Landscape Phage: Evolution from Phage Display to Nanobiotechnology
Abstract
:1. Introduction
2. The History of Filamentous Phages
3. Phage Engineering
4. Classification of Filamentous Phage Display Systems
5. Development of Landscape-Phage Libraries
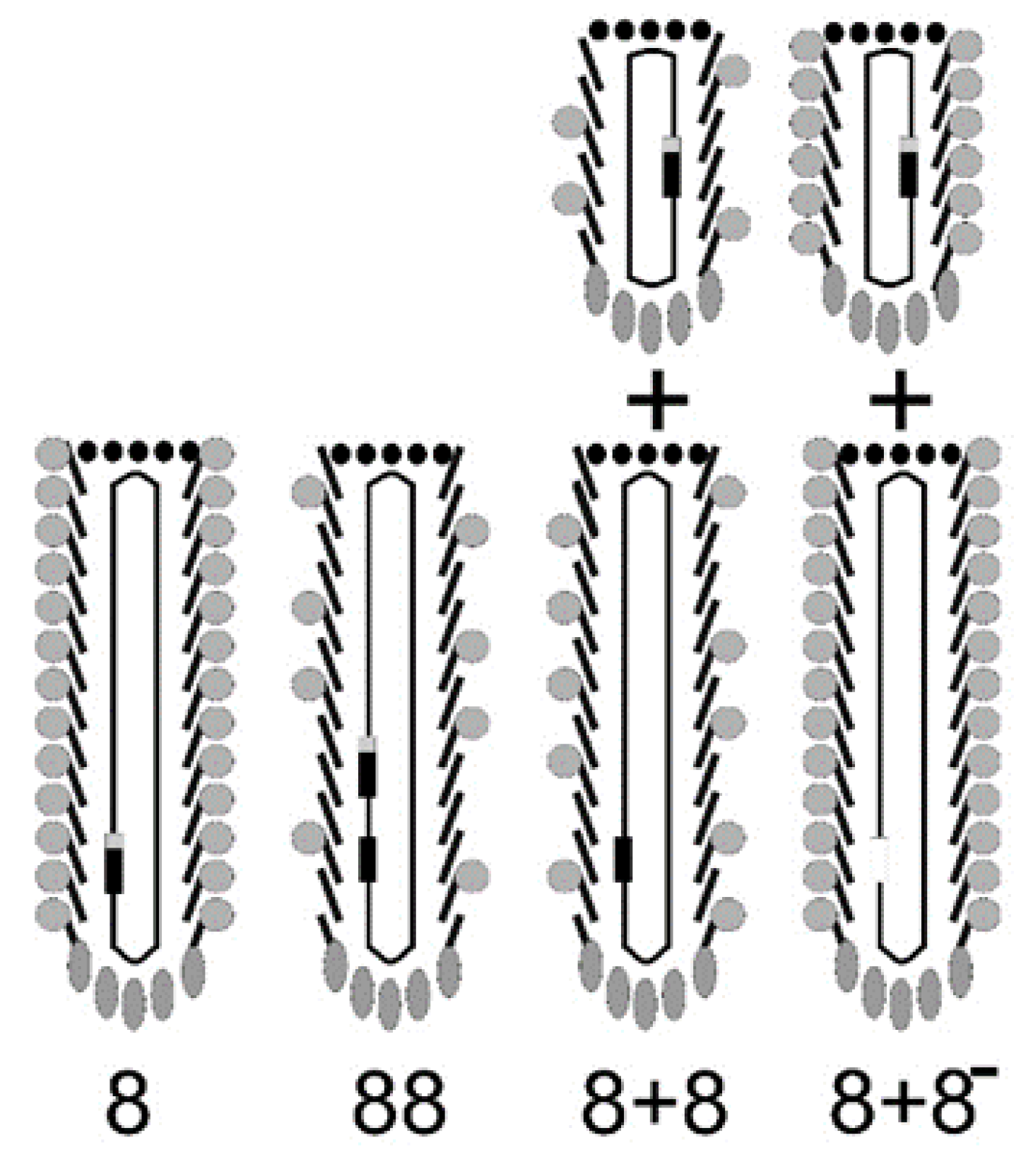
6. Landscape-Phage-Based Biosensors for Detection Monitoring of Biological Threats
7. Diagnostic-Therapeutic Cancer Cell-Targeted Landscape
8. Conclusions
Acknowledgments
Conflicts of Interest
References
- Webster, R. Filamentous Phage Biology. In Phage Display: A Laboratory Manual; Barbas, C.F., Burton, D.R., Silverman, G.J., Eds.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2001; pp. 1–37. [Google Scholar]
- Russel, M.; Model, P. Filamentous Phage. In The Bacteriophage, 2nd ed.; Calendar, R.L., Ed.; Oxford University Press, Inc.: Oxford, UK; New York, NY, USA, 2006; p. 746. [Google Scholar]
- Petrenko, V.A.; Smith, G.P. (Eds.) Phage Nanobiotechnology; RSC Publishing: Cambridge, UK, 2011; 273p. [Google Scholar]
- Stent, G.S. Molecular Biology of Bacterial Viruses; WH Freeman and Co.: San Francisco, CA, USA, 1963. [Google Scholar]
- Summers, W.C. Bacteriophage therapy. Annu. Rev. Microbiol. 2001, 55, 437–451. [Google Scholar] [CrossRef] [PubMed]
- Gale, E.P.; Reynolds, P.E.; Cundliffe, E.; Richmond, M.H.; Waring, M.J. The Molecular Basis of Antibiotic Action; Wiley-Interscience: Hoboken, NJ, USA, 1972. [Google Scholar]
- Cairns, J.; Stent, G.S.; Watson, J.D. (Eds.) Phage and the Origins of Molecular Biology, The Centennial Edition; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2007. [Google Scholar]
- Petrenko, V. Evolution of phage display: From bioactive peptides to bioselective nanomaterials. Expert Opin. Drug Deliv. 2008, 5, 825–836. [Google Scholar] [CrossRef] [PubMed]
- Glick, B.R.; Pasternak, J.J. Molecular Biotechnology: Principles and Applications of Recombinant DNA, 2nd ed.; ASM Press: Washington, DC, USA, 1998. [Google Scholar]
- Rodriguez, R.L.; David, T.; Denhardt, E. Vectors: A Survey of Molecular Cloning Vectors and Their Uses; Butterworth-Heinemann: London, UK, 1987; 592p. [Google Scholar]
- Petrenko, V.A.; Smith, G.P. Chapter 2: Vectors and modes of display. In Phage Display in Biotechnology and Drug Discovery; Gesyer, C.R., Sidhu, S.S., Eds.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2005; pp. 43–74. [Google Scholar]
- Smith, G.P.; Petrenko, V.A. Phage display. Chem. Rev. 1997, 97, 391–410. [Google Scholar] [CrossRef] [PubMed]
- Fellouse, F.A.; Pal, G. Methods for the Construction of Phage-Displayed Libraries. In Phage Display in Biotechnology and Drug Discovery; Sidhu, S.S., Geyer, C.R., Eds.; CRC Press: Boca Raton, FL, USA, 2015; pp. 75–96. [Google Scholar]
- Henry, K.A.; Arbabi-Ghahroudi, M.; Scott, J.K. Beyond phage display: Non-traditional applications of the filamentous bacteriophage as a vaccine carrier, therapeutic biologic, and bioconjugation scaffold. Front. Microbiol. 2015, 6, 755. [Google Scholar] [CrossRef] [PubMed]
- Barbas, C.F., III; Burton, D.R.; Scott, J.K.; Silverman, G.J. Phage Display: A. Laboratory Manual; Cold Spring Laboratory Press: Cold Spring Harbor, NY, USA, 2001. [Google Scholar]
- Sidhu, S.S.; Geyer, C.R. (Eds.) Phage Display in Biotechnology and Drug Discovery, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2015; 567p. [Google Scholar]
- Petrenko, V.A.; Smith, G.P.; Gong, X.; Quinn, T. A library of organic landscapes on filamentous phage. Protein Eng. 1996, 9, 797–801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrenko, V.A.; Smith, G.P. Vectors and modes of display. In Phage Display in Biotechnology and Drug Discovery, 2nd ed.; Sidhu, S.S., Geyer, C.R., Eds.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2015; p. 567. [Google Scholar]
- Kuzmicheva, G.A.; Jayanna, P.K.; Eroshkin, A.M.; Grishina, M.A.; Pereyaslavskaya, E.S.; Potemkin, V.A.; Petrenko, V.A. Mutations in fd phage major coat protein modulate affinity of the displayed peptide. Protein Eng. Des. Sel. 2009, 22, 631–639. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrenko, V.A. Landscape Phage as a Molecular Recognition Interface for Detection Devices. Microelectron. J. 2008, 39, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Lakshmanan, R.S.; Petrenko, V.A.; Chin, B.A. Phage-Based Pathogen Biosensors. In Phage Nanobiotechnology; Petrenko, V.A., Smith, G.P., Eds.; RSC Publishing: Cambridge, UK, 2011; p. 273. [Google Scholar]
- Ivanenkov, V.; Felici, F.; Menon, A.G. Uptake and intracellular fate of phage display vectors in mammalian cells. Biochim. Biophys. Acta 1999, 1448, 450–462. [Google Scholar] [CrossRef]
- Romanov, V.I.; Durand, D.B.; Petrenko, V.A. Phage display selection of peptides that affect prostate carcinoma cells attachment and invasion. Prostate 2001, 47, 239–251. [Google Scholar] [CrossRef] [PubMed]
- Huie, M.A.; Cheung, M.C.; Muench, M.O.; Becerril, B.; Kan, Y.W.; Marks, J.D. Antibodies to human fetal erythroid cells from a nonimmune phage antibody library. Proc. Natl. Acad. Sci. USA 2001, 98, 2682–2687. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Legendre, D.; Fastrez, J. Construction and exploitation in model experiments of functional selection of a landscape library expressed from a phagemid. Gene 2002, 290, 203–215. [Google Scholar] [CrossRef]
- Pires, D.P.; Cleto, S.; Sillankorva, S.; Azeredo, J.; Lu, T.K. Genetically Engineered Phages: A Review of Advances over the Last Decade. Microbiol. Mol. Biol. Rev. 2016, 80, 523–543. [Google Scholar] [CrossRef] [PubMed]
- Petrenko, V.A.; Smith, G.P.; Mazooji, M.M.; Quinn, T. Alpha-helically constrained phage display library. Protein Eng. 2002, 15, 943–950. [Google Scholar] [CrossRef] [PubMed]
- Marvin, D.A.; Hale, R.D.; Nave, C.; Citterich, M.H. Molecular-Models and Structural Comparisons of Native and Mutant Class-I Filamentous Bacteriophages Ff (Fd, F1, M13), If1 and Ike. J. Mol. Biol. 1994, 235, 260–286. [Google Scholar] [CrossRef]
- Parmley, S.F.; Smith, G.P. Antibody-selectable filamentous fd phage vectors: Affinity purification of target genes. Gene 1988, 73, 305–318. [Google Scholar] [CrossRef]
- Zacher, A.N.I.; Stock, C.A.; Golden, J.W.I.; Smith, G.P. A new filamentous phage cloning vector: Fd-tet. Gene 1980, 9, 127–140. [Google Scholar] [CrossRef]
- Scott, J.K.; Smith, G.P. Searching for peptide ligands with an epitope library. Science 1990, 249, 386–390. [Google Scholar] [CrossRef] [PubMed]
- Cwirla, S.E.; Peters, E.A.; Barrett, R.W.; Dower, W.J. Peptides on phage: A vast library of peptides for identifying ligands. Proc. Natl. Acad. Sci. USA 1990, 87, 6378–6382. [Google Scholar] [CrossRef] [PubMed]
- McCafferty, J.; Griffiths, A.D.; Winter, G.; Chiswell, D.J. Phage antibodies: Filamentous phage displaying antibody variable domains. Nature 1990, 348, 552–554. [Google Scholar] [CrossRef] [PubMed]
- Clackson, T.; Hoogenboom, H.R.; Griffiths, A.D.; Winter, G. Making antibody fragments using phage display libraries. Nature 1991, 352, 624–628. [Google Scholar] [CrossRef] [PubMed]
- Kuzmicheva, G.A.; Jayanna, P.K.; Sorokulova, I.B.; Petrenko, V.A. Diversity and censoring of landscape phage libraries. Protein Eng. Des. Sel. 2009, 22, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Iannolo, G.; Minenkova, O.; Petruzzelli, R.; Cesareni, G. Modifying filamentous phage capsid: Limits in the size of the major capsid protein. J. Mol. Biol. 1995, 248, 835–844. [Google Scholar] [CrossRef] [PubMed]
- Malik, P.; Terry, T.D.; Bellintani, F.; Perham, R.N. Factors limiting display of foreign peptides on the major coat protein of filamentous bacteriophage capsids and a potential role for leader peptidase. FEBS Lett. 1998, 436, 263–266. [Google Scholar] [CrossRef] [Green Version]
- Greenwood, J.; Willis, A.E.; Perham, R.N. Multiple display of foreign peptides on a filamentous bacteriophage. Peptides from Plasmodium falciparum circumsporozoite protein as antigens. J. Mol. Biol. 1991, 220, 821–827. [Google Scholar] [CrossRef]
- Malik, P.; Perham, R.N. New vectors for peptide display on the surface of filamentous bacteriophage. Gene 1996, 171, 49–51. [Google Scholar] [CrossRef]
- Malik, P.; Terry, T.D.; Gowda, L.R.; Langara, A.; Petukhov, S.A.; Symmons, M.F.; Welsh, L.C.; Marvin, D.A.; Perham, R.N. Role of capsid structure and membrane protein processing in determining the size and copy number of peptides displayed on the major coat protein of filamentous bacteriophage. J. Mol. Biol. 1996, 260, 9–21. [Google Scholar] [CrossRef] [PubMed]
- Iannolo, G.; Minenkova, O.; Gonfloni, S.; Castagnoli, L.; Cesareni, G. Construction, exploitation and evolution of a new peptide library displayed at high density by fusion to the major coat protein of filamentous phage. Biol. Chem. 1997, 378, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Ilyichev, A.A.; Minenkova, O.O.; Tatkov, S.I.; Karpyshev, N.N.; Eroshkin, A.M.; Petrenko, V.A.; Sandakhchiev, L.S. Construction of M13 viable bacteriophage with the insert of foreign peptides into the major coat protein. Dokl. Biochem. 1989, 307, 196–198. [Google Scholar]
- Minenkova, O.O.; Ilyichev, A.A.; Kishchenko, G.P.; Petrenko, V.A. Design of specific immunogens using filamentous phage as the carrier. Gene 1993, 128, 85–88. [Google Scholar] [CrossRef]
- Kishchenko, G.P.; Minenkova, O.O.; Ilichev, A.A.; Gruzdev, A.D.; Petrenko, V.A. Study of the structure of phage-M13 virions containing chimeric B-protein molecules. Mol. Biol.-Engl. Trans. 1991, 25, 1171–1176. [Google Scholar]
- Felici, F.; Castagnoli, L.; Musacchio, A.; Jappelli, R.; Cesareni, G. Selection of antibody ligands from a large library of oligopeptides expressed on a multivalent exposition vector. J. Mol. Biol. 1991, 222, 301–310. [Google Scholar] [CrossRef]
- Markland, W.; Roberts, B.L.; Saxena, M.J.; Guterman, S.K.; Ladner, R.C. Design, construction and function of a multicopy display vector using fusions to the major coat protein of bacteriophage M13. Gene 1991, 109, 13–19. [Google Scholar] [CrossRef]
- Il’ichev, A.A.; Minenkova, O.O.; Tat’kov, S.I.; Karpyshev, N.N.; Eroshkin, A.M.; Petrenko, V.A.; Sandakhchiev, L.S. Poluchenie zhiznesposobnogo varianta faga M13 so vstroennym chuzherodnym peptidom v osnovnoĭ belok obolochki. Dokl. Akad. Nauk. SSSR 1989, 307, 481–483. (In Russian) [Google Scholar] [PubMed]
- Kishchenko, G.; Batliwala, H.; Makowski, L. Structure of a foreign peptide displayed on the surface of bacteriophage M13. J. Mol. Biol. 1994, 241, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Petrenko, V.A.; Smith, G.P. Phages from landscape libraries as substitute antibodies. Protein Eng. 2000, 13, 589–592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kishchenko, G.P.; Minenkova, O.O.; Il’ichev, A.I.; Gruzdev, A.D.; Petrenko, V.A. Izuchenie struktury virionov faga M13, soderzhashchikh molekuly khimernykh B-belkov. Mol. Biol. 1991, 25, 1497–1503. (In Russian) [Google Scholar]
- Marvin, J.S.; Lowman, H.B. Chapter 15: Antibody Humanization and Affinity Maturation Using Phage Display. In Phage Display in Biotechnology and Drug Discovery, 2nd ed.; Geyer, C.R., Sidhu, S.S., Eds.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2005; pp. 347–371. [Google Scholar]
- Petrenko, V.A.; Sorokulova, I.B. Detection of biological threats. A challenge for directed molecular evolution. J. Microbiol. Methods 2004, 58, 147–168. [Google Scholar] [CrossRef] [PubMed]
- Gross, A.L.; Gillespie, J.W.; Petrenko, V.A. Promiscuous tumor targeting phage proteins. Protein Eng. Des. Sel. 2016, 29, 93–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrenko, V.A.; Jayanna, P.K. Phage-mediated Drug Delivery. In Phage Nanobiotechnology; Petrenko, V.A., Smith, G.P., Eds.; RSC Publishing: Cambridge, UK, 2011; pp. 55–82. [Google Scholar]
- Petrenko, V.A.; Jayanna, P.K. Phage protein-targeted cancer nanomedicines. FEBS Lett. 2014, 588, 341–349. [Google Scholar] [CrossRef] [PubMed]
- Petrenko, V.A.; Gillespie, J.W. Paradigm shift in bacteriophage-mediated delivery of anticancer drugs: From targeted ‘magic bullets’ to self-navigated ‘magic missiles’. Expert Opin. Drug Deliv. 2016, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Petrenko, V.A.; Vodyanoy, V.J. Phage display for detection of biological threat agents. J. Microbiol. Methods 2003, 53, 253–262. [Google Scholar] [CrossRef]
- Petrenko, V.A.; Brigati, J.R. Phage as Biospecific Probes. In Immunoassay and Other Bioanalytical Techniques; Van Emon, J.M., Ed.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2007. [Google Scholar]
- Brigati, J.R.; Samoylova, T.I.; Jayanna, P.K.; Petrenko, V.A. Phage display for generating peptide reagents. Curr. Protoc. Protein Sci. 2008. [Google Scholar] [CrossRef]
- Brigati, J.; Williams, D.D.; Sorokulova, I.B.; Nanduri, V.; Chen, I.H.; Turnbough, C.L., Jr.; Petrenko, V.A. Diagnostic probes for Bacillus anthracis spores selected from a landscape phage library. Clin. Chem. 2004, 50, 1899–1906. [Google Scholar] [CrossRef] [PubMed]
- Sorokulova, I.B.; Olsen, E.V.; Chen, I.H.; Fiebor, B.; Barbaree, J.M.; Vodyanoy, V.J.; Chin, B.A.; Petrenko, V.A. Landscape phage probes for Salmonella typhimurium. J. Microbiol. Methods 2005, 63, 55–72. [Google Scholar] [CrossRef] [PubMed]
- Yin, L.; Luo, Y.; Liang, B.; Wang, F.; Du, M.; Petrenko, V.A.; Qiu, H.J.; Liu, A. Specific ligands for classical swine fever virus screened from landscape phage display library. Antivir. Res. 2014, 109, 68–71. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Han, L.; Wang, F.; Li, X.Q.; Petrenko, V.A.; Liu, A.H. Sensitive colorimetric immunoassay of Vibrio parahaemolyticus based on specific nonapeptide probe screening from a phage display library conjugated with MnO2 nanosheets with peroxidase-like activity. Nanoscale 2018, 10, 2825–2833. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Han, L.; Wang, F.; Petrenko, V.A.; Liu, A.H. Gold nanoprobe functionalized with specific fusion protein selection from phage display and its application in rapid, selective and sensitive colorimetric biosensing of Staphylococcus aureus. Biosens. Bioelectron. 2016, 82, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Nanduri, V.; Sorokulova, I.B.; Samoylov, A.M.; Simonian, A.L.; Petrenko, V.A.; Vodyanoy, V. Phage as a molecular recognition element in biosensors immobilized by physical adsorption. Biosens. Bioelectron. 2007, 22, 986–992. [Google Scholar] [CrossRef] [PubMed]
- Olsen, E.V.; Sorokulova, I.B.; Petrenko, V.A.; Chen, I.H.; Barbaree, J.M.; Vodyanoy, V.J. Affinity-selected filamentous bacteriophage as a probe for acoustic wave biodetectors of Salmonella typhimurium. Biosens. Bioelectron. 2006, 21, 1434–1442. [Google Scholar] [CrossRef] [PubMed]
- Nanduri, V.; Balasubramanian, S.; Sista, S.; Vodyanoy, V.J.; Simonian, A.L. Highly sensitive phage-based biosensor for the detection of β-galactosidase. Anal. Chim. Acta 2007, 589, 166–172. [Google Scholar] [CrossRef] [PubMed]
- Knez, K.; Noppe, W.; Geukens, N.; Janssen, K.P.; Spasic, D.; Heyligen, J.; Vriens, K.; Thevissen, K.; Cammue, B.P.; Petrenko, V.; et al. Affinity comparison of p3 and p8 peptide displaying bacteriophages using surface plasmon resonance. Anal. Chem. 2013, 85, 10075–10082. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Liu, P.; Petrenko, V.A.; Liu, A.H. A Label-Free Electrochemical Impedance Cytosensor Based on Specific Peptide-Fused Phage Selected from Landscape Phage Library. Sci. Rep. 2016, 6, 10. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Liu, P.; Sun, L.; Li, C.; Petrenko, V.A.; Liu, A. Bio-mimetic nanostructure self-assembled from Au@Ag heterogeneous nanorods and phage fusion proteins for targeted tumor optical detection and photothermal therapy. Sci. Rep. 2014, 4, 6808. [Google Scholar] [CrossRef] [PubMed]
- Ogata, A.F.; Edgar, J.M.; Majumdar, S.; Briggs, J.S.; Patterson, S.V.; Tan, M.X.; Kudlacek, S.T.; Schneider, C.A.; Weiss, G.A.; Penner, R.M. Virus-Enabled Biosensor for Human Serum Albumin. Anal. Chem. 2017, 89, 1373–1381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, J.; Johnson, M.; Guntupalli, R.; Petrenko, V.A.; Chin, B.A. Detection of Bacillus anthracis spores in liquid using phage-based magnetoelastic micro-resonators. Sens. Actuators B Chem. 2007, 127, 559–566. [Google Scholar] [CrossRef]
- Wan, J.; Shu, H.; Huang, S.; Fiebor, B.; Chen, I.-H.; Petrenko, V.A.; Chin, B.A. Phage-based Magnetoelastic wireless biosensors for detecting Bacillus anthracis spores. IEEE Sens. J. 2007, 7, 470–477. [Google Scholar] [CrossRef]
- Lakshmanan, R.S.; Guntupalli, R.; Hu, J.; Kim, D.J.; Petrenko, V.A.; Barbaree, J.M.; Chin, B.A. Phage immobilized magnetoelastic sensor for the detection of Salmonella typhimurium. J. Microbiol. Methods 2007, 71, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Li, S.; Zhang, K.; Chen, I.H.; Petrenko, V.A.; Cheng, Z. Magnetostrictive Microcantilever as an Advanced Transducer for Biosensors. Sensors 2007, 7, 2929–2941. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samoylov, A.; Cochran, A.; Schemera, B.; Kutzler, M.; Donovan, C.; Petrenko, V.; Bartol, F.; Samoylova, T. Humoral immune responses against gonadotropin releasing hormone elicited by immunization with phage-peptide constructs obtained via phage display. J. Biotechnol. 2015, 216, 20–28. [Google Scholar] [CrossRef] [PubMed]
- Kouzmitcheva, G.A.; Petrenko, V.A.; Smith, G.P. Identifying diagnostic peptides for Lyme disease through epitope discovery. Clin. Diagn. Lab. Immunol. 2001, 8, 150–160. [Google Scholar] [CrossRef] [PubMed]
- Lang, Q.; Wang, F.; Yin, L.; Liu, M.; Petrenko, V.A.; Liu, A. Specific probe selection from landscape phage display library and its application in enzyme-linked immunosorbent assay of free prostate-specific antigen. Anal. Chem. 2014, 86, 2767–2774. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Xia, H.; Yin, L.; Petrenko, V.A.; Liu, A. Selected landscape phage probe as selective recognition interface for sensitive total prostate-specific antigen immunosensor. Biosens. Bioelectron. 2018, 106, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Wang, F.; Petrenko, V.A.; Liu, A. Peptide microarray with ligands at high density based on symmetrical carrier landscape phage for detection of cellulase. Anal. Chem. 2014, 86, 5844–5850. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Purra, M.; Ramos, V.; Petrenko, V.A.; Torchilin, V.P.; Borros, S. Double-targeted polymersomes and liposomes for multiple barrier crossing. Int. J. Pharm. 2016, 511, 946–956. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Hartner, W.C.; Gillespie, J.W.; Praveen, K.P.; Yang, S.; Mei, L.A.; Petrenko, V.A.; Torchilin, V.P. Enhanced tumor delivery and antitumor activity in vivo of liposomal doxorubicin modified with MCF-7-specific phage fusion protein. Nanomedicine 2014, 10, 421–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, T.; Yang, S.; Mei, L.A.; Parmar, C.K.; Gillespie, J.W.; Praveen, K.P.; Petrenko, V.A.; Torchilin, V.P. Paclitaxel-loaded PEG-PE-based micellar nanopreparations targeted with tumor-specific landscape phage fusion protein enhance apoptosis and efficiently reduce tumors. Mol. Cancer Ther. 2014, 13, 2864–2875. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, J.W.; Wei, L.; Petrenko, V.A. Selection of Lung Cancer-Specific Landscape Phage for Targeted Drug Delivery. Comb. Chem. High Throughput Screen. 2016, 19, 412–422. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, J.W.; Gross, A.L.; Puzyrev, A.T.; Bedi, D.; Petrenko, V.A. Combinatorial synthesis and screening of cancer cell-specific nanomedicines targeted via phage fusion proteins. Front. Microbiol. 2015, 6, 628. [Google Scholar] [CrossRef] [PubMed]
- Bedi, D.; Gillespie, J.W.; Petrenko, V.A., Jr.; Ebner, A.; Leitner, M.; Hinterdorfer, P.; Petrenko, V.A. Targeted delivery of siRNA into breast cancer cells via phage fusion proteins. Mol. Pharm. 2013, 10, 551–559. [Google Scholar] [CrossRef] [PubMed]
- Bedi, D.; Musacchio, T.; Fagbohun, O.A.; Gillespie, J.W.; Deinnocentes, P.; Bird, R.C.; Bookbinder, L.; Torchilin, V.P.; Petrenko, V.A. Delivery of siRNA into breast cancer cells via phage fusion protein-targeted liposomes. Nanomedicine 2011, 7, 315–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jayanna, P.K.; Bedi, D.; Deinnocentes, P.; Bird, R.C.; Petrenko, V.A. Landscape phage ligands for PC3 prostate carcinoma cells. Protein Eng. Des. Sel. 2010, 23, 423–430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jayanna, P.K.; Bedi, D.; Gillespie, J.W.; DeInnocentes, P.; Wang, T.; Torchilin, V.P.; Bird, R.C.; Petrenko, V.A. Landscape phage fusion protein-mediated targeting of nanomedicines enhances their prostate tumor cell association and cytotoxic efficiency. Nanomedicine 2010, 6, 538–546. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jayanna, P.K.; Torchilin, V.P.; Petrenko, V.A. Liposomes targeted by fusion phage proteins. Nanomedicine 2009, 5, 83–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fagbohun, O.A.; Bedi, D.; Grabchenko, N.I.; Deinnocentes, P.A.; Bird, R.C.; Petrenko, V.A. Landscape phages and their fusion proteins targeted to breast cancer cells. Protein Eng. Des. Sel. 2012, 25, 271–283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, T.; D’Souza, G.G.; Bedi, D.; Fagbohun, O.A.; Potturi, L.P.; Papahadjopoulos-Sternberg, B.; Petrenko, V.A.; Torchilin, V.P. Enhanced binding and killing of target tumor cells by drug-loaded liposomes modified with tumor-specific phage fusion coat protein. Nanomedicine 2010, 5, 563–574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, T.; Kulkarni, N.; Bedi, D.; D’Souza, G.G.; Papahadjopoulos-Sternberg, B.; Petrenko, V.A.; Torchilin, V.P. In vitro optimization of liposomal nanocarriers prepared from breast tumor cell specific phage fusion protein. J. Drug Target. 2011, 19, 597–605. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Petrenko, V.A.; Torchilin, V.P. Paclitaxel-loaded polymeric micelles modified with MCF-7 cell-specific phage protein: Enhanced binding to target cancer cells and increased cytotoxicity. Mol. Pharm. 2010, 7, 1007–1014. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Yang, S.; Petrenko, V.A.; Torchilin, V.P. Cytoplasmic delivery of liposomes into MCF-7 breast cancer cells mediated by cell-specific phage fusion coat protein. Mol. Pharm. 2010, 7, 1149–1158. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Kulkarni, N.; D’Souza, G.G.; Petrenko, V.A.; Torchilin, V.P. On the mechanism of targeting of phage fusion protein-modified nanocarriers: Only the binding peptide sequence matters. Mol. Pharm. 2011, 8, 1720–1728. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Dong, B.; Chen, B.; Jiang, Z.; Song, H. Selective photothermal therapy for breast cancer with targeting peptide modified gold nanorods. Dalton Trans. 2012, 41, 11134–11144. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Petrenko, V.A.; Torchilin, V.P. Optimization of Landscape Phage Fusion Protein-Modified Polymeric PEG-PE Micelles for Improved Breast Cancer Cell Targeting. J. Nanomed. Nanotechnol. 2012, 008. [Google Scholar] [CrossRef]
- Houten, N.E.; Scott, J.K. Chapter 6: Phage Libraries for Developing Antibody-Targeted Diagnostics and Vaccines. In Phage Display in Biotechnology and Drug Discovery, 2nd ed.; Geyer, C.R., Sidhu, S.S., Eds.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA; 2005; pp. 123–179. [Google Scholar]
- Smith, A.; Manoli, H.; Jaw, S.; Frutoz, K.; Epstein, A.L.; Khawli, L.A.; Theil, F.P. Unraveling the Effect of Immunogenicity on the PK/PD, Efficacy, and Safety of Therapeutic Proteins. J. Immunol. Res. 2016, 2016, 2342187. [Google Scholar] [CrossRef] [PubMed]
- Opella, S.J. The Roles of Structure, Dynamics and Assembly in the Display of Peptides on Filamentous Bacteriophage. In Phage Nanobiotechnology; Petrenko, V.A., Smith, G.P., Eds.; RSC Publishing: Cambridge, UK, 2011; pp. 12–32. [Google Scholar]
- Mount, J.D.; Samoylova, T.I.; Morrison, N.E.; Cox, N.R.; Baker, H.J.; Petrenko, V.A. Cell targeted phagemid rescued by preselected landscape phage. Gene 2004, 341, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Marvin, D.A.; Symmons, M.F.; Straus, S.K. Structure and assembly of filamentous bacteriophages. Prog. Biophys. Mol. Biol. 2014, 114, 80–122. [Google Scholar] [CrossRef] [PubMed]
- Vukovic, L.; Khatib, F.A.; Drake, S.P.; Madriaga, A.; Brandenburg, K.S.; Kral, P.; Onyuksel, H. Structure and dynamics of highly PEG-ylated sterically stabilized micelles in aqueous media. J. Am. Chem. Soc. 2011, 133, 13481–13488. [Google Scholar] [CrossRef] [PubMed]
- Papavoine, C.H.; Christiaans, B.E.; Folmer, R.H.; Konings, R.N.; Hilbers, C.W. Solution structure of the M13 major coat protein in detergent micelles: A basis for a model of phage assembly involving specific residues. J. Mol. Biol. 1998, 282, 401–419. [Google Scholar] [CrossRef] [PubMed]








| Name | Parent Phage | Antibiotic Resistance | Applications | Reference |
|---|---|---|---|---|
| f8-1 | fd-tet | Tet | Billion-clone 8-mer peptide library | [17] |
| f8-5 | fd-tet | Tet | Hundred-million-clone α-helical peptide library | [27] |
| f8-6 | fd-tet | Tet | Billion-clone 9-mer peptide library | [35] |
| PM54 | fd-tet | Tet | Small 6–16-mer peptide libraries | [36] |
| PM52 | fd-tet | Tet | Small 6–16-mer peptide libraries | [36] |
| fdAMPLAY8 | fd | Amp | Cloning of peptides | [37] |
| fdH | fd | None | Cloning of 4- and 6-mer peptides | [38] |
| fdISPLAY | fd | None | Cloning of peptides | [39,40] |
| PM48 | f1 | None | Ten-million-clone 8-mer peptide library; small 9-mer library | [36,41] |
| M13B | M13mp10 | Amp | Cloning of 5-mer peptides | [42,43] |
| Biosensor | Interface | Analyte | Sensitivity, Detection Range | Reference |
|---|---|---|---|---|
| Quartz crystal microbalance (QCM) | Phage coupled with phospholipid via biotin-streptavidin | β-galactosidase from Escherichia coli (β-gal) | Kd = 0.6 nM | [57] |
| Phage immobilized by physical adsorption | β-gal | Kd = 1.7 nM | [65] | |
| Salmonella typhimurium | 100 cells/mL | [66] | ||
| Surface plasmon resonance (SPR) spectroscopy | Phage immobilized by physical adsorption | β-gal | 1 pM to 1 nM | [67] |
| Enhanced green fluorescent protein (eGFP) | Phage in solution used in competition assay | 1.2 × 10−14 M (in competiton assay) | [68] | |
| Electrochemical impedance cytosensor | Phage immobilized on the electrode surface by physical adsorption | Colorectal carcinoma cells | 79 cells/mL, 2 × 102–2 × 108 cells/mL | [69,70] |
| Conjugate of the hybrid (8 + 8)-type M13 phage and electronically conductive polymer | Human serum albumin (HSA) | 100 nM to 5 µM | [71] | |
| Magnetoelastic particle resonators | Phage immobilized by physical adsorption | Bacillus anthracis spores | 102–103 cfu/mL | [21,72,73] |
| S. typhimurium | 102–104 cfu/mL | [74] | ||
| Magnetoelastic microcantilever | Phage immobilized by physical adsorption | S. typhimurium | Not determined | [75] |
| Colorimetric immunoassay | Conjugate of pVIII fusion protein and cysteamine (CS)–gold nanoparticles (CS–AuNPs) | Staphylococcus aureus (S. aureus) | 19 cfu/mL/mL | [64] |
| Conjugate of pVIII fusion protein and protein–MnO2 nanosheets | Vibrio parahaemolyticus | 15 cfu/mL, 20–104 cfu/mL | [63] | |
| Enzyme-linked immunosorbent assay (ELISA) | Phage immobilized by physical adsorption | B. anthracis spores | Not determined | [60] |
| β-gal | Kd = 30 nM | [49,57] | ||
| Neutravidin | Not determined | [49] | ||
| Streptavidin | [49] | |||
| Antibodies against gonadotropin-releasing hormone (GnRH) in patient sera | [76] | |||
| Lyme disease patient sera | [77] | |||
| Free prostate-specific antigen (f-PSA) | 0.16 ng/mL, 0.825–165 ng/mL | [78] | ||
| Total prostate-specific antigen (t-PSA) | 1.6 ng/mL, 3–330 ng/mL | [79] | ||
| Differential pulse voltammetry (DPV) analytical platform | Phage conjugated to the gold electrochemical immunosensor | 3 pg/mL, 0.01–100 ng/mL | ||
| Phage microarray | Phage conjugated with NHS-functionalized slide | Cellulytic endoglucanase I (EG I) | 5–500 nM | [80] |
© 2018 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrenko, V.A. Landscape Phage: Evolution from Phage Display to Nanobiotechnology. Viruses 2018, 10, 311. https://doi.org/10.3390/v10060311
Petrenko VA. Landscape Phage: Evolution from Phage Display to Nanobiotechnology. Viruses. 2018; 10(6):311. https://doi.org/10.3390/v10060311
Chicago/Turabian StylePetrenko, Valery A. 2018. "Landscape Phage: Evolution from Phage Display to Nanobiotechnology" Viruses 10, no. 6: 311. https://doi.org/10.3390/v10060311
APA StylePetrenko, V. A. (2018). Landscape Phage: Evolution from Phage Display to Nanobiotechnology. Viruses, 10(6), 311. https://doi.org/10.3390/v10060311





