Comparison of Different In Situ Hybridization Techniques for the Detection of Various RNA and DNA Viruses
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tissues and Viruses
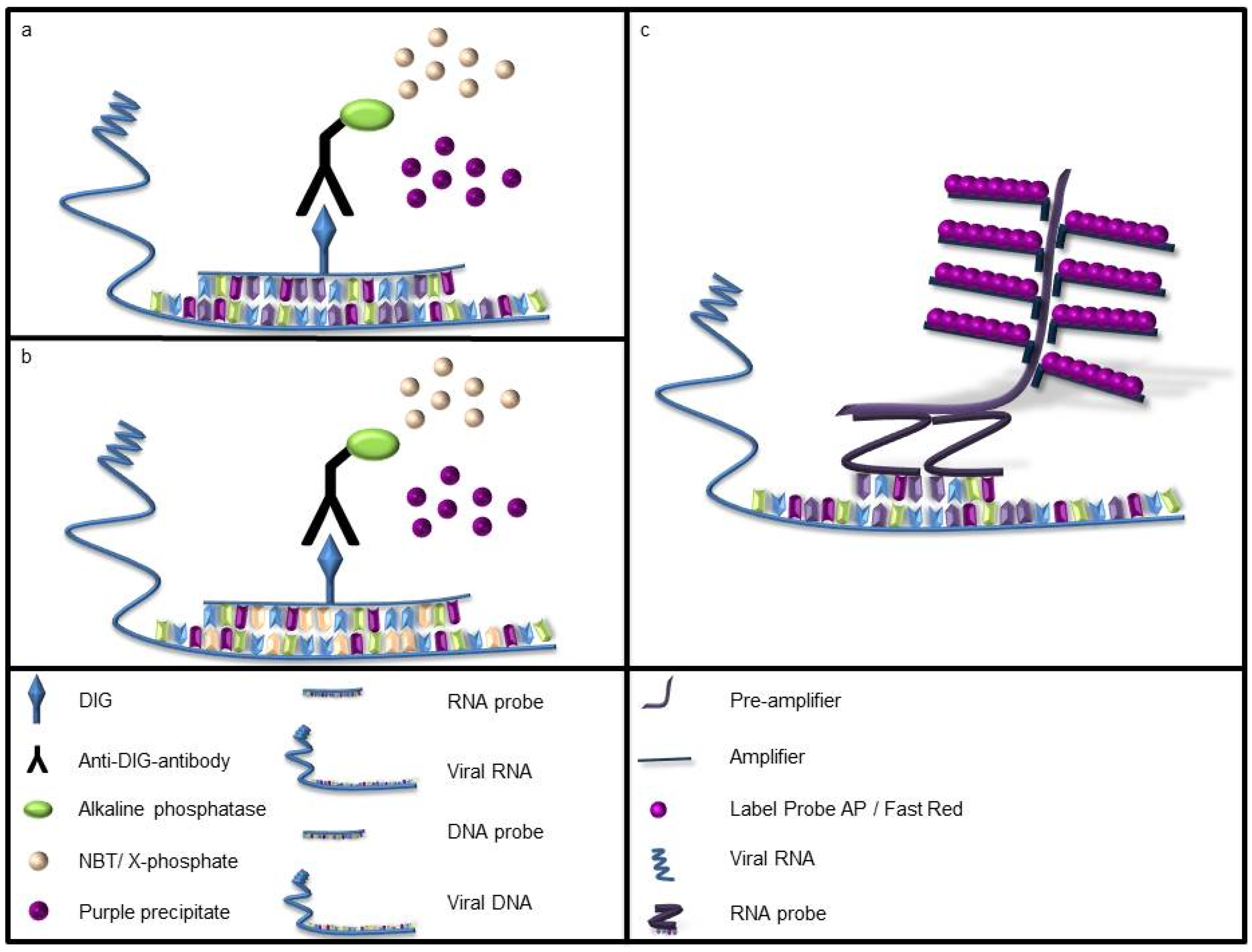
2.2. Probe Synthesis for CISH
2.3. In Situ Hybridization
2.4. Slide and Time Evaluation
3. Results
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Cassidy, A.; Jones, J. Developments in in situ hybridisation. Methods 2014, 70, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Gall, J.G.; Pardue, M.L. Formation and detection of RNA-DNA hybrid molecules in cytological preparations. Proc. Natl. Acad. Sci. USA 1969, 63, 378–383. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.C. Recent advances in fluorescence in situ hybridization. J. Radiat. Res. 1992, 33, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Lambros, M.B.; Natrajan, R.; Reis-Filho, J.S. Chromogenic and fluorescent in situ hybridization in breast cancer. Hum. Pathol. 2007, 38, 1105–1122. [Google Scholar] [CrossRef] [PubMed]
- Bodewes, R.; Lapp, S.; Hahn, K.; Habierski, A.; Förster, C.; König, M.; Wohlsein, P.; Osterhaus, A.D.; Baumgärtner, W. Novel canine bocavirus strain associated with severe enteritis in a dog litter. Vet. Microbiol. 2014, 174, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Pfankuche, V.M.; Bodewes, R.; Hahn, K.; Puff, C.; Beineke, A.; Habierski, A.; Osterhaus, A.D.; Baumgärtner, W. Porcine bocavirus infection associated with encephalomyelitis in a pig, Germany. Emerg. Infect. Dis. 2016, 22, 1310–1312. [Google Scholar] [CrossRef] [PubMed]
- Postel, A.; Hansmann, F.; Baechlein, C.; Fischer, N.; Alawi, M.; Grundhoff, A.; Derking, S.; Tenhundfeld, J.; Pfankuche, V.M.; Herder, V.; et al. Presence of atypical porcine pestivirus (APPV) genomes in newborn piglets correlates with congenital tremor. Sci. Rep. 2016, 6, 27735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hahn, K.; Habierski, A.; Herder, V.; Wohlsein, P.; Peters, M.; Hansmann, F.; Baumgärtner, W. Schmallenberg virus in central nervous system of ruminants. Emerg. Infect. Dis. 2013, 19, 154–155. [Google Scholar] [CrossRef] [PubMed]
- Pfaender, S.; Cavalleri, J.M.; Walter, S.; Doerrbecker, J.; Campana, B.; Brown, R.J.; Burbelo, P.D.; Postel, A.; Hahn, K.; Riebesehl, N.; et al. Clinical course of infection and viral tissue tropism of hepatitis C virus-like nonprimate hepaciviruses in horses. Hepatology 2015, 61, 447–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Prescott, J.; Feldmann, H.; Safronetz, D. Amending Koch’s postulates for viral disease: When “growth in pure culture” leads to a loss of virulence. Antivir. Res. 2017, 137, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Fredricks, D.N.; Relman, D.A. Sequence-based identification of microbial pathogens: A reconsideration of Koch’s postulates. Clin. Microbiol. Rev. 1996, 9, 18–33. [Google Scholar] [PubMed]
- Byrd, A.L.; Segre, J.A. Infectious disease. Adapting Koch’s postulates. Science 2016, 351, 224–226. [Google Scholar] [CrossRef] [PubMed]
- Gröters, S.; Alldinger, S.; Baumgärtner, W. Up-regulation of mRNA for matrix metalloproteinases-9 and -14 in advanced lesions of demyelinating canine distemper leukoencephalitis. Acta Neuropathol. 2005, 110, 369–382. [Google Scholar] [CrossRef] [PubMed]
- Gaedke, K.; Zurbriggen, A.; Baumgärtner, W. In vivo and in vitro detection of canine distemper virus nucleoprotein gene with digoxigenin-labelled RNA, double-stranded DNA probes and oligonucleotides by in situ hybridization. Zbl. Veterinarmed. B 1997, 44, 329–340. [Google Scholar] [CrossRef]
- Zurbriggen, A.; Müller, C.; Vandevelde, M. In situ hybridization of virulent canine distemper virus in brain tissue, using digoxigenin-labeled probes. Am. J. Vet. Res. 1993, 54, 1457–1461. [Google Scholar] [PubMed]
- Schaudien, D.; Polizopoulou, Z.; Koutinas, A.; Schwab, S.; Porombka, D.; Baumgärtner, W.; Herden, C. Leukoencephalopathy associated with parvovirus infection in Cretan hound puppies. J. Clin. Microbiol. 2010, 48, 3169–3175. [Google Scholar] [CrossRef] [PubMed]
- Hause, B.M.; Collin, E.A.; Peddireddi, L.; Yuan, F.; Chen, Z.; Hesse, R.A.; Gauger, P.C.; Clement, T.; Fang, Y.; Anderson, G. Discovery of a novel putative atypical porcine pestivirus in pigs in the USA. J. Gen. Virol. 2015, 96, 2994–2998. [Google Scholar] [CrossRef] [PubMed]
- Arruda, B.L.; Arruda, P.H.; Magstadt, D.R.; Schwartz, K.J.; Dohlman, T.; Schleining, J.A.; Patterson, A.R.; Visek, C.A.; Victoria, J.G. Identification of a divergent lineage porcine pestivirus in nursing piglets with congenital tremors and reproduction of disease following experimental inoculation. PLoS ONE 2016, 11, e0150104. [Google Scholar] [CrossRef] [PubMed]
- Kapoor, A.; Simmonds, P.; Gerold, G.; Qaisar, N.; Jain, K.; Henriquez, J.A.; Firth, C.; Hirschberg, D.L.; Rice, C.M.; Shields, S.; et al. Characterization of a canine homolog of hepatitis C virus. Proc. Natl. Acad. Sci. USA 2011, 108, 11608–11613. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfaender, S.; Walter, S.; Grabski, E.; Todt, D.; Bruening, J.; Romero-Brey, I.; Gather, T.; Brown, R.J.; Hahn, K.; Puff, C.; et al. Immune protection against reinfection with nonprimate hepacivirus. Proc. Natl. Acad. Sci. USA 2017, 114, E2430–E2439. [Google Scholar] [CrossRef] [PubMed]
- Baechlein, C.; Fischer, N.; Grundhoff, A.; Alawi, M.; Indenbirken, D.; Postel, A.; Baron, A.L.; Offinger, J.; Becker, K.; Beineke, A.; et al. Identification of a novel hepacivirus in domestic cattle from Germany. J. Virol. 2015, 89, 7007–7015. [Google Scholar] [CrossRef] [PubMed]
- Garigliany, M.M.; Bayrou, C.; Kleijnen, D.; Cassart, D.; Jolly, S.; Linden, A.; Desmecht, D. Schmallenberg virus: A new shamonda/sathuperi-like virus on the rise in Europe. Antivir. Res. 2012, 95, 82–87. [Google Scholar] [CrossRef] [PubMed]
- Varela, M.; Schnettler, E.; Caporale, M.; Murgia, C.; Barry, G.; McFarlane, M.; McGregor, E.; Piras, I.M.; Shaw, A.; Lamm, C.; et al. Schmallenberg virus pathogenesis, tropism and interaction with the innate immune system of the host. PLoS Pathog. 2013, 9, e1003133. [Google Scholar] [CrossRef] [PubMed]
- Gerhauser, I.; Weigand, M.; Hahn, K.; Herder, V.; Wohlsein, P.; Habierski, A.; Varela, M.; Palmarini, M.; Baumgärtner, W. Lack of Schmallenberg virus in ruminant brain tissues archived from 1961 to 2010 in Germany. J. Comp. Pathol. 2014, 150, 151–154. [Google Scholar] [CrossRef] [PubMed]
- Carmichael, L.E.; Schlafer, D.H.; Hashimoto, A. Pathogenicity of minute virus of canines (MVC) for the canine fetus. Cornell Vet. 1991, 81, 151–171. [Google Scholar] [PubMed]
- Harrison, L.R.; Styer, E.L.; Pursell, A.R.; Carmichael, L.E.; Nietfeld, J.C. Fatal disease in nursing puppies associated with minute virus of canines. J. Vet. Diagn. Investig. 1992, 4, 19–22. [Google Scholar] [CrossRef] [PubMed]
- Decaro, N.; Amorisco, F.; Lenoci, D.; Lovero, A.; Colaianni, M.L.; Losurdo, M.; Desario, C.; Martella, V.; Buonavoglia, C. Molecular characterization of canine minute virus associated with neonatal mortality in a litter of Jack Russell terrier dogs. J. Vet. Diagn. Investig. 2012, 24, 755–758. [Google Scholar] [CrossRef] [PubMed]
- Pollock, R.V. Experimental canine parvovirus infection in dogs. Cornell Vet. 1982, 72, 103–119. [Google Scholar] [PubMed]
- Kapoor, A.; Mehta, N.; Dubovi, E.J.; Simmonds, P.; Govindasamy, L.; Medina, J.L.; Street, C.; Shields, S.; Lipkin, W.I. Characterization of novel canine bocaviruses and their association with respiratory disease. J. Gen. Virol. 2012, 93, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Lau, S.K.; Woo, P.C.; Yeung, H.C.; Teng, J.L.; Wu, Y.; Bai, R.; Fan, R.Y.; Chan, K.H.; Yuen, K.Y. Identification and characterization of bocaviruses in cats and dogs reveals a novel feline bocavirus and a novel genetic group of canine bocavirus. J. Gen. Virol. 2012, 93, 1573–1582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blomström, A.L.; Belak, S.; Fossum, C.; McKillen, J.; Allan, G.; Wallgren, P.; Berg, M. Detection of a novel porcine boca-like virus in the background of porcine circovirus type 2 induced postweaning multisystemic wasting syndrome. Virus Res. 2009, 146, 125–129. [Google Scholar] [CrossRef] [PubMed]
- Opriessnig, T.; Langohr, I. Current state of knowledge on porcine circovirus type 2-associated lesions. Vet. Pathol. 2013, 50, 23–38. [Google Scholar] [CrossRef] [PubMed]
- Seeliger, F.A.; Brügmann, M.L.; Krüger, L.; Greiser-Wilke, I.; Verspohl, J.; Segales, J.; Baumgärtner, W. Porcine circovirus type 2-associated cerebellar vasculitis in postweaning multisystemic wasting syndrome (PMWS)-affected pigs. Vet. Pathol. 2007, 44, 621–634. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, B.; Krueger, L.; Seeliger, F.; Bruegmann, M.; Segalés, J.; Baumgaertner, W. Retrospective study on the occurrence of porcine circovirus 2 infection and associated entities in northern Germany. Vet. Microbiol. 2009, 138, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Bilk, S.; Schulze, C.; Fischer, M.; Beer, M.; Hlinak, A.; Hoffmann, B. Organ distribution of Schmallenberg virus RNA in malformed newborns. Vet. Microbiol. 2012, 159, 236–238. [Google Scholar] [CrossRef] [PubMed]
- Smits, S.L.; Osterhaus, A.D. Virus discovery: One step beyond. Curr. Opin. Virol. 2013. [Google Scholar] [CrossRef] [PubMed]
- Url, A.; Truyen, U.; Rebel-Bauder, B.; Weissenböck, H.; Schmidt, P. Evidence of parvovirus replication in cerebral neurons of cats. J. Clin. Microbiol. 2003, 41, 3801–3805. [Google Scholar] [CrossRef] [PubMed]
- Ford, J.; McEndaffer, L.; Renshaw, R.; Molesan, A.; Kelly, K. Parvovirus infection is associated with myocarditis and myocardial fibrosis in young dogs. Vet. Pathol. 2017, 54, 964–971. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, B.; Scheuch, M.; Höper, D.; Jungblut, R.; Holsteg, M.; Schirrmeier, H.; Eschbaumer, M.; Goller, K.V.; Wernike, K.; Fischer, M.; et al. Novel orthobunyavirus in cattle, Europe, 2011. Emerg. Infect. Dis. 2012, 18, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Martins Gomes de Castro, A.M.; Cortez, A.; Heinemann, M.B.; Brandão, P.E.; Richtzenhain, L.J. Genetic diversity of brazilian strains of porcine circovirus type 2 (PCV-2) revealed by analysis of the cap gene (ORF-2). Arch. Virol. 2007, 152, 1435–1445. [Google Scholar] [CrossRef] [PubMed]
- Roe, C.J.; Siddiqui, M.T.; Lawson, D.; Cohen, C. RNA in situ hybridization for Epstein-Barr virus and cytomegalovirus: Comparison with in situ hybridization and immunohistochemistry. Appl. Immunohistochem. Mol. Morphol. 2017. [Google Scholar] [CrossRef] [PubMed]
- Mardekian, S.K.; Solomides, C.C.; Gong, J.Z.; Peiper, S.C.; Wang, Z.X.; Bajaj, R. Comparison of chromogenic in situ hybridization and fluorescence in situ hybridization for the evaluation of MDM2 amplification in adipocytic tumors. J. Clin. Lab. Anal. 2015, 29, 462–468. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yue, L.; Zhang, Z.; Yuan, Z. Establishment of a fluorescent in situ hybridization assay for imaging hepatitis B virus nucleic acids in cell culture models. Emerg. Microbes Infect. 2017, 6, e98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koyuncu, O.O.; Perlman, D.H.; Enquist, L.W. Efficient retrograde transport of pseudorabies virus within neurons requires local protein synthesis in axons. Cell Host Microbe 2013, 13, 54–66. [Google Scholar] [CrossRef] [PubMed]
- Jo, W.K.; Pfankuche, V.M.; Petersen, H.; Frei, S.; Kummrow, M.; Lorenzen, S.; Ludlow, M.; Metzger, J.; Baumgärtner, W.; Osterhaus, A.; et al. New avian hepadnavirus in palaeognathous bird, Germany. Emerg. Infect. Dis. 2017, 23, 2089–2091. [Google Scholar] [CrossRef] [PubMed]


| Virus | Tissue |
|---|---|
| Atypical porcine pestivirus (APPV) (ss (+) RNA-virus); Family: Flaviviridae; Genus: Pestivirus | Cerebellum |
| Bovine hepacivirus (BovHepV) (ss (+) RNA-virus); Family: Flaviviridae; Genus: Hepacivirus | Liver |
| Equine hepacivirus (EqHV) (ss (+) RNA-virus); Family: Flaviviridae; Genus: Hepacivirus | Liver |
| Schmallenberg virus (SBV) (ss (−) RNA-virus); Family: Bunyaviridae; Genus: Orthobunyavirus | Cerebrum |
| Canine bocavirus 2 (CBoV-2) (ss (+) and (−) DNA-virus); Family: Parvoviridae; Genus: Bocaparvovirus | Small intestine |
| Porcine bocavirus (PBoV) (ss (+) and (−) DNA-virus); Family: Parvoviridae; Genus: Bocaparvovirus | Cervical spinal cord |
| Porcine circovirus 2 (PCV-2) (ss (ambisense) DNA-virus); Family: Circoviridae; Genus: Circovirus | Cerebrum, pulmonary lymph node, lung |
| Virus | Tissue | Probes | Estimated Time (Hands-on Time; Total Working Time) | Assay Result | Positive Region per Total Tissue Section (in %) |
|---|---|---|---|---|---|
| Atypical porcine pestivirus | Cerebellum | DIG-labelled RNA probe § | 15; 182 | − | sense: 0 anti-sense: 0 |
| FISH-RNA probe mix # | 3; 13 | + | 7.77 | ||
| Bovine hepacivirus | Liver | DIG-labelled RNA probe § | 15; 182 | − | sense: 0 anti-sense: 0 |
| FISH-RNA probe mix # | 3; 13 | + | 15.25 | ||
| Equine hepacivirus | Liver | DIG-labelled RNA probe § | 15; 182 | − | sense: 0 anti-sense: 0 |
| DIG-labelled RNA probe (synthetic) * | 7; 62 | − | sense: 0 anti-sense: 0 | ||
| FISH-RNA probe mix # | 3; 13 | + | 9.69 | ||
| Schmallenberg virus | Cerebrum | DIG-labelled RNA probe § | 15; 182 | + | sense: 0.20 anti-sense: 0.32 |
| FISH-RNA probe mix # | 3; 13 | + | 0.20 | ||
| Canine bocavirus 2 | Small intestine | DIG-labelled RNA probe § | 15; 182 | + | sense: 1.17 anti-sense: 0.38 |
| DIG-labelled DNA probe * | 7; 62 | + | sense: 0.79 anti-sense: 0.77 | ||
| FISH-RNA probe mix # | 3; 13 | + | 5.75 | ||
| Porcine bocavirus | Spinal cord | DIG-labelled RNA probe § | 15; 182 | − | sense: 0 anti-sense: 0 |
| DIG-labelled DNA probe * | 7; 62 | − | sense: 0 anti-sense: 0 | ||
| FISH-RNA probe mix # | 3; 13 | + | 0.10 | ||
| Porcine circovirus 2 | Pulmonary lymph node | DIG-labelled RNA probe § | 15; 182 | + | anti-sense: 1.42 sense: 0.89 |
| DIG-labelled DNA probe * | 7; 62 | + | anti-sense: 6.95 sense: 0.31 | ||
| FISH-RNA probe mix # | 3; 13 | + | 10.74 | ||
| Porcine circovirus 2 | Cerebrum | DIG-labelled RNA probe § | 15; 182 | + | anti-sense: 0.05 sense: 0.03 |
| DIG-labelled DNA probe * | 7; 62 | + | anti-sense: 0.04 sense: 0.04 | ||
| FISH-RNA probe mix # | 3; 13 | + | 0.18 | ||
| Porcine circovirus 2 | Lung | DIG-labelled RNA probe § | 15; 182 | + | anti-sense: 1.33 sense: 0.63 |
| DIG-labelled DNA probe * | 7; 62 | + | anti-sense: 0.83 sense: 0.31 | ||
| FISH-RNA probe mix # | 3; 13 | + | 2.12 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pfankuche, V.M.; Hahn, K.; Bodewes, R.; Hansmann, F.; Habierski, A.; Haverkamp, A.-K.; Pfaender, S.; Walter, S.; Baechlein, C.; Postel, A.; et al. Comparison of Different In Situ Hybridization Techniques for the Detection of Various RNA and DNA Viruses. Viruses 2018, 10, 384. https://doi.org/10.3390/v10070384
Pfankuche VM, Hahn K, Bodewes R, Hansmann F, Habierski A, Haverkamp A-K, Pfaender S, Walter S, Baechlein C, Postel A, et al. Comparison of Different In Situ Hybridization Techniques for the Detection of Various RNA and DNA Viruses. Viruses. 2018; 10(7):384. https://doi.org/10.3390/v10070384
Chicago/Turabian StylePfankuche, Vanessa M., Kerstin Hahn, Rogier Bodewes, Florian Hansmann, André Habierski, Ann-Kathrin Haverkamp, Stephanie Pfaender, Stephanie Walter, Christine Baechlein, Alexander Postel, and et al. 2018. "Comparison of Different In Situ Hybridization Techniques for the Detection of Various RNA and DNA Viruses" Viruses 10, no. 7: 384. https://doi.org/10.3390/v10070384
APA StylePfankuche, V. M., Hahn, K., Bodewes, R., Hansmann, F., Habierski, A., Haverkamp, A.-K., Pfaender, S., Walter, S., Baechlein, C., Postel, A., Steinmann, E., Becher, P., Osterhaus, A., Baumgärtner, W., & Puff, C. (2018). Comparison of Different In Situ Hybridization Techniques for the Detection of Various RNA and DNA Viruses. Viruses, 10(7), 384. https://doi.org/10.3390/v10070384





