Diagnosis of Feline Infectious Peritonitis: A Review of the Current Literature
Abstract
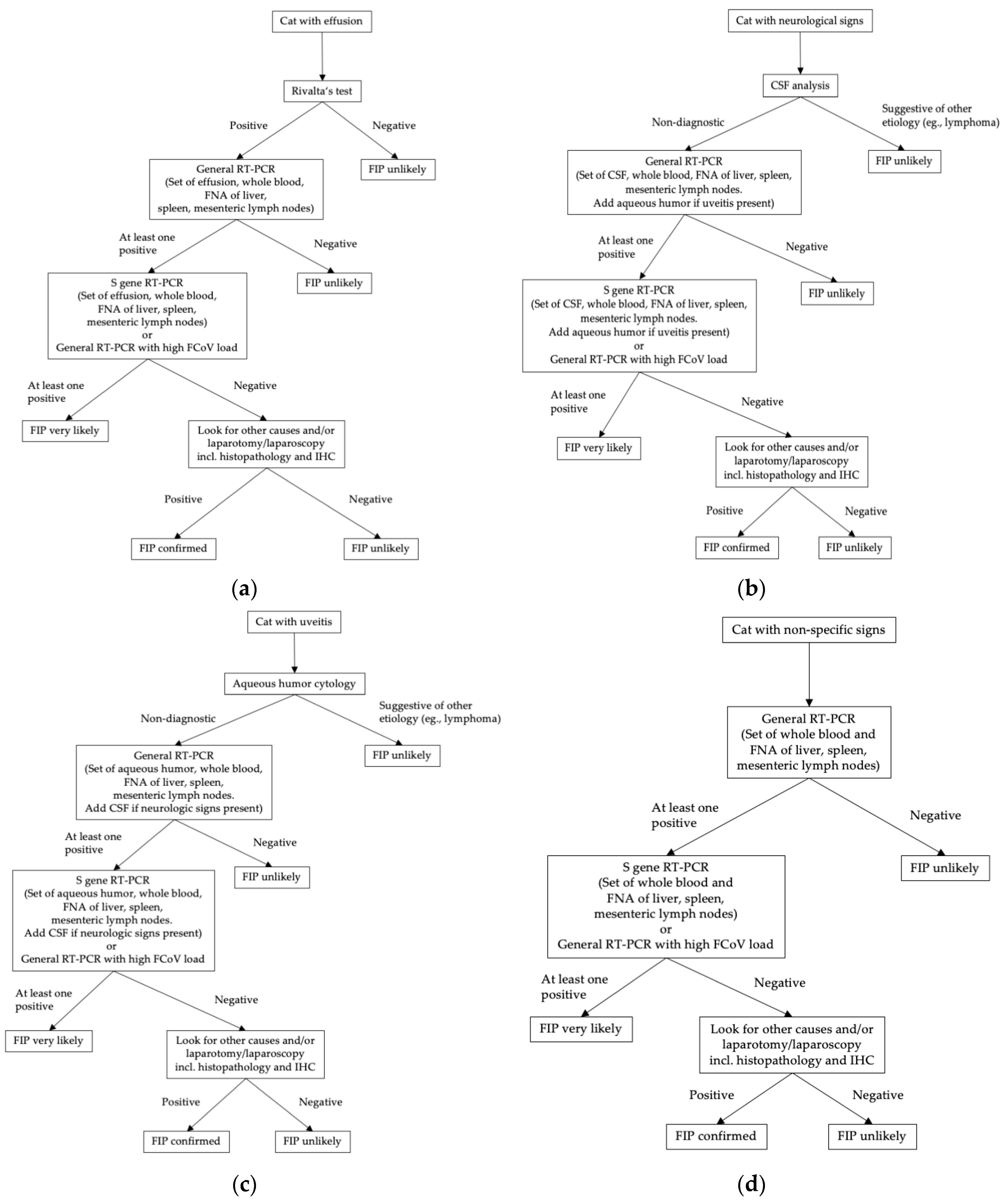
:1. Introduction
2. General Laboratory Testing
2.1. Blood
2.2. Effusion
2.3. Cerebrospinal Fluid (CSF)
2.4. Aqueous Humor
3. Detection of Anti-FCoV Antibodies
3.1. Blood
3.2. Effusion
3.3. CSF
4. Detection of Immune Complexes
5. Detection of FCoV Antigen in Macrophages by Immunostaining
5.1. Tissue
5.2. Effusion
5.3. CSF
5.4. Aqueous Humor
6. Detection of FCoV RNA by Reverse Transcriptase Polymerase Chain Reaction (RT-PCR)
6.1. Tissue
6.2. Blood
6.3. Effusion
6.4. CSF
6.5. Aqueous Humor
7. Detection of FCoV Mutations
7.1. Tissue
7.2. Blood
7.3. Effusion
7.4. CSF
7.5. Aqueous Humor
8. Conclusions
Funding
Conflicts of Interest
References
- Pedersen, N.C.; Allen, C.E.; Lyons, L.A. Pathogenesis of feline enteric coronavirus infection. J. Feline Med. Surg. 2008, 10, 529–541. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, N.C.; Sato, R.; Foley, J.E.; Poland, A.M. Common virus infections in cats, before and after being placed in shelters, with emphasis on feline enteric coronavirus. J. Feline Med. Surg. 2004, 6, 83–88. [Google Scholar] [CrossRef] [PubMed]
- Vogel, L.; Van der Lubben, M.; te Lintelo, E.G.; Bekker, C.P.; Geerts, T.; Schuijff, L.S.; Grinwis, G.C.; Egberink, H.F.; Rottier, P.J. Pathogenic characteristics of persistent feline enteric coronavirus infection in cats. Vet. Res. 2010, 41, 71–82. [Google Scholar] [CrossRef]
- Vennema, H.; Poland, A.; Foley, J.; Pedersen, N.C. Feline infectious peritonitis viruses arise by mutation from endemic feline enteric coronaviruses. Virology 1998, 243, 150–157. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, N.C.; Boyle, J.F.; Floyd, K. Infection studies in kittens, using feline infectious peritonitis virus propagated in cell culture. Am. J. Vet. Res. 1981, 42, 363–367. [Google Scholar] [PubMed]
- Pedersen, N.C.; Boyle, J.F.; Floyd, K.; Fudge, A.; Barker, J. An enteric coronavirus infection of cats and its relationship to feline infectious peritonitis. Am. J. Vet. Res. 1981, 42, 368–377. [Google Scholar] [PubMed]
- Riemer, F.; Kuehner, K.A.; Ritz, S.; Sauter-Louis, C.; Hartmann, K. Clinical and laboratory features of cats with feline infectious peritonitis-a retrospective study of 231 confirmed cases (2000–2010). J. Feline Med. Surg. 2016, 18, 348–356. [Google Scholar] [CrossRef]
- Pesteanu-Somogyi, L.D.; Radzai, C.; Pressler, B.M. Prevalence of feline infectious peritonitis in specific cat breeds. J. Feline Med. Surg. 2006, 8, 1–5. [Google Scholar] [CrossRef]
- Rohrbach, B.W.; Legendre, A.M.; Baldwin, C.A.; Lein, D.H.; Reed, W.M.; Wilson, R.B. Epidemiology of feline infectious peritonitis among cats examined at veterinary medical teaching hospitals. J. Am. Vet. Med. Assoc. 2001, 218, 1111–1115. [Google Scholar] [CrossRef]
- Foley, J.; Pedersen, N. The inheritance of susceptibility to feline infectious peritonitis in purebred catteries. Feline Pract. 1996, 24, 14–22. [Google Scholar]
- Giori, L.; Giordano, A.; Giudice, C.; Grieco, V.; Paltrinieri, S. Performances of different diagnostic tests for feline infectious peritonitis in challenging clinical cases. J. Small Anim. Pract. 2011, 52, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, N.C. A review of feline infectious peritonitis virus infection: 1963–2008. J. Feline Med. Surg. 2009, 11, 225–258. [Google Scholar] [CrossRef] [PubMed]
- Kipar, A.; Meli, M.L. Feline infectious peritonitis: Still an enigma? Vet. Pathol. 2014, 51, 505–526. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.; Foster, D.J.; Child, G.; Lamb, W.A. Inflammatory cerebrospinal fluid analysis in cats: Clinical diagnosis and outcome. J. Feline Med. Surg. 2005, 7, 77–93. [Google Scholar] [CrossRef]
- Stranieri, A.; Giordano, A.; Paltrinieri, S.; Giudice, C.; Cannito, V.; Lauzi, S. Comparison of the performance of laboratory tests in the diagnosis of feline infectious peritonitis. J. Vet. Diagn. Investig. 2018, 30, 459–463. [Google Scholar] [CrossRef] [PubMed]
- Hartmann, K.; Binder, C.; Hirschberger, J.; Cole, D.; Reinacher, M.; Schroo, S.; Frost, J.; Egberink, H.; Lutz, H.; Hermanns, W. Comparison of different tests to diagnose feline infectious peritonitis. J. Vet. Intern. Med. 2003, 17, 781–790. [Google Scholar] [CrossRef]
- Paltrinieri, S.; Comazzi, S.; Spagnolo, V.; Giordano, A. Laboratory changes consistent with feline infectious peritonitis in cats from multicat environments. J. Vet. Med. A. Physiol. Pathol. Clin. Med. 2002, 49, 503–510. [Google Scholar] [CrossRef]
- Paltrinieri, S.; Grieco, V.; Comazzi, S.; Cammarata Parodi, M. Laboratory profiles in cats with different pathological and immunohistochemical findings due to feline infectious peritonitis (fip). J. Feline Med. Surg. 2001, 3, 149–159. [Google Scholar] [CrossRef]
- Sparkes, A.H.; Gruffydd-Jones, T.J.; Harbour, D.A. Feline infectious peritonitis: A review of clinicopathological changes in 65 cases, and a critical assessment of their diagnostic value. Vet. Rec. 1991, 129, 209–212. [Google Scholar] [CrossRef]
- Sparkes, A.H.; Gruffydd-Jones, T.J.; Harbour, D.A. An appraisal of the value of laboratory tests in the diagnosis of feline infectious peritonitis. J. Am. Anim. Hosp. Assoc. 1994, 30, 345–350. [Google Scholar]
- Norris, J.M.; Bosward, K.L.; White, J.D.; Baral, R.M.; Catt, M.J.; Malik, R. Clinicopathological findings associated with feline infectious peritonitis in sydney, australia: 42 cases (1990–2002). Aust. Vet. J. 2005, 83, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Tsai, H.Y.; Chueh, L.L.; Lin, C.N.; Su, B.L. Clinicopathological findings and disease staging of feline infectious peritonitis: 51 cases from 2003 to 2009 in taiwan. J. Feline Med. Surg. 2011, 13, 74–80. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, U.; Deitz, K.; Hostetter, S. Positive predictive value of albumin: Globulin ratio for feline infectious peritonitis in a mid-western referral hospital population. J. Feline Med. Surg. 2012, 14, 903–905. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.S.; Tappin, S.W.; Dodkin, S.J.; Papasouliotis, K.; Casamian-Sorrosal, D.; Tasker, S. Serum protein electrophoresis in 155 cats. J. Feline Med. Surg. 2010, 12, 643–653. [Google Scholar] [CrossRef]
- Duthie, S.; Eckersall, P.D.; Addie, D.D.; Lawrence, C.E.; Jarrett, O. Value of alpha 1-acid glycoprotein in the diagnosis of feline infectious peritonitis. Vet. Rec. 1997, 141, 299–303. [Google Scholar] [CrossRef]
- Giordano, A.; Spagnolo, V.; Colombo, A.; Paltrinieri, S. Changes in some acute phase protein and immunoglobulin concentrations in cats affected by feline infectious peritonitis or exposed to feline coronavirus infection. Vet. J. 2004, 167, 38–44. [Google Scholar] [CrossRef]
- Hazuchova, K.; Held, S.; Neiger, R. Usefulness of acute phase proteins in differentiating between feline infectious peritonitis and other diseases in cats with body cavity effusions. J. Feline Med. Surg. 2017, 19, 809–816. [Google Scholar] [CrossRef]
- Paltrinieri, S.; Giordano, A.; Tranquillo, V.; Guazzetti, S. Critical assessment of the diagnostic value of feline alpha1-acid glycoprotein for feline infectious peritonitis using the likelihood ratios approach. J. Vet. Diagn. Investig. 2007, 19, 266–272. [Google Scholar]
- Selting, K.A.; Ogilvie, G.K.; Lana, S.E.; Fettman, M.J.; Mitchener, K.L.; Hansen, R.A.; Richardson, K.L.; Walton, J.A.; Scherk, M.A. Serum alhpa 1-acid glycoprotein concentrations in healthy and tumor-bearing cats. J. Vet. Intern. Med. 2000, 14, 503–506. [Google Scholar]
- Ceron, J.J.; Eckersall, P.D.; Martynez-Subiela, S. Acute phase proteins in dogs and cats: Current knowledge and future perspectives. Vet. Clin. Pathol. 2005, 34, 85–99. [Google Scholar] [CrossRef]
- Paltrinieri, S.; Metzger, C.; Battilani, M.; Pocacqua, V.; Gelain, M.E.; Giordano, A. Serum alpha1-acid glycoprotein (agp) concentration in non-symptomatic cats with feline coronavirus (fcov) infection. J. Feline Med. Surg. 2007, 9, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Paltrinieri, S.; Marchini, I.; Gelain, M.E. Flow cytometric detection of alpha-1-acid glycoprotein on feline circulating leucocytes. Aust. Vet. J. 2012, 90, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Fischer, Y.; Wess, G.; Hartmann, K. Pericardial effusion in a cat with feline infectious peritonitis. Schweizer Archiv Tierheilkunde 2012, 154, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, N.C. An update on feline infectious peritonitis: Diagnostics and therapeutics. Vet. J. 2014, 201, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Hirschberger, J. Cytology of body cavity effusions. Tierärztliche Prax. 1995, 23, 192–199. [Google Scholar]
- Hirschberger, J.; Koch, S. Validation of the determination of the activity of adenosine deaminase in the body effusions of cats. Res. Vet. Sci. 1995, 59, 226–229. [Google Scholar] [CrossRef]
- Hirschberger, J.; Sauer, G. Clinical and chemical investigations of body cavity effusions. Tieraerztliche Prax. 1991, 19, 431–434. [Google Scholar]
- Shelly, S.; Scarlett-Kranz, J.; Blue, J. Protein electrophoresis on effusions from cats as a diagnostic test for feline infectious peritonitis. J. Am. Anim. Hosp. Assoc. 1988, 24, 495–500. [Google Scholar]
- Fischer, Y.; Sauter-Louis, C.; Hartmann, K. Diagnostic accuracy of the rivalta test for feline infectious peritonitis. Vet. Clin. Pathol. 2012, 41, 558–567. [Google Scholar] [CrossRef]
- Berti-Bock, G.; Vial, F.; Premuda, L.; Rulliere, R. Exudates, transudates and the rivalta reaction (1895). Current status and historical premises. Minerva. Med. 1979, 70, 3573–3580. [Google Scholar]
- Giordano, A.; Stranieri, A.; Rossi, G.; Paltrinieri, S. High diagnostic accuracy of the sysmex xt-2000iv delta total nucleated cells on effusions for feline infectious peritonitis. Vet. Clin. Pathol. 2015, 44, 295–302. [Google Scholar] [CrossRef] [PubMed]
- Stranieri, A.; Paltrinieri, S.; Giordano, A. Diagnosing feline infectious peritonitis using the sysmex xt-2000iv based on frozen supernatants from cavitary effusions. J. Vet. Diagn. Investig. 2017, 29, 321–324. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Crawford, A.H.; Stoll, A.L.; Sanchez-Masian, D.; Shea, A.; Michaels, J.; Fraser, A.R.; Beltran, E. Clinicopathologic features and magnetic resonance imaging findings in 24 cats with histopathologically confirmed neurologic feline infectious peritonitis. J. Vet. Intern. Med. 2017, 31, 1477–1486. [Google Scholar] [CrossRef] [PubMed]
- Steinberg, T.A.; Boettcher, I.C.; Matiasek, K.; Hirschvogel, K.; Hartmann, K.; Kunz, A.; Fischer, A. Use of albumin quotient and igg index to differentiate blood- vs brain-derived proteins in the cerebrospinal fluid of cats with feline infectious peritonitis. Vet. Clin. Pathol. 2008, 37, 207–216. [Google Scholar] [CrossRef] [PubMed]
- Foley, J.E.; Lapointe, J.M.; Koblik, P.; Poland, A.; Pedersen, N.C. Diagnostic features of clinical neurologic feline infectious peritonitis. J. Vet. Intern. Med. 1998, 12, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Boettcher, I.C.; Steinberg, T.; Matiasek, K.; Greene, C.E.; Hartmann, K.; Fischer, A. Use of anti-coronavirus antibody testing of cerebrospinal fluid for diagnosis of feline infectious peritonitis involving the central nervous system in cats. J. Am. Vet. Med. Assoc. 2007, 230, 199–205. [Google Scholar] [CrossRef]
- Ziolkowska, N.; Pazdzior-Czapula, K.; Lewczuk, B.; Mikulska-Skupien, E.; Przybylska-Gornowicz, B.; Kwiecinska, K.; Ziolkowski, H. Feline infectious peritonitis: Immunohistochemical features of ocular inflammation and the distribution of viral antigens in structures of the eye. Vet. Pathol. 2017, 54, 933–944. [Google Scholar] [CrossRef]
- Doherty, M.J. Ocular manifestations of feline infectious peritonitis. J. Am. Vet. Med. Assoc. 1971, 159, 417–424. [Google Scholar]
- Peiffer, R.L., Jr.; Wilcock, B.P. Histopathologic study of uveitis in cats: 139 cases (1978–1988). J. Am. Vet. Med. Assoc. 1991, 198, 135–138. [Google Scholar]
- Linn-Pearl, R.N.; Powell, R.M.; Newman, H.A.; Gould, D.J. Validity of aqueocentesis as a component of anterior uveitis investigation in dogs and cats. Vet. Ophthalmol. 2015, 18, 326–334. [Google Scholar] [CrossRef]
- Wiggans, K.T.; Vernau, W.; Lappin, M.R.; Thomasy, S.M.; Maggs, D.J. Diagnostic utility of aqueocentesis and aqueous humor analysis in dogs and cats with anterior uveitis. Vet. Ophthalmol. 2014, 17, 212–220. [Google Scholar] [CrossRef] [Green Version]
- Lappin, M.R. Feline infectious uveitis. J. Feline Med. Surg. 2000, 2, 159–163. [Google Scholar] [CrossRef]
- Kipar, A.; Bellmann, S.; Gunn-Moore, D.A.; Leukert, W.; Kohler, K.; Menger, S.; Reinacher, M. Histopathological alterations of lymphatic tissues in cats without feline infectious peritonitis after long-term exposure to fip virus. Vet. Microbiol. 1999, 69, 131–137. [Google Scholar] [CrossRef]
- Meli, M.; Kipar, A.; Muller, C.; Jenal, K.; Gonczi, E.; Borel, N.; Gunn-Moore, D.; Chalmers, S.; Lin, F.; Reinacher, M.; et al. High viral loads despite absence of clinical and pathological findings in cats experimentally infected with feline coronavirus (fcov) type i and in naturally fcov-infected cats. J. Feline Med. Surg. 2004, 6, 69–81. [Google Scholar] [CrossRef] [Green Version]
- Gunn-Moore, D.A.; Caney, S.M.; Gruffydd-Jones, T.J.; Helps, C.R.; Harbour, D.A. Antibody and cytokine responses in kittens during the development of feline infectious peritonitis (fip). Vet. Immunol. Immunopathol. 1998, 65, 221–242. [Google Scholar] [CrossRef]
- Pedersen, N.C.; Boyle, J.F. Immunologic phenomena in the effusive form of feline infectious peritonitis. Am. J. Vet. Res. 1980, 41, 868–876. [Google Scholar]
- Pedersen, N.C.; Eckstrand, C.; Liu, H.; Leutenegger, C.; Murphy, B. Levels of feline infectious peritonitis virus in blood, effusions, and various tissues and the role of lymphopenia in disease outcome following experimental infection. Vet. Microbiol. 2015, 175, 157–166. [Google Scholar] [CrossRef]
- Desmarets, L.M.; Vermeulen, B.L.; Theuns, S.; Conceicao-Neto, N.; Zeller, M.; Roukaerts, I.D.; Acar, D.D.; Olyslaegers, D.A.; Van Ranst, M.; Matthijnssens, J.; et al. Experimental feline enteric coronavirus infection reveals an aberrant infection pattern and shedding of mutants with impaired infectivity in enterocyte cultures. Sci. Rep. 2016, 6, 20022. [Google Scholar] [CrossRef]
- Stoddart, M.E.; Whicher, J.T.; Harbour, D.A. Cats inoculated with feline infectious peritonitis virus exhibit a biphasic acute phase plasma protein response. Vet. Rec. 1988, 123, 622–624. [Google Scholar]
- Pedersen, N.C. Serologic studies of naturally occurring feline infectious peritonitis. Am. J. Vet. Res. 1976, 37, 1449–1453. [Google Scholar]
- Addie, D.D.; Jarrett, O. Feline coronavirus antibodies in cats. Vet. Rec. 1992, 131, 202–203. [Google Scholar] [CrossRef] [PubMed]
- Addie, D.D.; le Poder, S.; Burr, P.; Decaro, N.; Graham, E.; Hofmann-Lehmann, R.; Jarrett, O.; McDonald, M.; Meli, M.L. Utility of feline coronavirus antibody tests. J. Feline Med. Surg. 2015, 17, 152–162. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osterhaus, A.D.; Horzinek, M.C.; Reynolds, D.J. Seroepidemiology of feline infectious peritonitis virus infections using transmissible gastroenteritis virus as antigen. Zentralblatt für Veterinärmedizin Reihe B 1977, 24, 835–841. [Google Scholar] [CrossRef] [PubMed]
- Horzinek, M.C.; Osterhaus, A.D. Feline infectious peritonitis: A worldwide serosurvey. Am. J. Vet. Res. 1979, 40, 1487–1492. [Google Scholar]
- Kummrow, M.; Meli, M.L.; Haessig, M.; Goenczi, E.; Poland, A.; Pedersen, N.C.; Hofmann-Lehmann, R.; Lutz, H. Feline coronavirus serotypes 1 and 2: Seroprevalence and association with disease in switzerland. Clin. Diagn. Lab. Immunol. 2005, 12, 1209–1215. [Google Scholar] [CrossRef] [Green Version]
- Addie, D.D.; McLachlan, S.A.; Golder, M.; Ramsey, I.; Jarrett, O. Evaluation of an in-practice test for feline coronavirus antibodies. J. Feline Med. Surg. 2004, 6, 63–67. [Google Scholar] [CrossRef] [Green Version]
- Pratelli, A. Comparison of serologic techniques for the detection of antibodies against feline coronaviruses. J. Vet. Diagn. Investig. 2008, 20, 45–50. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, N.C. The history and interpretation of feline coronavirus serology. Feline Pract. 1995, 23, 46–51. [Google Scholar]
- Barlough, J.E.; Stoddart, C.A. Cats and coronaviruses. J. Am. Vet. Med. Assoc. 1988, 193, 796–800. [Google Scholar]
- Addie, D.D.; Dennis, J.M.; Toth, S.; Callanan, J.J.; Reid, S.; Jarrett, O. Long-term impact on a closed household of pet cats of natural infection with feline coronavirus, feline leukaemia virus and feline immunodeficiency virus. Vet. Rec. 2000, 146, 419–424. [Google Scholar] [CrossRef]
- Addie, D.D.; Jarrett, O. A study of naturally occurring feline coronavirus infections in kittens. Vet. Rec. 1992, 130, 133–137. [Google Scholar] [CrossRef]
- Addie, D.D.; Toth, S.; Murray, G.D.; Jarrett, O. Risk of feline infectious peritonitis in cats naturally infected with feline coronavirus. Am. J. Vet. Res. 1995, 56, 429–434. [Google Scholar]
- Sparkes, A.H.; Gruffydd-Jones, T.J.; Howard, P.E.; Harbour, D.A. Coronavirus serology in healthy pedigree cats. Vet. Rec. 1992, 131, 35–36. [Google Scholar] [CrossRef]
- Scott, F.W. Fip antibody test-interpretation and recommendations. J. Am. Vet. Med. Assoc. 1979, 175, 1164–1168. [Google Scholar]
- Gerber, J.D.; Ingersoll, J.D.; Gast, A.M.; Christianson, K.K.; Selzer, N.L.; Landon, R.M.; Pfeiffer, N.E.; Sharpee, R.L.; Beckenhauer, W.H. Protection against feline infectious peritonitis by intranasal inoculation of a temperature-sensitive fipv vaccine. Vaccine 1990, 8, 536–542. [Google Scholar] [CrossRef]
- Wasmoen, T.L.; Kadakia, N.P.; Unfer, R.C.; Fickbohm, B.L.; Cook, C.P.; Chu, H.J.; Acree, W.M. Protection of cats from infectious peritonitis by vaccination with a recombinant raccoon poxvirus expressing the nucleocapsid gene of feline infectious peritonitis virus. Adv. Exp. Med. Biol. 1995, 380, 221–228. [Google Scholar]
- Haijema, B.J.; Volders, H.; Rottier, P.J. Live, attenuated coronavirus vaccines through the directed deletion of group-specific genes provide protection against feline infectious peritonitis. J. Virol. 2004, 78, 3863–3871. [Google Scholar] [CrossRef] [Green Version]
- Hirschberger, J.; Hartmann, K.; Wilhelm, N.; Frost, J.; Lutz, H.; Kraft, W. Clinical symptoms and diagnosis of feline infectious peritonitis. Tierarztliche Prax. 1995, 23, 92–99. [Google Scholar]
- Kennedy, M.A.; Abd-Eldaim, M.; Zika, S.E.; Mankin, J.M.; Kania, S.A. Evaluation of antibodies against feline coronavirus 7b protein for diagnosis of feline infectious peritonitis in cats. Am. J. Vet. Res. 2008, 69, 1179–1182. [Google Scholar] [CrossRef]
- Kennedy, M.A.; Brenneman, K.; Millsaps, R.K.; Black, J.; Potgieter, L.N. Correlation of genomic detection of feline coronavirus with various diagnostic assays for feline infectious peritonitis. J. Vet. Diagn. Investig. 1998, 10, 93–97. [Google Scholar] [CrossRef] [Green Version]
- Meli, M.L.; Burr, P.; Decaro, N.; Graham, E.; Jarrett, O.; Lutz, H.; McDonald, M.; Addie, D.D. Samples with high virus load cause a trend toward lower signal in feline coronavirus antibody tests. J. Feline Med. Surg. 2013, 15, 295–299. [Google Scholar] [CrossRef]
- Hartmann, K. Feline infectious peritonitis. Vet. Clin. N. Am. Small. Anim. Pract. 2005, 35, 39–79. [Google Scholar] [CrossRef]
- Addie, D.; Jarrett, O. Isolation of Immune Complexes in Feline Infectious Peritonitis. In Proceedings of the IXth International Congress of Virology Abstracts, Glasgow, UK, 8–13 August 1993; p. 60. [Google Scholar]
- Negrin, A.; Lamb, C.R.; Cappello, R.; Cherubini, G.B. Results of magnetic resonance imaging in 14 cats with meningoencephalitis. J. Feline Med. Surg. 2007, 9, 109–116. [Google Scholar] [CrossRef]
- Addie, D.D.; Jarrett, O. Use of a reverse-transcriptase polymerase chain reaction for monitoring the shedding of feline coronavirus by healthy cats. Vet. Rec. 2001, 148, 649–653. [Google Scholar] [CrossRef]
- Addie, D.D.; Schaap, I.A.; Nicolson, L.; Jarrett, O. Persistence and transmission of natural type i feline coronavirus infection. J. Gen. Virol. 2003, 84, 2735–2744. [Google Scholar] [CrossRef]
- Soma, T.; Ishii, H. Detection of feline coronavirus antibody, feline immunodeficiency virus antibody, and feline leukemia virus antigen in ascites from cats with effusive feline infectious peritonitis. J. Vet. Med. Sci. 2004, 66, 89–90. [Google Scholar] [CrossRef] [Green Version]
- Addie, D.; Belak, S.; Boucraut-Baralon, C.; Egberink, H.; Frymus, T.; Gruffydd-Jones, T.; Hartmann, K.; Hosie, M.J.; Lloret, A.; Lutz, H.; et al. Feline infectious peritonitis. Abcd guidelines on prevention and management. J. Feline Med. Surg. 2009, 11, 594–604. [Google Scholar] [CrossRef]
- Lorusso, E.; Mari, V.; Losurdo, M.; Lanave, G.; Trotta, A.; Dowgier, G.; Colaianni, M.L.; Zatelli, A.; Elia, G.; Buonavoglia, D.; et al. Discrepancies between feline coronavirus antibody and nucleic acid detection in effusions of cats with suspected feline infectious peritonitis. Res. Vet. Sci. 2017. [Google Scholar] [CrossRef]
- Soma, T.; Saito, N.; Kawaguchi, M.; Sasai, K. Feline coronavirus antibody titer in cerebrospinal fluid from cats with neurological signs. J. Vet. Med. Sci. 2018, 80, 59–62. [Google Scholar] [CrossRef] [Green Version]
- Weiss, R.C.; Scott, F.W. Pathogenesis of feline infetious peritonitis: Pathologic changes and immunofluorescence. Am. J. Vet. Res. 1981, 42, 2036–2048. [Google Scholar]
- Weiss, R.C.; Scott, F.W. Antibody-mediated enhancement of disease in feline infectious peritonitis: Comparisons with dengue hemorrhagic fever. Comp. Immunol. Microbiol. Infect. Dis. 1981, 4, 175–189. [Google Scholar] [CrossRef]
- Paltrinieri, S.; Cammarata Parodi, M.; Cammarata, G.; Comazzi, S. Some aspects of humoral and cellular immunity in naturally occuring feline infectious peritonitis. Vet. Immunol. Immunopathol. 1998, 65, 205–220. [Google Scholar] [CrossRef]
- Jacobse-Geels, H.E.; Daha, M.R.; Horzinek, M.C. Isolation and characterization of feline c3 and evidence for the immune complex pathogenesis of feline infectious peritonitis. J. Immunol. 1980, 125, 1606–1610. [Google Scholar] [PubMed]
- Jacobse-Geels, H.E.; Daha, M.R.; Horzinek, M.C. Antibody, immune complexes, and complement activity fluctuations in kittens with experimentally induced feline infectious peritonitis. Am. J. Vet. Res. 1982, 43, 666–670. [Google Scholar]
- Horzinek, M.C.; Osterhaus, A.D. The virology and pathogenesis of feline infectious peritonitis. Brief review. Arch. Virol. 1979, 59, 1–15. [Google Scholar] [CrossRef]
- Weiss, R.C.; Dodds, W.J.; Scott, F.W. Disseminated intravascular coagulation in experimentally induced feline infectious peritonitis. Am. J. Vet. Res. 1980, 41, 663–671. [Google Scholar]
- Horzinek, M.C.; Ederveen, J.; Egberink, H.; Jacobse-Geels, H.E.; Niewold, T.; Prins, J. Virion polypeptide specificity of immune complexes and antibodies in cats inoculated with feline infectious peritonitis virus. Am. J. Vet. Res. 1986, 47, 754–761. [Google Scholar]
- Pfeiffer, N. Prospects and problems of coronaviral serology. In Proceedings of the Symposium New Perspectives on Prevention of Feline Infectious Peritonitis, Orlando, FL, USA; 1991; pp. 30–34. [Google Scholar]
- Schroo, S. Kompetitiver Elisa zum Nachweis von Löslichen Immunkomplexen in Serum und Exsudaten Fip-Verdächtiger Katzen. Ph.D. Thesis, Justus-Liebig-Universität Gießen, Gießen, Germany, 1994. [Google Scholar]
- Stoddart, C.A.; Scott, F.W. Intrinsic resistance of feline peritoneal macrophages to coronavirus infection correlates with in vivo virulence. J. Virol. 1989, 63, 436–440. [Google Scholar]
- Kipar, A.; Bellmann, S.; Kremendahl, J.; Kohler, K.; Reinacher, M. Cellular composition, coronavirus antigen expression and production of specific antibodies in lesions in feline infectious peritonitis. Vet. Immunol. Immunopathol. 1998, 65, 243–257. [Google Scholar] [CrossRef]
- Kipar, A.; May, H.; Menger, S.; Weber, M.; Leukert, W.; Reinacher, M. Morphologic features and development of granulomatous vasculitis in feline infectious peritonitis. Vet. Pathol. 2005, 42, 321–330. [Google Scholar] [CrossRef]
- Weiss, R.C.; Scott, F.W. Pathogenesis of feline infectious peritonitis: Nature and development of viremia. Am. J. Vet. Res. 1981, 42, 382–390. [Google Scholar] [PubMed]
- Cornelissen, E.; Dewerchin, H.L.; Van Hamme, E.; Nauwynck, H.J. Absence of surface expression of feline infectious peritonitis virus (fipv) antigens on infected cells isolated from cats with fip. Vet. Microbiol. 2007, 121, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Gunn-Moore, D.A.; Gruffydd-Jones, T.J.; Harbour, D.A. Detection of feline coronaviruses by culture and reverse transcriptase-polymerase chain reaction of blood samples from healthy cats and cats with clinical feline infectious peritonitis. Vet. Microbiol. 1998, 62, 193–205. [Google Scholar] [CrossRef]
- Kipar, A.; Baptiste, K.; Barth, A.; Reinacher, M. Natural fcov infection: Cats with fip exhibit significantly higher viral loads than healthy infected cats. J. Feline Med. Surg. 2006, 8, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, N.C.; Liu, H.; Scarlett, J.; Leutenegger, C.M.; Golovko, L.; Kennedy, H.; Kamal, F.M. Feline infectious peritonitis: Role of the feline coronavirus 3c gene in intestinal tropism and pathogenicity based upon isolates from resident and adopted shelter cats. Virus. Res. 2012, 165, 17–28. [Google Scholar] [CrossRef] [PubMed]
- Kipar, A.; Meli, M.L.; Baptiste, K.E.; Bowker, L.J.; Lutz, H. Sites of feline coronavirus persistence in healthy cats. J. Gen. Virol. 2010, 91, 1698–1707. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Addie, D.D.; Paltrinieri, S.; Pedersen, N.C. Recommendations from workshops of the second international feline coronavirus/feline infectious peritonitis symposium. J. Feline Med. Surg. 2004, 6, 125–130. [Google Scholar] [CrossRef] [Green Version]
- Hok, K. Demonstration of feline infectious peritonitis virus in conjunctival epithelial cells from cats. A simple and reliable method for clinical veterinary virology screening. Apmis 1989, 97, 820–824. [Google Scholar] [CrossRef]
- Walter, J.; Dohse, K.; Rudolph, R. Eine modifikation der abc-methode (avidin-biotin-peroxidase-complex) für den nachweis von viralen antigenen bei der infektion der katze durch ein coronavirus (fip) und der infektion des hundes durch das parvovirus-typ 2. J. Vet. Med. Ser. B 1989, 36, 321–332. [Google Scholar] [CrossRef]
- Hok, K. A comparison between immunofluorescence staining on smears from membrana nictitans (m3 test), immunohistopathology and routine pathology in cats with suspected feline infectious peritonitis (fip). Acta. Vet. Scand. 1991, 32, 171–176. [Google Scholar]
- Hok, K. Demonstration of feline corona virus (fcv) antigen in organs of cats suspected of feline infectious peritonitis (fip) disease. Apmis 1990, 98, 659–664. [Google Scholar] [CrossRef] [PubMed]
- Tammer, R.; Evensen, O.; Lutz, H.; Reinacher, M. Immunohistological demonstration of feline infectious peritonitis virus antigen in paraffin-embedded tissues using feline ascites or murine monoclonal antibodies. Vet. Immunol. Immunopathol. 1995, 49, 177–182. [Google Scholar] [CrossRef]
- Rissi, D.R. A retrospective study of the neuropathology and diagnosis of naturally occurring feline infectious peritonitis. J. Vet. Diagn. Investig. 2018, 30, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Giordano, A.; Paltrinieri, S.; Bertazzolo, W.; Milesi, E.; Parodi, M. Sensitivity of tru-cut and fine needle aspiration biopsies of liver and kidney for diagnosis of feline infectious peritonitis. Vet. Clin. Pathol. 2005, 34, 368–374. [Google Scholar] [CrossRef]
- Felten, S.; Hartmann, K.; Doerfelt, S.; Sangl, L.; Hirschberger, J.; Matiasek, K. Immunocytochemistry of mesenteric lymph node fine-needle aspirates in the diagnosis of feline infectious peritonitis. J. Vet. Diagn. Investig. 2019, 31, 210–216. [Google Scholar] [CrossRef] [Green Version]
- Dunbar, D.; Kwok, W.; Graham, E.; Armitage, A.; Irvine, R.; Johnston, P.; McDonald, M.; Montgomery, D.; Nicolson, L.; Robertson, E.; et al. Diagnosis of non-effusive feline infectious peritonitis by reverse transcriptase quantitative pcr from mesenteric lymph node fine-needle aspirates. J. Feline Med. Surg. 2018, 1098612x18809165. [Google Scholar] [CrossRef] [Green Version]
- Declercq, J.; De Bosschere, H.; Schwarzkopf, I.; Declercq, L. Papular cutaneous lesions in a cat associated with feline infectious peritonitis. Vet. Dermatol. 2008, 19, 255–258. [Google Scholar] [CrossRef]
- Bauer, B.S.; Kerr, M.E.; Sandmeyer, L.S.; Grahn, B.H. Positive immunostaining for feline infectious peritonitis (fip) in a sphinx cat with cutaneous lesions and bilateral panuveitis. Vet. Ophthalmol. 2013, 16 (Suppl. 1), 160–163. [Google Scholar] [CrossRef]
- Rota, A.; Paltrinieri, S.; Jussich, S.; Ubertalli, G.; Appino, S. Priapism in a castrated cat associated with feline infectious peritonitis. J. Feline Med. Surg. 2008, 10, 181–184. [Google Scholar] [CrossRef]
- Stephenson, N.; Swift, P.; Moeller, R.B.; Worth, S.J.; Foley, J. Feline infectious peritonitis in a mountain lion (puma concolor), california, USA. J. Wildl. Dis. 2013, 49, 408–412. [Google Scholar] [CrossRef]
- Mwase, M.; Shimada, K.; Mumba, C.; Yabe, J.; Squarre, D.; Madarame, H. Positive immunolabelling for feline infectious peritonitis in an african lion (panthera leo) with bilateral panuveitis. J. Comp. Pathol. 2015, 152, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Cammarata Parodi, M.; Cammarata, G.; Paltrinieri, S.; Lavazza, A.; Ape, F. Using direct immunofluorescence to detect coronaviruses in peritoneal and pleural effusions. J. Small Anim. Pract. 1993, 34, 609–613. [Google Scholar] [CrossRef] [Green Version]
- Paltrinieri, S.; Cammarata Parodi, M.; Cammarata, G. In vivo diagnosis of feline infectious peritonitis by comparison of protein content, cytology, and direct immunofluorescence test on peritoneal and pleural effusions. J. Vet. Diagn. Investig. 1999, 11, 358–361. [Google Scholar] [CrossRef]
- Felten, S.; Matiasek, K.; Gruendl, S.; Sangl, L.; Wess, G.; Hartmann, K. Investigation into the utility of an immunocytochemical assay in body cavity effusions for diagnosis of feline infectious peritonitis. J. Feline Med. Surg. 2017, 19, 410–418. [Google Scholar] [CrossRef] [PubMed]
- Litster, A.L.; Pogranichniy, R.; Lin, T.L. Diagnostic utility of a direct immunofluorescence test to detect feline coronavirus antigen in macrophages in effusive feline infectious peritonitis. Vet. J. 2013, 198, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Ives, E.J.; Vanhaesebrouck, A.E.; Cian, F. Immunocytochemical demonstration of feline infectious peritonitis virus within cerebrospinal fluid macrophages. J. Feline Med. Surg. 2013, 15, 1149–1153. [Google Scholar] [CrossRef] [PubMed]
- Gruendl, S.; Matiasek, K.; Matiasek, L.; Fischer, A.; Felten, S.; Jurina, K.; Hartmann, K. Diagnostic utility of cerebrospinal fluid immunocytochemistry for diagnosis of feline infectious peritonitis manifesting in the central nervous system. J. Feline Med. Surg. 2017, 19, 576–585. [Google Scholar] [CrossRef]
- Jinks, M.R.; English, R.V.; Gilger, B.C. Causes of endogenous uveitis in cats presented to referral clinics in north carolina. Vet. Ophthalmol. 2016, 19 (Suppl. 1), 30–37. [Google Scholar] [CrossRef]
- Felten, S.; Matiasek, K.; Gruendl, S.; Sangl, L.; Hartmann, K. Utility of an immunocytochemical assay using aqueous humor in the diagnosis of feline infectious peritonitis. Vet. Ophthalmol. 2018, 21, 27–34. [Google Scholar] [CrossRef] [Green Version]
- Li, X.; Scott, F.W. Detection of feline coronaviruses in cell cultures and in fresh and fixed feline tissues using polymerase chain reaction. Vet. Microbiol. 1994, 42, 65–77. [Google Scholar] [CrossRef]
- Pedersen, N.C.; Evermann, J.F.; McKeirnan, A.J.; Ott, R.L. Pathogenicity studies of feline coronavirus isolates 79-1146 and 79-1683. Am. J. Vet. Res. 1984, 45, 2580–2585. [Google Scholar] [PubMed]
- Barker, E.N.; Stranieri, A.; Helps, C.R.; Porter, E.L.; Davidson, A.D.; Day, M.J.; Knowles, T.; Kipar, A.; Tasker, S. Limitations of using feline coronavirus spike protein gene mutations to diagnose feline infectious peritonitis. Vet. Res. 2017, 48, 60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kipar, A.; Meli, M.L.; Failing, K.; Euler, T.; Gomes-Keller, M.A.; Schwartz, D.; Lutz, H.; Reinacher, M. Natural feline coronavirus infection: Differences in cytokine patterns in association with the outcome of infection. Vet. Immunol. Immunopathol. 2006, 112, 141–155. [Google Scholar] [CrossRef] [PubMed]
- Porter, E.; Tasker, S.; Day, M.J.; Harley, R.; Kipar, A.; Siddell, S.G.; Helps, C.R. Amino acid changes in the spike protein of feline coronavirus correlate with systemic spread of virus from the intestine and not with feline infectious peritonitis. Vet. Res. 2014, 45, 49–59. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Addie, D.D.; Belák, S.; Boucraut-Baralon, C.; Egberink, H.; Frymus, T.; Gruffydd-Jones, T.; Hartmann, K.; Hofmann-Lehmann, R.; Hosie, M.J.; Lloret, A.; et al. Feline Infectious Peritonitis. Available online: http://www.abcdcatsvets.org/feline-infectious-peritonitis/ (accessed on 8 August 2019).
- Hornyak, A.; Balint, A.; Farsang, A.; Balka, G.; Hakhverdyan, M.; Rasmussen, T.B.; Blomberg, J.; Belak, S. Detection of subgenomic mrna of feline coronavirus by real-time polymerase chain reaction based on primer-probe energy transfer (p-sg-qpcr). J. Virol. Methods 2012, 181, 155–163. [Google Scholar] [CrossRef]
- Freiche, G.M.; Guidez, C.L.; Duarte, M.; Le Poder, Y.B. Sequencing of 3c and spike genes in feline infectious peritonitis: Which samples are the most relevant for analysis? A retrospective study of 33 cases from 2008 to 2014. J. Vet. Intern. Med. 2016, 30, 436. [Google Scholar]
- Felten, S.; Emmler, L.; Matiasek, K.; Balzer, H.-J.; Pantchev, N.; Leutenegger, C.M.; Hartmann, K. Detection of mutated and non-mutated feline coronavirus in tissues and body fluids of cats with feline infectious peritonitis. In Proceedings of the 2018 ISCAID Symposium, Portland, OR, USA, 30 September–3 October 2018; p. 76. [Google Scholar]
- Sangl, L.; Matiasek, K.; Felten, S.; Grundl, S.; Bergmann, M.; Balzer, H.J.; Pantchev, N.; Leutenegger, C.M.; Hartmann, K. Detection of feline coronavirus mutations in paraffin-embedded tissues in cats with feline infectious peritonitis and controls. J. Feline Med. Surg. 2019, 21, 133–142. [Google Scholar] [CrossRef] [Green Version]
- Herrewegh, A.A.; de Groot, R.J.; Cepica, A.; Egberink, H.F.; Horzinek, M.C.; Rottier, P.J. Detection of feline coronavirus rna in feces, tissues, and body fluids of naturally infected cats by reverse transcriptase pcr. J. Clin. Microbiol. 1995, 33, 684–689. [Google Scholar]
- Herrewegh, A.A.; Mahler, M.; Hedrich, H.J.; Haagmans, B.L.; Egberink, H.F.; Horzinek, M.C.; Rottier, P.J.; de Groot, R.J. Persistence and evolution of feline coronavirus in a closed cat-breeding colony. Virology 1997, 234, 349–363. [Google Scholar] [CrossRef] [Green Version]
- Egberink, H.F.; Herrewegh, A.P.; Schuurman, N.M.; van der Linde-Sipman, J.S.; Horzinek, M.C.; de Groot, R.J. Fip, easy to diagnose? Vet. Q. 1995, 17 (Suppl. 1), 24–25. [Google Scholar] [CrossRef]
- Fehr, D.; Bolla, S.; Herrewegh, A.A.; Horzinek, M.C.; Lutz, H. Detection of feline coronavirus using rt pcr: Basis for the study of the pathogenesis of feline infectious peritonitis (fip). Schweiz. Arch. Tierheilkd. 1996, 138, 74–79. [Google Scholar] [PubMed]
- Fish, E.J.; Diniz, P.P.V.; Juan, Y.C.; Bossong, F.; Collisson, E.W.; Drechsler, Y.; Kaltenboeck, B. Cross-sectional quantitative rt-pcr study of feline coronavirus viremia and replication in peripheral blood of healthy shelter cats in southern california. J. Feline Med. Surg. 2018, 20, 295–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simons, F.A.; Vennema, H.; Rofina, J.E.; Pol, J.M.; Horzinek, M.C.; Rottier, P.J.; Egberink, H.F. A mrna pcr for the diagnosis of feline infectious peritonitis. J. Virol. Methods 2005, 124, 111–116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doenges, S.J.; Weber, K.; Dorsch, R.; Fux, R.; Hartmann, K. Comparison of real-time reverse transcriptase polymerase chain reaction of peripheral blood mononuclear cells, serum and cell-free body cavity effusion for the diagnosis of feline infectious peritonitis. J. Feline Med. Surg. 2017, 19, 344–350. [Google Scholar] [CrossRef] [PubMed]
- Sharif, S.; Arshad, S.S.; Hair-Bejo, M.; Omar, A.-R.; Zeenathul, N.A.; Rahman, N.-A.; Alazawy, A. Evaluation of feline coronavirus viraemia in clinically healthy and ill cats with feline infectious peritonitis. J. Anim. Vet. Adv. 2011, 10, 18–22. [Google Scholar] [CrossRef] [Green Version]
- Can-Sahna, K.; Soydal Ataseven, V.; Pinar, D.; Oguzoglu, T.C. The detection of feline coronaviruses in blood samples from cats by mrna rt-pcr. J. Feline Med. Surg. 2007, 9, 369–372. [Google Scholar] [CrossRef] [Green Version]
- Ritz, S.; Egberink, H.; Hartmann, K. Effect of feline interferon-omega on the survival time and quality of life of cats with feline infectious peritonitis. J. Vet. Intern. Med. 2007, 21, 1193–1197. [Google Scholar] [CrossRef]
- Rottier, P.J.; Nakamura, K.; Schellen, P.; Volders, H.; Haijema, B.J. Acquisition of macrophage tropism during the pathogenesis of feline infectious peritonitis is determined by mutations in the feline coronavirus spike protein. J. Virol. 2005, 79, 14122–14130. [Google Scholar] [CrossRef] [Green Version]
- Dewerchin, H.L.; Cornelissen, E.; Nauwynck, H.J. Replication of feline coronaviruses in peripheral blood monocytes. Arch. Virol. 2005, 150, 2483–2500. [Google Scholar] [CrossRef] [Green Version]
- Stranieri, A.; Lauzi, S.; Giordano, A.; Paltrinieri, S. Reverse transcriptase loop-mediated isothermal amplification for the detection of feline coronavirus. J. Virol. Methods 2017, 243, 105–108. [Google Scholar] [CrossRef]
- Felten, S.; Weider, K.; Doenges, S.; Gruendl, S.; Matiasek, K.; Hermanns, W.; Mueller, E.; Matiasek, L.; Fischer, A.; Weber, K.; et al. Detection of feline coronavirus spike gene mutations as a tool to diagnose feline infectious peritonitis. J. Feline Med. Surg. 2017, 19, 321–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Felten, S.; Leutenegger, C.M.; Balzer, H.J.; Pantchev, N.; Matiasek, K.; Wess, G.; Egberink, H.; Hartmann, K. Sensitivity and specificity of a real-time reverse transcriptase polymerase chain reaction detecting feline coronavirus mutations in effusion and serum/plasma of cats to diagnose feline infectious peritonitis. BMC Vet. Res. 2017, 13, 228. [Google Scholar] [CrossRef] [PubMed]
- Gamble, D.A.; Lobbiani, A.; Gramegna, M.; Moore, L.E.; Colucci, G. Development of a nested pcr assay for detection of feline infectious peritonitis virus in clinical specimens. J. Clin. Microbiol. 1997, 35, 673–675. [Google Scholar] [PubMed]
- Gunther, S.; Felten, S.; Wess, G.; Hartmann, K.; Weber, K. Detection of feline coronavirus in effusions of cats with and without feline infectious peritonitis using loop-mediated isothermal amplification. J. Virol. Methods 2018, 256, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Longstaff, L.; Porter, E.; Crossley, V.J.; Hayhow, S.E.; Helps, C.R.; Tasker, S. Feline coronavirus quantitative reverse transcriptase polymerase chain reaction on effusion samples in cats with and without feline infectious peritonitis. J. Feline Med. Surg. 2017, 19, 240–245. [Google Scholar] [CrossRef] [Green Version]
- Soma, T.; Wada, M.; Taharaguchi, S.; Tajima, T. Detection of ascitic feline coronavirus rna from cats with clinically suspected feline infectious peritonitis. J. Vet. Med. Sci. 2013, 75, 1389–1392. [Google Scholar] [CrossRef] [Green Version]
- Doenges, S.J.; Weber, K.; Dorsch, R.; Fux, R.; Fischer, A.; Matiasek, L.A.; Matiasek, K.; Hartmann, K. Detection of feline coronavirus in cerebrospinal fluid for diagnosis of feline infectious peritonitis in cats with and without neurological signs. J. Feline Med. Surg. 2016, 18, 104–109. [Google Scholar] [CrossRef] [Green Version]
- Webb, A.A.; Muir, G.D. The blood-brain barrier and its role in inflammation. J. Vet. Intern. Med. 2000, 14, 399–411. [Google Scholar] [CrossRef]
- Felten, S.; Sangl, L.; Matiasek, K.; Leutenegger, C.M.; Pantchev, N.; Balzer, H.-J.; Hartmann, K. Comparison of different diagnostic methods in aqueous humor to diagnose feline infectious peritonitis. In Proceedings of the 2016 ISCAID Symposium, Bristol, UK, 16–19 October 2016. [Google Scholar]
- Denison, M.R.; Graham, R.L.; Donaldson, E.F.; Eckerle, L.D.; Baric, R.S. Coronaviruses: An rna proofreading machine regulates replication fidelity and diversity. RNA Biol. 2011, 8, 270–279. [Google Scholar] [CrossRef] [Green Version]
- Kiss, I.; Kecskemeti, S.; Tanyi, J.; Klingeborn, B.; Belak, S. Preliminary studies on feline coronavirus distribution in naturally and experimentally infected cats. Res. Vet. Sci. 2000, 68, 237–242. [Google Scholar] [CrossRef]
- Hora, A.S.; Tonietti, P.O.; Taniwaki, S.A.; Asano, K.M.; Maiorka, P.; Richtzenhain, L.J.; Brandao, P.E. Feline coronavirus 3c protein: A candidate for a virulence marker? Biomed. Res. Int. 2016, 2016, 8560691. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poland, A.M.; Vennema, H.; Foley, J.E.; Pedersen, N.C. Two related strains of feline infectious peritonitis virus isolated from immunocompromised cats infected with a feline enteric coronavirus. J. Clin. Microbiol. 1996, 34, 3180–3184. [Google Scholar] [PubMed]
- Borschensky, C.M.; Reinacher, M. Mutations in the 3c and 7b genes of feline coronavirus in spontaneously affected fip cats. Res. Vet. Sci. 2014, 97, 333–340. [Google Scholar] [PubMed]
- Bank-Wolf, B.R.; Stallkamp, I.; Wiese, S.; Moritz, A.; Tekes, G.; Thiel, H.J. Mutations of 3c and spike protein genes correlate with the occurrence of feline infectious peritonitis. Vet. Microbiol. 2014, 173, 177–188. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, N.C.; Liu, H.; Dodd, K.A.; Pesavento, P.A. Significance of coronavirus mutants in feces and diseased tissues of cats suffering from feline infectious peritonitis. Viruses 2009, 1, 166–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, H.W.; de Groot, R.J.; Egberink, H.F.; Rottier, P.J. Feline infectious peritonitis: Insights into feline coronavirus pathobiogenesis and epidemiology based on genetic analysis of the viral 3c gene. J. Gen. Virol. 2010, 91, 415–420. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, L.E.; Huang, W.P.; Tang, D.J.; Wang, Y.T.; Chen, C.T.; Chueh, L.L. 3c protein of feline coronavirus inhibits viral replication independently of the autophagy pathway. Res. Vet. Sci. 2013, 95, 1241–1247. [Google Scholar]
- Balint, A.; Farsang, A.; Zadori, Z.; Hornyak, A.; Dencso, L.; Almazan, F.; Enjuanes, L.; Belak, S. Molecular characterization of feline infectious peritonitis virus strain df-2 and studies of the role of orf3abc in viral cell tropism. J. Virol. 2012, 86, 6258–6267. [Google Scholar] [CrossRef] [Green Version]
- Balint, A.; Farsang, A.; Zadori, Z.; Belak, S. Comparative in vivo analysis of recombinant type ii feline coronaviruses with truncated and completed orf3 region. PLoS ONE 2014, 9, e88758. [Google Scholar] [CrossRef]
- Dedeurwaerder, A.; Desmarets, L.M.; Olyslaegers, D.A.; Vermeulen, B.L.; Dewerchin, H.L.; Nauwynck, H.J. The role of accessory proteins in the replication of feline infectious peritonitis virus in peripheral blood monocytes. Vet. Microbiol. 2013, 162, 447–455. [Google Scholar] [CrossRef]
- Herrewegh, A.A.; Vennema, H.; Horzinek, M.C.; Rottier, P.J.; de Groot, R.J. The molecular genetics of feline coronaviruses: Comparative sequence analysis of the orf7a/7b transcription unit of different biotypes. Virology 1995, 212, 622–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takano, T.; Tomiyama, Y.; Katoh, Y.; Nakamura, M.; Satoh, R.; Hohdatsu, T. Mutation of neutralizing/antibody-dependent enhancing epitope on spike protein and 7b gene of feline infectious peritonitis virus: Influences of viral replication in monocytes/macrophages and virulence in cats. Virus. Res. 2011, 156, 72–80. [Google Scholar] [CrossRef] [PubMed]
- Dedeurwaerder, A.; Olyslaegers, D.A.; Desmarets, L.M.; Roukaerts, I.D.; Theuns, S.; Nauwynck, H.J. Orf7-encoded accessory protein 7a of feline infectious peritonitis virus as a counteragent against ifn-alpha-induced antiviral response. J. Gen. Virol. 2014, 95, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, M.; Boedeker, N.; Gibbs, P.; Kania, S. Deletions in the 7a orf of feline coronavirus associated with an epidemic of feline infectious peritonitis. Vet. Microbiol. 2001, 81, 227–234. [Google Scholar] [CrossRef]
- Lin, C.N.; Su, B.L.; Huang, H.P.; Lee, J.J.; Hsieh, M.W.; Chueh, L.L. Field strain feline coronaviruses with small deletions in orf7b associated with both enteric infection and feline infectious peritonitis. J. Feline Med. Surg. 2009, 11, 413–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heald-Sargent, T.; Gallagher, T. Ready, set, fuse! The coronavirus spike protein and acquisition of fusion competence. Viruses 2012, 4, 557–580. [Google Scholar] [CrossRef] [Green Version]
- Bosch, B.J.; van der Zee, R.; de Haan, C.A.; Rottier, P.J. The coronavirus spike protein is a class i virus fusion protein: Structural and functional characterization of the fusion core complex. J. Virol. 2003, 77, 8801–8811. [Google Scholar] [CrossRef] [Green Version]
- Jaimes, J.A.; Whittaker, G.R. Feline coronavirus: Insights into viral pathogenesis based on the spike protein structure and function. Virology 2018, 517, 108–121. [Google Scholar] [CrossRef]
- Chang, H.W.; Egberink, H.F.; Halpin, R.; Spiro, D.J.; Rottier, P.J. Spike protein fusion peptide and feline coronavirus virulence. Emerg. Infect. Dis. 2012, 18, 1089–1095. [Google Scholar] [CrossRef]
- Licitra, B.N.; Millet, J.K.; Regan, A.D.; Hamilton, B.S.; Rinaldi, V.D.; Duhamel, G.E.; Whittaker, G.R. Mutation in spike protein cleavage site and pathogenesis of feline coronavirus. Emerg. Infect. Dis. 2013, 19, 1066–1073. [Google Scholar] [CrossRef]
- Lewis, C.S.; Porter, E.; Matthews, D.; Kipar, A.; Tasker, S.; Helps, C.R.; Siddell, S.G. Genotyping coronaviruses associated with feline infectious peritonitis. J. Gen. Virol. 2015, 96, 1358–1368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Felten, S.; Leutenegger, C.M.; Balzer, H.J.; Pantchev, N.; Matiasek, K.; Sangl, L.; Doenges, S.; Gruendl, S.; Fischer, A.; Hartmann, K. Evaluation of a discriminative realtime rt-pcr in cerebrospinal fluid for the diagnosis of feline infectious peritonitis. In Proceedings of the 27th ECVIM-CA Congress, St. Julian’s, Malta, 14–16 September 2017. [Google Scholar]
| Study | Number of Samples | Antibody Assay | Sensitivity | Specificity | Reference Standard for FIP | Control Group |
|---|---|---|---|---|---|---|
| [19] | FIP (n = 39) Controls (n = 39) | IFAT | 28–74% | 64–92% | Histopathology | Diseases other than FIP |
| [78] | FIP (n = 70) Controls (n = 214) | IFAT | 30–79% | 64–98% | Histopathology | Diseases other than FIP |
| [16] | FIP (n = 97) Controls (n = 245) | IFAT | 85% | 57% | Histopathology | Diseases other than FIP |
| [87] | FIP (n = 88) | IFAT | 100% | n. d. | Clinical suspicion | None |
| [79] | FIP (n = 19) Controls (n = 20) | Western blot | 100% | 25–45% | Combination of tests | Healthy or diseases other than FIP |
| Study | Sample Type | Number of Samples | Antibody Assay | Sensitivity | Specificity | Reference Standard for FIP | Control Group |
|---|---|---|---|---|---|---|---|
| [16] | Ascites or pleural effusion | FIP (n = 119) Controls (n = 74) | IFAT | 86% | 85% | Histopathology | Diseases other than FIP |
| [87] | Ascites | FIP (n = 88) | IFAT | 100% | n. d. | Clinical suspicion | None |
| [89] | Ascites or pleural effusion | FIP (n = 61) | IFAT | 84% | n. d. | Combination of tests (according to ABCD guidelines) | None |
| Study | Number of Samples | Antibody Assay | Sensitivity | Specificity | Reference Standard for FIP | Control Group |
|---|---|---|---|---|---|---|
| [45] | FIP without neurological signs (n = 8) | IFAT | 0% | n. d. | Histopathology | None |
| [45] | FIP with neurological signs (n = 16) Controls with neurological signs (n = 3) | IFAT | 94% | 100% | Histopathology | Neurologic diseases other than FIP |
| [46] | FIP without neurological signs (n = 13) Controls without neurological signs (n = 15) | IFAT | 31% | 100% | Histopathology | Diseases other than FIP |
| [46] | FIP with neurological signs (n = 10) Controls with neurological signs (n = 29) | IFAT | 60% | 93% | Histopathology | Diseases other than FIP |
| [90] | FIP with neurological signs (n = 271) | IFAT | 10% | n. d. | Clinical suspicion | None |
| Study | Sample Material | Number of Samples | Assay | Sensitivity | Specificity | Reference Standard for FIP | Control Cats |
|---|---|---|---|---|---|---|---|
| [16] | Serum | FIP (n = 29) Controls (n = 83) | Competitive ELISA | 48% | 91% | Histopathology | Diseases other than FIP |
| Study | Sample Material | Number of Samples | Assay | Sensitivity | Specificity | Reference Standard for FIP | Control Cats |
|---|---|---|---|---|---|---|---|
| [115] | Various tissues | FIP (n = 102) Controls (n = 6) | IHC | 98% | 100% | Histopathology | Diseases other than FIP |
| [117] | TCB liver | FIP (n = 25) | IHC | 24% | n. d. | Histopathology/IHC of other organs | None |
| [117] | TCB kidney | FIP (n = 18) | IHC | 31% | n. d. | Histopathology/IHC of other organs | None |
| [117] | FNA liver | FIP (n = 22) | ICC | 17–31% | n. d. | Histopathology/IHC of other organs | None |
| [117] | FNA kidney | FIP (n = 24) | ICC | 11–20% | n. d. | Histopathology/IHC of other organs | None |
| [116] | Various tissues | FIP (n = 26) | IHC | 100% | n. d. | Histopathology | None |
| [118] | FNA mesenteric lymph nodes | FIP (n = 30) Controls (n = 11) | ICC | 53% | 91% | Histopathology/IHC of other organs | Diseases other than FIP |
| Study | Sample Material | Number of Samples | Assay | Sensitivity | Specificity | Reference Standard for FIP | Control Cats |
|---|---|---|---|---|---|---|---|
| [125] | Ascites or pleural effusion | FIP (n = 21) Controls (n = 11) | IFA | 95% | 100% | Histopathology | Diseases other than FIP |
| [78] | Ascites or pleural effusion | FIP (n = 49) Controls (n = 50) | IFA | 69% | 100% | Histopathology | Diseases other than FIP |
| [126] | Ascites or pleural effusion | FIP (n = 79) Controls (n = 31) | IFA | 95% | 100% | Histopathology/IHC | Diseases other than FIP |
| [16] | Ascites or pleural effusion | FIP (n = 109) Controls (n = 62) | IFA | 57% | 100% | Histopathology | Diseases other than FIP |
| [128] | Ascites or pleural effusion | FIP (n = 10) Controls (n = 7) | IFA | 100% | 71% | Histopathology | Diseases other than FIP |
| [127] | Ascites, pleural or pericardial effusion | FIP (n = 27) Controls (n = 29) | ICC | 85% | 72% | Histopathology/IHC | Diseases other than FIP |
| Study | Number of Samples | Assay | Sensitivity | Specificity | Reference Standard for FIP | Control Cats |
|---|---|---|---|---|---|---|
| [130] | FIP (n = 20) Controls (n = 18) | ICC | 85% | 83% | Histopathology/IHC | Diseases other than FIP |
| [130] | FIP with neurological signs (n = 9) Controls with neurological signs (n = 16) | ICC | 78% | 88% | Histopathology/IHC | Diseases other than FIP |
| [130] | FIP without neurological signs (n = 11) Controls without neurological signs (n = 2) | ICC | 91% | 50% | Histopathology/IHC | Diseases other than FIP |
| Study | Number of Samples | Assay | Sensitivity | Specificity | Reference Standard for FIP | Control Cats |
|---|---|---|---|---|---|---|
| [132] | FIP (n = 26) Controls (n = 12) | ICC | 64% | 82% | Histopathology/IHC | Diseases other than FIP |
| Study | Sample Material | Number of Samples | RT-PCR Assay | Sensitivity | Specificity | Reference Standard for FIP | Control Cats |
|---|---|---|---|---|---|---|---|
| [133] | Liver, kidney, spleen | FIP (n = 8) Controls (n = 84) | RT-PCR | 88% | 39% | Histopathology | Diseases other than FIP |
| [133] | Liver, kidney, spleen | Experimentally induced FIP (n = 13) | RT-PCR | 100% | n. d. | Experimen-tal infection | None |
| [107] | Hemolymphatic tissue | FIP (n = 15) Controls (n = 13) | Real-time RT-PCR | 60–87% | 54–67% | Histopathology/IHC | Healthy |
| [15] | Mesenteric lymph nodes, spleen, small intestine, lung | FIP (n = 11) Controls (n = 8) | RT-nPCR | 91% | 50% | Histopathology/IHC | Diseases other than FIP |
| [119] | FNA of mesenteric lymph nodes | FIP (n = 20) Controls (n = 26) | RT-qPCR | 90% | 96% | Histopathology or diagnostic algorithm | Healthy or diseases other than FIP |
| [139] | Various tissues | FIP (n = 32) Controls (n = 9) | Real-time RT-PCR for mRNA | 60–100% | 100% | Histopathology/IHC | Healthy |
| [137] | Various tissues | FIP (n = 45) Controls (n = 41) | Real-time RT-PCR | 96% | 78% | Histopathology/IHC | Diseases other than FIP |
| [140] | FNA of various tissues | FIP (n = 11) | RT-PCR | 100% | n. d. | Histopathology | None |
| [135] | Various tissues | FIP (n = 57) Controls (n = 45) | RT-qPCR | 98% | 73% | Histopathology/IHC | Healthy or diseases other than FIP |
| [142] | Pooled tissues | FIP (n = 34) Controls (n = 30) | Real-time RT-PCR | 94% | 90% | Histopathology/IHC | Diseases other than FIP |
| [141] | Various tissues | FIP (n = 20) | Real-time RT-PCR | 65–95% | n. d. | Histopathology/IHC | None |
| Study | Sample Material | Number of Samples | RT-PCR Assay | Sensitivity | Specificity | Reference Standard for FIP | Control Cats |
|---|---|---|---|---|---|---|---|
| [143] | Serum, plasma | FIP (n = 17) Controls (n = 15) | RT-nPCR | 56–75% | 75–88% | Histopathology | Healthy or diseases other than FIP |
| [145] | Plasma | FIP (n = 42) Controls (n = 141) | RT-nPCR | 71% | 89% | Histopathology | Healthy or diseases other than FIP |
| [106] | Serum, plasma, whole blood | FIP (n = 47) Controls (n = 69) | RT-snPCR | 67–87% | 10–20% | Histopathology | Healthy |
| [80] | Serum, plasma, whole blood | FIP (n = 6) Controls (n = 5) | RT-nPCR | 20–75% | 100% | Histopathology | Diseases other than FIP |
| [16] | Serum | FIP (n = 17) Controls (n = 8) | RT-nPCR | 53% | 88% | Histopathology | Diseases other than FIP |
| [148] | PBMC | FIP (n = 81) Controls (n = 17) | RT-PCR for mRNA | 93% | 100% | Histopathology | Healthy or diseases other than FIP |
| [148] | PBMC | FIP (n = 651) Controls (n = 424) | RT-PCR for mRNA | 46% | 95% | Histopathology or only clinical suspicion | Healthy or diseases other than FIP |
| [151] | Whole blood | FIP (n = 1) Controls (n = 25) | RT-PCR for mRNA | 100% | 48% | Clinical suspicion | Healthy |
| [150] | Whole blood | FIP (n = 10) Controls (n = 40) | RT-PCR | 100% | 33% | Clinical suspicion | Healthy |
| [150] | Whole blood | FIP (n = 10) Controls (n = 40) | RT-PCR for mRNA | 100% | 85% | Clinical suspicion | Healthy |
| [139] | WBC | FIP (n = 2) | Real-time RT-PCR for mRNA | 100% | n. d. | Histopathology/IHC | Healthy |
| [149] | Serum, PBMC | FIP (n = 43) Controls (n = 49) | Real-time RT-PCR | 15–29% | 100% | Histopathology or detection of FCoV antigen in effusion | Diseases other than FIP |
| [155] | Whole blood | FCoV-positive(n = 4) FCoV-negative (n = 5) | RT-LAMP | 25–50% | 100% | RT-nPCR | Diseases other than FIP |
| [156] | Serum/plasma | FIP (n = 32) Controls (n = 21) | RT-nPCR | 9% | 100% | Histopathology, IHC or detection of FCoV antigen in effusion | Diseases other than FIP |
| [157] | Serum/plasma | FIP (n = 14) Controls (n = 3) | Real-time RT-PCR | 14% | 100% | Histopathology or IHC | Diseases other than FIP |
| [15] | Whole blood | FIP (n = 8) Controls (n = 8) | RT-nPCR | 75% | 100% | Histopathology/IHC | Diseases other than FIP |
| [147] | Buffy coat | Controls (n = 205) | RT-qPCR | n. d. | 96% | None | Healthy |
| [147] | Buffy coat | Controls (n = 205) | RT-qPCR for mRNA | n. d. | 99.5% | None | Healthy |
| [141] | Buffy coat, serum or whole blood | FIP (n = 20) | Real-time RT-PCR | 36–77% | n. d. | Histopathology/IHC | None |
| Study | Sample Material | Number of Samples | RT-PCR Assay | Sensitivity | Specificity | Reference Standard for FIP | Control Cats |
|---|---|---|---|---|---|---|---|
| [143] | Ascites | FIP (n = 5) Controls (n = 1) | RT-nPCR | 100% | 100% | Histopathology | Healthy or diseases other than FIP |
| [158] | Ascites or pleural effusion | FIP (n = 12) Controls (n = 11) | RT-nPCR | 91% | 94% | Histopathology or laboratory fluid analysis | Diseases other than FIP |
| [80] | Ascites or pleural effusion | FIP (n = 5) Controls (n = 3) | RT-nPCR | 100% | 100% | Histopathology (some cats) | Diseases other than FIP |
| [16] | Ascites or pleural effusion | FIP (n = 5) Controls (n = 1) | RT-nPCR | 100% | 100% | Histopathology | Diseases other than FIP |
| [22] | Ascites or pleural effusion | FIP (n = 27) | RT-nPCR | 96% | n. d. | Histopathology | None |
| [160] | Ascites, pleural or pericardial effusion | FIP (n = 20) Controls (n = 23) | Real-time RT-PCR | 85% | 100% | Histopathology/IHC | Diseases other than FIP |
| [149] | Ascites or pleural effusion | FIP (n = 36) Controls (n = 33) | Real-time RT-PCR | 89% | 100% | Histopathology or detection of FCoV antigen in effusion | Diseases other than FIP |
| [155] | Effusion | FCoV-positive (n = 5) FCoV-negative (n = 3) | RT-LAMP | 40% | 100% | RT-nPCR | Diseases other than FIP |
| [159] | Ascites or pleural effusion | FIP (n = 34) Controls (n = 37) | RT-LAMP | 35–59% | 95–97% | Histopathology or detection of FCoV antigen in effusion or tissue | Diseases other than FIP |
| [89] | Ascites or pleural effusion | FIP (n = 61) | RT-qPCR | 85% | n. d. | Combination of tests (according to ABCD guidelines) | None |
| [135] | Ascites, pleural or pericardial effusion | FIP (n = 35) Controls (n = 28) | RT-qPCR | 91% | 96% | Histopathology/IHC | Healthy or diseases other than FIP |
| [156] | Ascites, pleural or pericardial effusion | FIP (n = 50) Controls (n = 51) | RT-nPCR | 72% | 100% | Histopathology, IHC or detection of FCoV antigen in effusion | Diseases other than FIP |
| [157] | Ascites or pleural effusion | FIP (n = 35) Controls (n = 24) | Real-time RT-PCR | 97% | 88% | Histopathology and/or IHC | Diseases other than FIP |
| [15] | Effusion | FIP (n = 10) Controls (n = 6) | RT-nPCR | 100% | 83% | Histopathology/IHC | Diseases other than FIP |
| [141] | Effusion | FIP (n = 14) | Real-time RT-PCR | 86% | n. d. | Histopathology/IHC | None |
| Study | Sample Material | Number of Samples | RT-PCR Assay | Sensitivity | Specificity | Reference Standard for FIP | Control Cats |
|---|---|---|---|---|---|---|---|
| [45] | CSF | FIP (n = 24) Controls (n = 3) | RT-PCR | 21% | 100% | Histopathology | Neurologic diseases other than FIP |
| [45] | CSF | FIP with neurological signs (n = 16) Controls with neurological signs (n = 3) | RT-PCR | 31% | 100% | Histopathology | Neurologic diseases other than FIP |
| [162] | CSF | FIP (n = 19) Controls (n = 15) | Real-time RT-PCR | 42% | 100% | Histopathology or detection of FCoV antigen in effusion | Diseases other than FIP |
| [162] | CSF | FIP with neurological and/or ocular signs (n = 7) Controls with neurological and/or ocular signs (n = 3) | Real-time RT-PCR | 86% | 100% | Histopathology or detection of FCoV antigen in effusion | Diseases other than FIP |
| [135] | CSF | FIP (n = 14) Controls (n = 19) | RT-qPCR | 50% | 100% | Histopathology/IHC | Healthy or diseases other than FIP |
| [141] | CSF | FIP (n = 16) | Real-time RT-PCR | 63% | n. d. | Histopathology/IHC | None |
| Study | Sample Material | Number of Samples | Assay | Sensitivity | Specificity | Reference Standard for FIP | Control Cats |
|---|---|---|---|---|---|---|---|
| [135] | Aqueous humor | FIP (n = 2) | RT-qPCR | 50% | n. d. | IHC | Healthy or diseases other than FIP |
| [141] | Aqueous humor | FIP (n = 20) | Real-time RT-PCR | 25% | n. d. | IHC | None |
| [164] | Aqueous humor | FIP (n = 25) Controls (n = 11) | Real-time RT-PCR | 40% | 100% | IHC | Diseases other than FIP |
| Study | Sample Type | Number of Samples | Assay | Sensitivity | Specificity | Reference Standard for FIP | Control Cats |
|---|---|---|---|---|---|---|---|
| [185] | Tissue or ascites | FIP (n = 118) | RT-nPCR plus S gene sequencing | 96% | n. d. | Histopathology | None |
| [137] | Tissue | FIP (n = 47) Controls (n = 10) | RT-qPCR plus pyrosequencing | 91% (M1058L) | 11% | Histopathology/IHC | Diseases other than FIP |
| [187] | Tissue | FIP (n = 3) | RT-qPCR plus sequencing | 100% | n. d. | Histopathology/IHC | None |
| [140] | FNA of various tissues | FIP (n = 9) | RT-PCR plus sequencing | 89% | n. d. | Histopathology | None |
| [135] | Tissue | FIP (n = 225) Controls (n = 258) | RT-qPCR plus pyrosequencing ± Sanger sequencing | 81% | 95% | Histopathology/IHC | Healthy or diseases other than FIP |
| [15] | Tissue | FIP (n = 10) Controls (n = 8) | RT-nPCR plus sequencing | 70% | 88% | Histopathology/IHC | Diseases other than FIP |
| [142] | Pooled tissues | FIP (n = 34) Controls (n = 30) | Real-time RT-PCR | 71% | 100% | Histopathology/IHC | Diseases other than FIP |
| [141] | FNA or IB of various tissues | FIP (n = 20) | Real-time RT-PCR | 15–50% | n. d. | Histopathology/IHC | None |
| Study | Sample Type | Number of Samples | Assay | Sensitivity | Specificity | Reference Standard for FIP | Control Cats |
|---|---|---|---|---|---|---|---|
| [156] | Serum/plasma | FIP (n = 31) Controls (n = 21) | RT-nPCR plus S gene sequencing | 7% | n. d. | Histopathology, IHC or detection of FCoV antigen in effusion | Diseases other than FIP |
| [157] | Serum/plasma | FIP (n = 14) Controls (n = 3) | Real-time RT-PCR | 0% | n. d. | Histopathology and/or IHC | Diseases other than FIP |
| [15] | Whole blood | FIP (n = 7) Controls (n = 7) | RT-nPCR plus S gene sequencing | 43% | n. d. | Histopathology/IHC | Diseases other than FIP |
| [141] | Buffy coat, serum or whole blood | FIP (n = 20) | Real-time RT-PCR | 0–23% | n. d. | Histopathology/IHC | None |
| Study | Sample Type | Number of Samples | Assay | Sensitivity | Specificity | Reference Standard for FIP | Control Cats |
|---|---|---|---|---|---|---|---|
| [170] | Ascites | FIP (n = 15) | RT-nPCR plus S gene sequencing | 83% | n. d. | n. d. | None |
| [160] | Ascites, pleural or pericardial effusion | FIP (n = 20) Controls (n = 23) | RT-qPCR plus pyrosequencing | 60% | n. d. | Histopathology/IHC | Diseases other than FIP |
| [135] | CSF, aqueous humor, ascites, pleural or pericardial effusion | FIP (n = 47) Controls (n = 50) | RT-qPCR plus pyrosequencing | 60% | 98% | Histopathology/IHC | Healthy or diseases other than FIP |
| [156] | Ascites, pleural or pericardial effusion | FIP (n = 49) Controls (n = 51) | RT-nPCR plus S gene sequencing | 65% | n. d. | Histopathology, IHC or detection of FCoV antigen in effusion | Diseases other than FIP |
| [157] | Ascites or pleural effusion | FIP (n = 35) Controls (n = 24) | Real-time RT-PCR | 69% | 96% | Histopathology and/or IHC | Diseases other than FIP |
| [15] | Body cavity effusions | FIP (n = 10) Controls (n = 6) | RT-nPCR plus S gene sequencing | 40% | 83% | Histopathology/IHC | Diseases other than FIP |
| [141] | Body cavity effusions | FIP (n = 14) | Real-time RT-PCR | 64% | n. d. | Histopathology/IHC | None |
| Study | Sample Type | Number of Samples | Assay | Sensitivity | Specificity | Reference Standard for FIP | Control Cats |
|---|---|---|---|---|---|---|---|
| [188] | CSF | FIP (n = 31) Controls (n = 29) | Real-time RT-PCR | 10% | n. d. | Histopathology/IHC | Diseases other than FIP |
| [188] | CSF | FIP with neurological signs (n = 6) Controls with neurological signs (n = 10) | Real-time RT-PCR | 17% | n. d. | Histopathology/IHC | Diseases other than FIP |
| [141] | CSF | FIP (n = 16) | Real-time RT-PCR | 44% | n. d. | Histopathology/IHC | None |
| Study | Sample Type | Number of Samples | Assay | Sensitivity | Specificity | Reference Standard for FIP | Control Cats |
|---|---|---|---|---|---|---|---|
| [141] | Aqueous humor | FIP (n = 20) | Real-time RT-PCR | 10% | n. d. | Histopathology/IHC | None |
| [164] | Aqueous humor | FIP (n = 25) Controls (n = 11) | Real-time RT-PCR | 17% | n. d. | Histopathology/IHC | Diseases other than FIP |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Felten, S.; Hartmann, K. Diagnosis of Feline Infectious Peritonitis: A Review of the Current Literature. Viruses 2019, 11, 1068. https://doi.org/10.3390/v11111068
Felten S, Hartmann K. Diagnosis of Feline Infectious Peritonitis: A Review of the Current Literature. Viruses. 2019; 11(11):1068. https://doi.org/10.3390/v11111068
Chicago/Turabian StyleFelten, Sandra, and Katrin Hartmann. 2019. "Diagnosis of Feline Infectious Peritonitis: A Review of the Current Literature" Viruses 11, no. 11: 1068. https://doi.org/10.3390/v11111068
APA StyleFelten, S., & Hartmann, K. (2019). Diagnosis of Feline Infectious Peritonitis: A Review of the Current Literature. Viruses, 11(11), 1068. https://doi.org/10.3390/v11111068




