol. J. 2011, 8, 106. [Google Scholar] [CrossRef] [PubMed]
- Chen, I.C.; Wang, S.-M.; Yu, C.-K.; Liu, C.-C. Subneutralizing antibodies to enterovirus 71 induce antibody-dependent enhancement of infection in newborn mice. Med. Microbiol. Immunol. 2013, 202, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.M.; Lei, H.Y.; Huang, M.C.; Wu, J.M.; Chen, C.T.; Wang, J.N.; Wang, J.R.; Liu, C.C. Therapeutic efficacy of milrinone in the management of enterovirus 71-induced pulmonary edema. Pediatr. Pulmonol. 2005, 39, 219–223. [Google Scholar] [CrossRef] [PubMed]
- Han, J.F.; Cao, R.Y.; Tian, X.; Yu, M.; Qin, E.D.; Qin, C.F. Producing infectious enterovirus type 71 in a rapid strategy. Virol. J. 2010, 7, 116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, P.; Bao, L.; Xu, L.; Li, F.; Lv, Q.; Deng, W.; Xu, Y.; Qin, C. Neurotropism in vitro and mouse models of severe and mild infection with clinical strains of enterovirus 71. Viruses 2017, 9, 351. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ji, L.; Yuan, X.; Jin, Y.; Cardona, C.J.; Xing, Z. Differential regulation of tlr signaling on the induction of antiviral interferons in human intestinal epithelial cells infected with enterovirus 71. PLoS ONE 2016, 11, e0152177. [Google Scholar] [CrossRef] [PubMed]
- Lui, Y.L.E.; Timms, P.; Hafner, L.M.; Tan, T.L.; Tan, K.H.; Tan, E.L. Characterisation of enterovirus 71 replication kinetics in human colorectal cell line, HT29. SpringerPlus 2013, 2, 267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.F.; Chou, C.T.; Lei, H.Y.; Liu, C.C.; Wang, S.M.; Yan, J.J.; Su, I.J.; Wang, J.R.; Yeh, T.M.; Chen, S.H.; et al. A mouse-adapted enterovirus 71 strain causes neurological disease in mice after oral infection. J. Virol. 2004, 78, 7916–7924. [Google Scholar] [CrossRef] [PubMed]
- Xing, Z.; Huang, H.-I.; Chio, C.-C.; Lin, J.-Y. Inhibition of EV71 by curcumin in intestinal epithelial cells. PLoS ONE 2018, 13, e0191617. [Google Scholar]
- Ang, L.Y.E.; Too, H.K.I.; Tan, E.L.; Chow, T.-K.V.; Shek, P.-C.L.; Tham, E.; Alonso, S. Antiviral activity of lactobacillus reuteri protectis against coxsackievirus A and enterovirus 71 infection in human skeletal muscle and colon cell lines. Virol. J. 2016, 13, 111. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Zheng, Z.; Shu, B.; Meng, J.; Zhang, Y.; Zheng, C.; Ke, X.; Gong, P.; Hu, Q.; Wang, H.; et al. Sumo modification stabilizes enterovirus 71 polymerase 3D to facilitate viral replication. J. Virol. 2016, 90, 10472–10485. [Google Scholar] [CrossRef] [PubMed]
- Du, X.; Wang, H.; Xu, F.; Huang, Y.; Liu, Z.; Liu, T.E. Enterovirus 71 induces apoptosis of SH-SY5Y human neuroblastoma cells through stimulation of endogenous microRNA let-7b expression. Mol. Med. Rep. 2015, 12, 953–959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, L.J.; Jiang, T.; Zhang, F.J.; Han, J.F.; Liu, J.; Zhao, H.; Li, X.F.; Liu, R.J.; Deng, Y.Q.; Wu, X.Y.; et al. Global transcriptomic analysis of human neuroblastoma cells in response to enterovirus type 71 infection. PLoS ONE 2013, 8, e65948. [Google Scholar] [CrossRef] [PubMed]
- Too, I.H.K.; Yeo, H.; Sessions, O.M.; Yan, B.; Libau, E.A.; Howe, J.L.C.; Lim, Z.Q.; Suku-Maran, S.; Ong, W.-Y.; Chua, K.B.; et al. Enterovirus 71 infection of motor neuron-like NSC-34 cells undergoes a non-lytic exit pathway. Sci. Rep. 2016, 6, 36983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shih, S.-R.; Weng, K.-F.; Stollar, V.; Li, M.-L. Viral protein synthesis is required for enterovirus 71 to induce apoptosis in human glioblastoma cells. J. Neurovirol. 2008, 14, 53–61. [Google Scholar] [CrossRef] [PubMed]
- Tse, H.; Victorio, C.B.L.; Xu, Y.; Ng, Q.; Chow, V.T.K.; Chua, K.B. Phenotypic and genotypic characteristics of novel mouse cell line (NIH/3T3)-adapted human enterovirus 71 strains (EV71:TLLm and EV71:TLLmv). PLoS ONE 2014, 9, e92719. [Google Scholar]
- Wang, B.; Xi, X.; Lei, X.; Zhang, X.; Cui, S.; Wang, J.; Jin, Q.; Zhao, Z. Enterovirus 71 protease 2Apro targets MAVS to inhibit anti-viral type I interferon responses. PLoS Pathog. 2013, 9, e1003231. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xiao, F.; Wan, P.; Pan, P.; Zhang, Y.; Liu, F.; Wu, K.; Liu, Y.; Wu, J. Ev71 3d protein binds with nlrp3 and enhances the assembly of inflammasome complex. PLoS Pathog. 2017, 13, e1006123. [Google Scholar] [CrossRef] [PubMed]
- Khong, W.X.; Foo, D.G.; Trasti, S.L.; Tan, E.L.; Alonso, S. Sustained high levels of interleukin-6 contribute to the pathogenesis of enterovirus 71 in a neonate mouse model. J. Virol. 2011, 85, 3067–3076. [Google Scholar] [CrossRef] [PubMed]
- Chang, G.H.; Lin, L.; Luo, Y.J.; Cai, L.J.; Wu, X.Y.; Xu, H.M.; Zhu, Q.Y. Sequence analysis of six enterovirus 71 strains with different virulences in humans. Virus Res. 2010, 151, 66–73. [Google Scholar] [CrossRef] [PubMed]
- Zaini, Z.; McMinn, P. A single mutation in capsid protein VP1 (Q145E) of a genogroup C4 strain of human enterovirus 71 generates a mouse-virulent phenotype. J. Gen. Virol. 2012, 93, 1935–1940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chua, B.H.; Phuektes, P.; Sanders, S.A.; Nicholls, P.K.; McMinn, P.C. The molecular basis of mouse adaptation by human enterovirus 71. J. Gen. Virol. 2008, 89, 1622–1632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.C.; Yu, C.-K.; Wang, Y.-F.; Liu, C.-C.; Su, I.-J.; Lei, H.-Y. A murine oral enterovirus 71 infection model with central nervous system involvement. J. Gen. Virol. 2004, 85, 69–77. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, W.; Duo, J.; Liu, J.; Ma, C.; Zhang, L.; Wei, Q.; Qin, C. A mouse muscle-adapted enterovirus 71 strain with increased virulence in mice. Microbes Infect. 2011, 13, 862–870. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.W.; Chang, K.C.; Kao, C.M.; Chang, S.P.; Tung, Y.Y.; Chen, S.H. Lymphocyte and antibody responses reduce enterovirus 71 lethality in mice by decreasing tissue viral loads. J. Virol. 2009, 83, 6477–6483. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.L.; Lee, Y.-P.; Liu, C.-C.; Yeh, T.-M.; Wang, S.-M.; Chen, S.-H.; Wang, Y.-F.; Su, I.-J.; Lei, H.-Y.; Wang, J.-R.; et al. Type I interferons protect mice against enterovirus 71 infection. J. Gen. Virol. 2005, 86, 3263–3269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.H.; Li, C.M.; Ling, P.; Shen, F.H.; Chen, S.H.; Liu, C.C.; Yu, C.K.; Chen, S.H. Ribavirin reduces mortality in enterovirus 71-infected mice by decreasing viral replication. J. Infect. Dis. 2008, 197, 854–857. [Google Scholar] [CrossRef] [PubMed]
- Ong, K.C.; Devi, S.; Cardosa, M.J.; Wong, K.T. Formaldehyde-inactivated whole-virus vaccine protects a murine model of enterovirus 71 encephalomyelitis against disease. J. Virol. 2010, 84, 661–665. [Google Scholar] [CrossRef] [PubMed]
- Ong, K.C.; Badmanathan, M.; Devi, S.; Leong, K.L.; Cardosa, M.J.; Wong, K.T. Pathologic characterization of a murine model of human enterovirus 71 encephalomyelitis. J. Neuropathol. Exp. Neurol. 2008, 67, 532–542. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.H.; Tsai, C.C.; Wang, L.C.; Chang, K.C.; Tung, Y.Y.; Su, I.J.; Chen, S.H. Enterovirus 71 infection increases expression of interferon-gamma-inducible protein 10 which protects mice by reducing viral burden in multiple tissues. J. Gen. Virol. 2013, 94, 1019–1027. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Dong, W.; Quan, X.; Ma, C.; Qin, C.; Zhang, L. Transgenic expression of human p-selectin glycoprotein ligand-1 is not sufficient for enterovirus 71 infection in mice. Arch. Virol. 2011, 157, 539–543. [Google Scholar] [CrossRef] [PubMed]
- Fujii, K.; Nagata, N.; Sato, Y.; Ong, K.C.; Wong, K.T.; Yamayoshi, S.; Shimanuki, M.; Shitara, H.; Taya, C.; Koike, S. Transgenic mouse model for the study of enterovirus 71 neuropathogenesis. Proc. Natl. Acad. Sci. USA 2013, 110, 14753–14758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.-W.; Yu, S.-L.; Shao, H.-Y.; Lin, H.-Y.; Liu, C.-C.; Hsiao, K.-N.; Chitra, E.; Tsou, Y.-L.; Chang, H.-W.; Sia, C.; et al. Human SCARB2 transgenic mice as an infectious animal model for enterovirus 71. PLoS ONE 2013, 8, e57591. [Google Scholar] [CrossRef] [PubMed]
- Khong, W.X.; Yan, B.; Yeo, H.; Tan, E.L.; Lee, J.J.; Ng, J.K.; Chow, V.T.; Alonso, S. A non-mouse-adapted enterovirus 71 (EV71) strain exhibits neurotropism, causing neurological manifestations in a novel mouse model of EV71 infection. J. Virol. 2012, 86, 2121–2131. [Google Scholar] [CrossRef] [PubMed]
- Caine, E.A.; Partidos, C.D.; Santangelo, J.D.; Osorio, J.E. Adaptation of enterovirus 71 to adult interferon deficient mice. PLoS ONE 2013, 8, e59501. [Google Scholar] [CrossRef] [PubMed]
- Arita, M.; Ami, Y.; Wakita, T.; Shimizu, H. Cooperative effect of the attenuation determinants derived from poliovirus sabin 1 strain is essential for attenuation of enterovirus 71 in the NOD/SCID mouse infection model. J. Virol. 2008, 82, 1787–1797. [Google Scholar] [CrossRef] [PubMed]
- Lyu, K.; Wang, G.C.; He, Y.L.; Han, J.F.; Ye, Q.; Qin, C.F.; Chen, R. Crystal structures of enterovirus 71 (EV71) recombinant virus particles provide insights into vaccine design. J. Biol. Chem. 2015, 290, 3198–3208. [Google Scholar] [CrossRef] [PubMed]
- Ooi, M.H.; Wong, S.C.; Lewthwaite, P.; Cardosa, M.J.; Solomon, T. Clinical features, diagnosis, and management of enterovirus 71. Lancet Neurol. 2010, 9, 1097–1105. [Google Scholar] [CrossRef]
- Chang, L.Y.; King, C.C.; Hsu, K.H.; Ning, H.C.; Tsao, K.C.; Li, C.C.; Huang, Y.C.; Shih, S.R.; Chiou, S.T.; Chen, P.Y.; et al. Risk factors of enterovirus 71 infection and associated hand, foot, and mouth disease/herpangina in children during an epidemic in taiwan. Pediatrics 2002, 109, e88. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, Y.; Shimojima, M.; Tano, Y.; Miyamura, T.; Wakita, T.; Shimizu, H. Human p-selectin glycoprotein ligand-1 is a functional receptor for enterovirus 71. Nat. Med. 2009, 15, 794–797. [Google Scholar] [CrossRef] [PubMed]
- Yamayoshi, S.; Yamashita, Y.; Li, J.; Hanagata, N.; Minowa, T.; Takemura, T.; Koike, S. Scavenger receptor B2 is a cellular receptor for enterovirus 71. Nat. Med. 2009, 15, 798–801. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.L.; Chou, Y.T.; Wu, C.N.; Ho, M.S. Annexin ii binds to capsid protein vp1 of enterovirus 71 and enhances viral infectivity. J. Virol. 2011, 85, 11809–11820. [Google Scholar] [CrossRef] [PubMed]
- Su, P.Y.; Wang, Y.F.; Huang, S.W.; Lo, Y.C.; Wang, Y.H.; Wu, S.R.; Shieh, D.B.; Chen, S.H.; Wang, J.R.; Lai, M.D.; et al. Cell surface nucleolin facilitates enterovirus 71 binding and infection. J. Virol. 2015, 89, 4527–4538. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.W.; Poh, C.L.; Sam, I.C.; Chan, Y.F. Enterovirus 71 uses cell surface heparan sulfate glycosaminoglycan as an attachment receptor. J. Virol. 2013, 87, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Ren, X.X.; Ma, L.; Liu, Q.W.; Li, C.; Huang, Z.; Wu, L.; Xiong, S.D.; Wang, J.H.; Wang, H.B. The molecule of DC-SIGN captures enterovirus 71 and confers dendritic cell-mediated viral trans-infection. Virol. J. 2014, 11, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fukuhara, M.; Iwami, S.; Sato, K.; Nishimura, Y.; Shimizu, H.; Aihara, K.; Koyanagi, Y. Quantification of the dynamics of enterovirus 71 infection by experimental-mathematical investigation. J. Virol. 2013, 87, 701–705. [Google Scholar] [CrossRef] [PubMed]
- Yu, P.; Gao, Z.; Zong, Y.; Bao, L.; Xu, L.; Deng, W.; Li, F.; Lv, Q.; Gao, Z.; Xu, Y.; et al. Histopathological features and distribution of EV71 antigens and SCARB2 in human fatal cases and a mouse model of enterovirus 71 infection. Virus Res. 2014, 189, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.S.; Yao, Y.C.; Lin, S.C.; Lee, Y.P.; Wang, Y.F.; Wang, J.R.; Liu, C.C.; Lei, H.Y.; Yu, C.K. Retrograde axonal transport: A major transmission route of enterovirus 71 in mice. J. Virol. 2007, 81, 8996–9003. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.T.; Munisamy, B.; Ong, K.C.; Kojima, H.; Noriyo, N.; Chua, K.B.; Ong, B.B.; Nagashima, K. The distribution of inflammation and virus in human enterovirus 71 encephalomyelitis suggests possible viral spread by neural pathways. J. Neuropathol. Exp. Neurol. 2008, 67, 162–169. [Google Scholar] [CrossRef] [PubMed]
- Ren, R.; Racaniello, V.R. Poliovirus spreads from muscle to the central nervous system by neural pathways. J. Infect. Dis. 1992, 166, 747–752. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, J.; Li, Q. Immune evasion of enteroviruses under innate immune monitoring. Front. Microbiol. 2018, 9, 1866. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Ennis, J.; Turner, J.D.; Chu, J.J. Single dose of an adenovirus vectored mouse interferon-alpha protects mice from lethal EV71 challenge. Antivir. Res. 2016, 134, 207–215. [Google Scholar] [CrossRef] [PubMed]
- Lin, H.; Huang, L.; Zhou, J.; Lin, K.; Wang, H.; Xue, X.; Xia, C. Efficacy and safety of interferon-α2b spray in the treatment of hand, foot, and mouth disease: A multicenter, randomized, double-blind trial. Arch. Virol. 2016, 161, 3073–3080. [Google Scholar] [CrossRef] [PubMed]
- Meng, G.; Li, M.; Li, Y.; Wang, X.; Chen, Q.; Wei, H.J.C.J.C.E.P. Severe hand-foot-mouth disease: A report of 3 autopsied cases. Chin. J. Clin. Exp. Pathol. 2011, 27, 48–51. [Google Scholar]
- Wang, S.M.; Lei, H.Y.; Yu, C.K.; Wang, J.R.; Su, I.J.; Liu, C.C. Acute chemokine response in the blood and cerebrospinal fluid of children with enterovirus 71-associated brainstem encephalitis. J. Infect. Dis. 2008, 198, 1002–1006. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.L.; Chow, Y.H.; Huang, L.M.; Hsieh, S.M.; Cheng, P.Y.; Hu, K.C.; Chiang, B.L. A CpG-adjuvanted intranasal enterovirus 71 vaccine elicits mucosal and systemic immune responses and protects human SCARB2-transgenic mice against lethal challenge. Sci. Rep. 2018, 8, 10713. [Google Scholar] [CrossRef] [PubMed]
- Chumakov, M.; Voroshilova, M.; Shindarov, L.; Lavrova, I.; Gracheva, L.; Koroleva, G.; Vasilenko, S.; Brodvarova, I.; Nikolova, M.; Gyurova, S.; et al. Enterovirus 71 isolated from cases of epidemic poliomyelitis-like disease in Bulgaria. Arch. Virol. 1979, 60, 329–340. [Google Scholar] [CrossRef] [PubMed]
- Nagy, G.; Takatsy, S.; Kukan, E.; Mihaly, I.; Domok, I. Virological diagnosis of enterovirus type 71 infections: Experiences gained during an epidemic of acute CNS diseases in hungary in 1978. Arch. Virol. 1982, 71, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Shih, S.R.; Ho, M.S.; Lin, K.H.; Wu, S.L.; Chen, Y.T.; Wu, C.N.; Lin, T.Y.; Chang, L.Y.; Tsao, K.C.; Ning, H.C.; et al. Genetic analysis of enterovirus 71 isolated from fatal and non-fatal cases of hand, foot and mouth disease during an epidemic in taiwan, 1998. Virus Res. 2000, 68, 127–136. [Google Scholar] [CrossRef]
- Yan, J.J.; Su, I.J.; Chen, P.F.; Liu, C.C.; Yu, C.K.; Wang, J.R. Complete genome analysis of enterovirus 71 isolated from an outbreak in taiwan and rapid identification of enterovirus 71 and coxsackievirus A16 by RT-PCR. J. Med. Virol. 2001, 65, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Zou, Q.; Chen, L.; Zhang, H.; Wang, Y. Molecular analysis of virulent determinants of enterovirus 71. PLoS ONE 2011, 6, e26237. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.W.; Wang, Y.F.; Yu, C.K.; Su, I.J.; Wang, J.R. Mutations in VP2 and VP1 capsid proteins increase infectivity and mouse lethality of enterovirus 71 by virus binding and RNA accumulation enhancement. Virology 2012, 422, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Yeh, M.T.; Wang, S.W.; Yu, C.K.; Lin, K.H.; Lei, H.Y.; Su, I.J.; Wang, J.R. A single nucleotide in stem loop II of 5′-untranslated region contributes to virulence of enterovirus 71 in mice. PLoS ONE 2011, 6, e27082. [Google Scholar] [CrossRef] [PubMed]
- Cordey, S.; Petty, T.J.; Schibler, M.; Martinez, Y.; Gerlach, D.; van Belle, S.; Turin, L.; Zdobnov, E.; Kaiser, L.; Tapparel, C. Identification of site-specific adaptations conferring increased neural cell tropism during human enterovirus 71 infection. PLoS Pathog. 2012, 8, e1002826. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, K.; Sudaka, Y.; Takashino, A.; Imura, A.; Fujii, K.; Koike, S. Amino acid variation at VP1-145 of enterovirus 71 determines attachment receptor usage and neurovirulence in human scavenger receptor B2 transgenic mice. J. Virol. 2018. [Google Scholar] [CrossRef] [PubMed]
- Tseligka, E.D.; Sobo, K.; Stoppini, L.; Cagno, V.; Abdul, F.; Piuz, I.; Meylan, P.; Huang, S.; Constant, S.; Tapparel, C. A VP1 mutation acquired during an enterovirus 71 disseminated infection confers heparan sulfate binding ability and modulates ex vivo tropism. PLoS Pathog. 2018, 14, e1007190. [Google Scholar] [CrossRef] [PubMed]
- McMinn, P.C. An overview of the evolution of enterovirus 71 and its clinical and public health significance. FEMS Microbiol. Rev. 2002, 26, 91–107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, K.D.; Yang, M.Y.; Li, C.C.; Lin, S.F.; Chong, M.C.; Wang, C.L.; Chen, R.F.; Lin, T.Y. Altered cellular but not humoral reactions in children with complicated enterovirus 71 infections in Taiwan. J. Infect. Dis. 2001, 183, 850–856. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.Y.; Chang, I.S.; Chen, W.J.; Huang, Y.C.; Chen, G.W.; Shih, S.R.; Juang, J.L.; Shih, H.M.; Hsiung, C.A.; Lin, T.Y.; et al. HLA-A33 is associated with susceptibility to enterovirus 71 infection. Pediatrics 2008, 122, 1271–1276. [Google Scholar] [CrossRef] [PubMed]
- Li, J.A.; Chen, Z.B.; Lv, T.G.; Han, Z.L.; Liu, P.P. Impact of endothelial nitric oxide synthase gene polymorphism on severity of enterovirus 71-infection in Chinese children. Clin. Biochem. 2013, 46, 1842–1847. [Google Scholar] [CrossRef] [PubMed]
- Zou, R.; Zhang, G.; Li, S.; Wang, W.; Yuan, J.; Li, J.; Wang, Y.; Lin, Y.; Deng, Y.; Zhou, B.; et al. A functional polymorphism in IFNAR1 gene is associated with susceptibility and severity of HFMD with EV71 infection. Sci. Rep. 2015, 5, 18541. [Google Scholar] [CrossRef] [PubMed]
- Zhu, F.; Xu, W.; Xia, J.; Liang, Z.; Liu, Y.; Zhang, X.; Tan, X.; Wang, L.; Mao, Q.; Wu, J.; et al. Efficacy, safety, and immunogenicity of an enterovirus 71 vaccine in china. N. Engl. J. Med. 2014, 370, 818–828. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Liu, L.; Mo, Z.; Wang, X.; Xia, J.; Liang, Z.; Zhang, Y.; Li, Y.; Mao, Q.; Wang, J.; et al. An inactivated enterovirus 71 vaccine in healthy children. N. Engl. J. Med. 2014, 370, 829–837. [Google Scholar] [CrossRef] [PubMed]
- Wei, M.; Meng, F.; Wang, S.; Li, J.; Zhang, Y.; Mao, Q.; Hu, Y.; Liu, P.; Shi, N.; Tao, H.; et al. 2-year efficacy, immunogenicity, and safety of vigoo enterovirus 71 vaccine in healthy Chinese children: A randomized open-label study. J. Infect. Dis. 2017, 215, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Yi, E.J.; Shin, Y.J.; Kim, J.H.; Kim, T.G.; Chang, S.Y. Enterovirus 71 infection and vaccines. Clin. Exp. Vaccine Res. 2017, 6, 4–14. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhao, B.; Chen, X.; Song, N.; Wu, J.; Li, G.; Yu, P.; Han, Y.; Liu, J.; Qin, C. GS-9620 inhibits enterovirus 71 replication mainly through the NF-kB and PI3K-AKT signaling pathways. Antivir. Res. 2018, 153, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Zhang, X.; Wang, F.; Wei, H.; Ma, H.; Sui, M.; Lu, J.; Wang, H.; Dumler, J.S.; Sheng, G.; et al. Clinical efficacy of therapy with recombinant human interferon alpha1b in hand, foot, and mouth disease with enterovirus 71 infection. PLoS ONE 2016, 11, e0148907. [Google Scholar]
- Qiu, S.; Liu, N.; Jia, L.; Yang, G.; Su, W.; Li, J.; Song, L.; Yang, C.; Wang, J.; Zhang, C.; et al. A new treatment for neurogenic inflammation caused by EV71 with CR2-targeted complement inhibitor. Virol. J. 2012, 9, 285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, W.; Wu, Y.; Bi, J.; Lu, X.; Hou, A.; Zhou, Y.; Sun, B.; Kong, W.; Barbier, J.; Cintrat, J.-C.; et al. Antiviral effects of Retro-2cycl and Retro-2.1 against Enterovirus 71 in vitro and in vivo. Antivir. Res. 2017, 144, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zhou, F.; Gu, B.; Ding, C.; Feng, D.; Xie, F.; Wang, J.; Zhang, C.; Cao, Q.; Deng, Y.; et al. In vitro and in vivo evaluation of ribavirin and pleconaril antiviral activity against enterovirus 71 infection. Arch. Virol. 2012, 157, 669–679. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Yang, X.; Guan, C.; Wen, T.; Duan, Y.; Zhang, W.; Li, X.; Wang, Y.; Zhao, Z.; Liu, S. Andrographolide sulfonate reduces mortality in enterovirus 71 infected mice by modulating immunity. Int. Immunopharmacol. 2018, 55, 142–150. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Li, X.; Fan, X.; Ma, C.; Qin, C.; Zhang, L. Adoptive transfer of macrophages from adult mice reduces mortality in mice infected with human enterovirus 71. Arch. Virol. 2013, 158, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.W.; Huang, B.S.; Hsu, C.W.; Peng, C.W.; Cheng, M.L.; Kao, J.Y.; Way, T.D.; Yin, H.C.; Wang, S.S. Efficacy and safety evaluation of a chlorine dioxide solution. Int. J. Environ. Res. Public Health 2017, 14, 329. [Google Scholar] [CrossRef] [PubMed]
- Wong, T.W.; Huang, H.J.; Wang, Y.F.; Lee, Y.P.; Huang, C.C.; Yu, C.K. Methylene blue-mediated photodynamic inactivation as a novel disinfectant of enterovirus 71. J. Antimicrob. Chemother. 2010, 65, 2176–2182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, B.; Xu, L.; Zhu, R.; Tang, J.; Wu, Y.; Su, R.; Yin, Z.; Liu, D.; Jiang, Y.; Wen, C.; et al. A bispecific broadly neutralizing antibody against enterovirus 71 and coxsackievirus A16 with therapeutic potential. Antivir. Res. 2019, 161, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Chang, H.-W.; Lin, Y.-W.; Ho, H.-M.; Lin, M.-H.; Liu, C.-C.; Shao, H.-Y.; Chong, P.; Sia, C.; Chow, Y.-H. Protective efficacy of VP1-specific neutralizing antibody associated with a reduction of viral load and pro-inflammatory cytokines in human SCARB2-transgenic mice. PLoS ONE 2013, 8, e69858. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.-T.; Lin, S.-J.; Wang, H.-C.; Chen, P.-C.; Lin, J.-J.; Chiang, J.-R.; Chang, C.-L.; Shih, D.Y.-C.; Lo, C.-F.; Wang, D.-Y. Establishment of an animal challenge model as a potency assay for an inactivated enterovirus type 71 vaccine. Biologicals 2016, 44, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Cao, R.-Y.; Han, J.-F.; Jiang, T.; Tian, X.; Yu, M.; Deng, Y.-Q.; Qin, E.D.; Qin, C.-F. In vitro and in vivo characterization of a new enterovirus type 71-specific human intravenous immunoglobulin manufactured from selected plasma donors. J. Clin. Virol. 2011, 51, 246–249. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Yao, P.-P.; Xia, Y.; Qian, L.; Yang, Z.-N.; Xie, R.-H.; Sun, Y.-S.; Lu, H.-J.; Miao, Z.-P.; Li, C.; et al. Enterovirus 71 infection causes severe pulmonary lesions in gerbils, meriones unguiculatus, which can be prevented by passive immunization with specific antisera. PLoS ONE 2015, 10, e0119173. [Google Scholar] [CrossRef] [PubMed]
- Ho, B.-C.; Yu, I.S.; Lu, L.-F.; Rudensky, A.; Chen, H.-Y.; Tsai, C.-W.; Chang, Y.-L.; Wu, C.-T.; Chang, L.-Y.; Shih, S.-R.; et al. Inhibition of miR-146a prevents enterovirus-induced death by restoring the production of type I interferon. Nat. Commun. 2014, 5, 3344. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Cell Line | Species | Tissue Origin | EV71 Genotype | CPE # | References |
|---|---|---|---|---|---|
| RD | Human | Rhabdomyosarcoma | A, B, C, D | + | [23,24,25,26,27,28,29,30] |
| SK-N-SH | Neuroblastoma | C2, D | + | [24,27] | |
| SH-SY5Y | Neuroblastoma | C4 | + | [31,32] | |
| NSC-34 | motor neuron cell line | B2, B4 & C2 | − | [33] | |
| SF268 | glioblastoma | * | + | [34] | |
| Neuro-2A | Mouse | Neuroblastoma | D | + | [24,35] |
| HT29 | Human | intestinal epithelial cell | B4, C | + | [25,26,28] |
| Caco-2 | colorectal carcinoma | B, C2 | + | [27,29] | |
| C2BBel | mature intestinal epithelial cell (derived from Caco-2) | C | + | [28] | |
| HeLa | Human | cervical carcinoma | EV71:BS (Accession NO KF514878), C4 | + | [35,36] |
| HEK293T | Human | embryonic kidney | EV71:BS (Accession NO KF514878), C4 | + | [35,37] |
| THP-1 | Human | monocytic cell line | C2, C4 | [19,37] | |
| human PBMC | C2 | [19] | |||
| Vero | Monkey | African green monkey kidney | A, EV71:BS (Accession NO KF514878) | + | [30,35] |
| COS-7 | African green monkey kidney (SV40 transformed) | EV71:BS (Accession NO KF514878) | + | [35] | |
| NIH/3T3 | Mouse | mouse embryonic fibroblast | mouse-adapted strain (EV:TLLm) | + | [35] |
| Mouse Strain | Host Age @ | Route | Genotype | Titer/Mouse | Phenotype | Virus-Replicating Tissue | Reference |
|---|---|---|---|---|---|---|---|
| Balb/C | 1-day-old | i.p. | B4 | 1 × 104 PFU | limb paralysis & death | intestine | [38] |
| 2-day-old | i.p./i.c. | C4 | 103 TCID50 | * | brain, liver and intestine | [39] | |
| 5-day-old | i.p. | C4 (mouse adapted strain) | 104 TCID50 | limb paralysis & death | spleen, heart, liver, muscle and brain | [40] | |
| 7-day-old | i.m. i.p. i.c. | B3 (CHO cell adapted strain; CHO-26M) (mouse adapted strain; MP-26M) | 103 TCID50 | death | muscle (CHO-26M) blood, spleen, heart, liver, muscle and brain (MP-26M) | [41] | |
| 9-day-old | i.p. | C4 | 104.5 TCID50 | limb paralysis | skeletal muscle, spinal cord, brainstem, lung, and jejunum | [24] | |
| ICR | 1-day-old | oral | C (mouse adapted strain) | 106–107 PFU | paralysis & skin lesion | skin, intestine and spinal cord | [42] |
| 1-day-old | i.p. | C4 (Fuyang-0805a) | 2 × 105 TCID50 | none | muscles, intestines, lungs, and brain | [43] | |
| 7-day-old | oral | C (mouse adapted strain M2) | 5 × 106 PFU | death | * | [44] | |
| 7-day-old | i.p. | C2 (mouse adapted strain MP4) | 2.5 × 106 PFU | death | brain, spinal cord, lung and muscle | [21] | |
| 3-day-old | i.p. | C2 (clinical isolate) | 4 × 106 PFU | death | * | [45] | |
| 7-day-old | oral | C2 (mouse adapted strain MP4) | 5 × 105 PFU | death | * | [45] | |
| 12–14-day-old | i.p. | C (mouse adapted strain M2) | 5 × 104–105 PFU | paralysis & death | * | [46] | |
| 14-day-old | i.p. | B3 (mouse adapted strain) | 100 CCID50 | paralysis | brain and muscle | [47] | |
| 14-day- & 28-day-old | i.p. i.m. oral s.c. | B3 (mouse adapted strain) | 105 CCID50 | paralysis | mainly in CNS | [48] | |
| C57BL/6 | 9-day-old | oral | C (mouse adapted strain M2) | 3 × 107 PFU | * | * | [44] |
| C57BL/6J | 14-day-old | i.p. | C (mouse adapted strain M2) | 3 × 105 PFU | hunched posture, paralysis & death | brain | [49] |
| PSGL-1 Tg | 10-day-old | i.p. | C4 (clinical isolate) | 108 TCID50 | no disease onset | brain, spinal cord and skeletal muscle | [50] |
| C4 (mouse adapted strain MP10) | 5 × 106 TCID50 | hindlimb paralysis | * | ||||
| hSCARB2 Tg | 3-week-old | i.v. | C (Isehara strain) | 102–6 TCID50 | ataxia, paralysis & death | CNS | [51] |
| i.c. | A, B and C | 102–6 TCID50 | |||||
| i.p. | C (Isehara strain) | 103–6 TCID50 | |||||
| oral | C (Isehara strain) | 106–7 TCID50 | |||||
| pEF-1α-hSCARB2 | 1-day-old | s.c. | B | 1 × 107 PFU | HFMD-like syndrome & paralysis | CNS, intestine, muscle and skin | [52] |
| 7-day-old | s.c. | C | 3 × 104 PFU | ||||
| AG129 | younger than 2-week-old | i.p. | B4 | 5 × 105–107 PFU | limb paralysis & death | CNS | [53] |
| oral | 106–107 PFU | ||||||
| AG129 | 10-week-old | i.p. | B2 (mouse adapted strain) | 1.3 × 105 TCID50/mL | limb paralysis & death | ND | [54] |
| A129 | |||||||
| G129 | 7-day-old | i.p. | B5 (clinical isolates) | 108 PFU | limb paralysis & death | ND | [4] |
| stat-1 KO | 7-day-old | i.p. | B5 (clinical isolates) | 108 PFU | limb paralysis & death | CNS | [4] |
| pSCARB2x stat-1 KO | younger than 2-week-old | i.p. | C2 (clinical isolates) | 106–108 PFU | [5] | ||
| NOD/SCID | 3-day-old | oral | B5 (clinical isolates) | 108 PFU | hair loss, skin lesion, limb paralysis & death | muscle and spleen | [4] |
| 7-day-old | i.p. | 107–108 PFU | |||||
| 3–4 week-old | i.v./i.c. | B1 (mouse adapted strain) | 106.5 CCID50 | skeletal muscle and heart | [55] | ||
| IP10 KO | 14-day-old | i.p. | C (mouse adapted strain M2) | 3 × 105 PFU | The death rate was higher than C57BL/6J | ND | [49] |
| Mouse Strains | Promoter | Virus Strain (Genotype) | Host Age | Virus Dose | Inoculation Route | % Earlier symptom ** (Tg vs. non-Tg) | % Limb Paralysis (Tg vs. non-Tg) | % Death (Tg vs. non-Tg) | References |
|---|---|---|---|---|---|---|---|---|---|
| pEF-1α- hSCARB2 | ubiquitous EF-1 Promoter | E59 (B4) | 1-day-old | 3 × 104 PFU | s.c. | 100 vs. 57.1 | 18.8 vs. 14.2 | 0 vs. 0 | [52] |
| N-2838 (B5) | 100 vs.75 | 44.4 vs. 12.5 | 0 vs. 0 | ||||||
| 5476 (C2) | 7-day-old | 107 PFU | s.c. | Nd @ | 100 vs. 57.1 | 100 vs. 0 | |||
| N3340 (C4) | Nd @ | 100 vs. 100 | 100 vs. 0 | ||||||
| pSC2- hSCARB2 | native promoter | Isehara (C2) | 3-wk-old | 106 TCID50 | i.c. | N/A # | 100 vs. 0 * | [51] | |
| i.v. | N/A # | 89 vs. 0 * | |||||||
| i.p. | N/A # | 39 vs. 0 * | |||||||
| oral | N/A # | 5 vs. 0 * | |||||||
| Therapeutic Approaches | Drug Candidates | Drug Delivery Methods | Human or Animal Model | Host Age at Virus Inoculation | Virus Inoculation | EV71 Clinical Isolates * or Adapted Strains # | Virus Genotype | Ref. |
|---|---|---|---|---|---|---|---|---|
| GROUP 1 Passive immunization | bispecific anti-EV71 & CA16 Ab Bs(scFv) | i.p. injection at 24 h after inoculation | BALB/c | 1-day-old | i.c. | EV71/pSVA-MP4 # | B3 | [104] |
| VP1-specific mAb | i.p. injection at 3, 24, and 48 h after inoculation | SCARB2-Tg | 1-day & 7-day-old | s.c. | E59 & 5746-TW98 * | B4 & C2 | [105] | |
| human plasma or IVIG | in vitro pre-mix with EV71 at 37 °C for 1 h | ICR | 2-day-old | i.p. | KM593929 * | C4 | [106] | |
| human IVIG | 3 i.p. injections at 4 h, 1- and 2-days after inoculation | Kunming mice | 7-day-old | i.p. | AH08/06 * | C4 | [107] | |
| EV71-specific Ab | i.p. injection 1 day before and 1 day after (ICR), or 2 and 4 days after inoculation (C57BL/6) | ICR & C57BL/6 | 7-day & 9-day-old | oral | M2 # | C2 | [44] | |
| adult immune sera | i.p. injection at 1hr before and 24 h after inoculation | young gerbils | 21-day-old | i.p. or i.m. | strain 58301 * | C4 | [108] | |
| human IVIG | i.v. infusion at a dosage of 1 g/kg/day for 2 days | human patients | <2-yr-old | natural infection | N/A | N/A | [17] | |
| GROUP 2 Immune modulators | Poly (I:C) | i.p. injection at 12 h before inoculation | ICR | 3-day-old | i.p. or oral | MP4 # | C2 | [45] |
| Ad-IFN-a | one intranasal shot within 12 h post-inoculation | BALB/c | 6-day-old | i.p. | strain 41 * | B4 | [71] | |
| antagomir -146a | i.p. injection before or after virus inoculation | C57BL/6 | 7-day-old | i.p. | mEV71 # | B2 | [109] | |
| GS-9620 | oral uptake at 2, 26, 50 h post-inoculation | ICR | 10-day-old | i.p. | MP10 # | C4 | [95] | |
| GROUP 3 Anti-inflammation | anti-IL6 Ab | i.p. co-injection of Ab and virus (day 0) | BALB/c | 1-day-old | i.p. | strain 41 * | B4 | [38] |
| complement inhibitor (CR2-crry) | treatment post-inoculation | ICR | 7-day-old | i.c. | BrCr * | A | [97] | |
| GROUP 4 Others | Retro-2cycl | i.p. injection following EV71 inoculation | BALB/c | <1-day-old | i.c. | KJ508817 * | C4 | [98] |
| Pleconaril | daily i.p. injection for 5 days | ICR | 1-day-old | i.p. | BrCr * | A | [99] | |
| andrographolide sulfonate | i.p. injection once daily until 10 days post-inoculation | ICR | 7-day-old | i.p. | BJ09/07 # | C4 | [100] | |
| adoptive transfer of macrophage | i.p. injection with adult macrophage at 1 day post-inoculation | ICR | 10-day-old | i.p. | MP10 # | C4 | [101] | |
| Milrinone | i.v. injection within 2–6 h after admission at dosage 0.35–0.55 mg/kg/min for 72 h. | human patients | <2-yr-old | natural infection | N/A | N/A | [22] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shih, C.; Liao, C.-C.; Chang, Y.-S.; Wu, S.-Y.; Chang, C.-S.; Liou, A.-T. Immunocompetent and Immunodeficient Mouse Models for Enterovirus 71 Pathogenesis and Therapy. Viruses 2018, 10, 674. https://doi.org/10.3390/v10120674
Shih C, Liao C-C, Chang Y-S, Wu S-Y, Chang C-S, Liou A-T. Immunocompetent and Immunodeficient Mouse Models for Enterovirus 71 Pathogenesis and Therapy. Viruses. 2018; 10(12):674. https://doi.org/10.3390/v10120674
Chicago/Turabian StyleShih, Chiaho, Chun-Che Liao, Ya-Shu Chang, Szu-Yao Wu, Chih-Shin Chang, and An-Ting Liou. 2018. "Immunocompetent and Immunodeficient Mouse Models for Enterovirus 71 Pathogenesis and Therapy" Viruses 10, no. 12: 674. https://doi.org/10.3390/v10120674
APA StyleShih, C., Liao, C. -C., Chang, Y. -S., Wu, S. -Y., Chang, C. -S., & Liou, A. -T. (2018). Immunocompetent and Immunodeficient Mouse Models for Enterovirus 71 Pathogenesis and Therapy. Viruses, 10(12), 674. https://doi.org/10.3390/v10120674