Bovine Foamy Virus: Shared and Unique Molecular Features In Vitro and In Vivo
Abstract
:1. Introduction
2. Specific Topics and Highlights in BFV Biology and Virus-Host Interaction
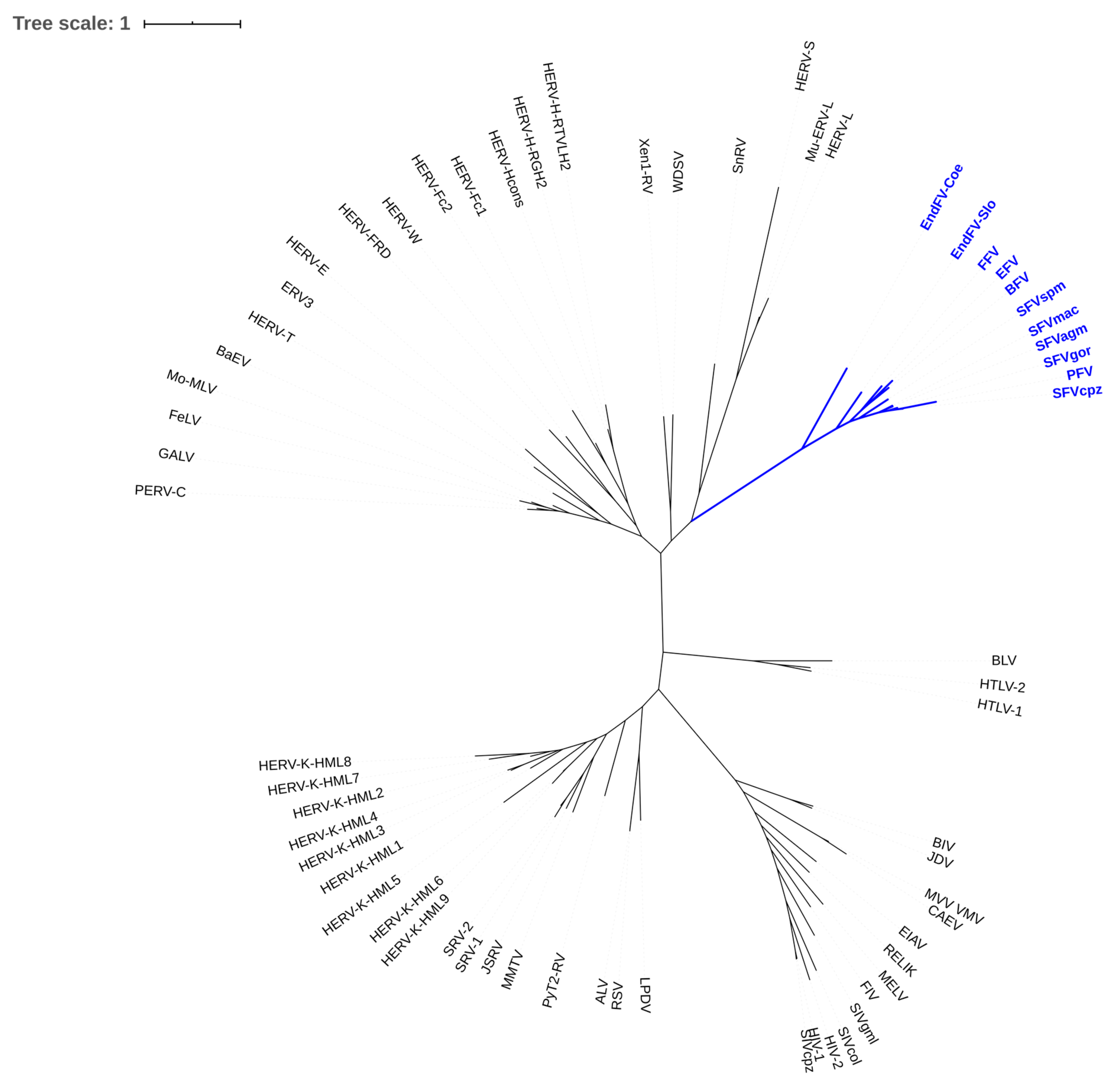
2.1. Historic View
2.2. Excellent, Well Established Non-Primate FV Model of Transactivation, Gene Expression and Gene Function
2.2.1. Function of Tas
2.2.2. Function of Bet
2.2.3. Function and Localization of Gag
2.3. BFV-Host Interactions: Restriction Factors, Innate Immunity, miRNAs and Tight Cell Association
2.3.1. Restriction Factors
2.3.2. Innate Immunity
2.3.3. miRNA Expression as an Additional Layer to Control Host Gene Expression and Innate Immunity
2.3.4. Highly Cell-Associated Spread, at Least in Cell Cultures—What Is Behind This Phenotype?
2.4. BFV-Host Interactions at the Organismal and Populational Level
2.4.1. BFV Epidemiology and Naturally Occurring Co-Infections
2.4.2. BFV Transmission Route
2.4.3. Interspecies and Zoonotic Transmission of BFV as Part of the Human Food Chain
2.4.4. BFV Replication in Naturally and Experimentally Infected Animals
2.5. Utilization of BFV as Viral Vector for Translational Applications
3. Conclusions and Outlook
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Khan, A.S.; Bodem, J.; Buseyne, F.; Gessain, A.; Johnson, W.; Kuhn, J.H.; Kuzmak, J.; Lindemann, D.; Linial, M.L.; Löchelt, M.; et al. Spumaretroviruses: Updated taxonomy and nomenclature. Virology 2018, 516, 158–164. [Google Scholar] [CrossRef] [PubMed]
- Han, G.Z.; Worobey, M. An endogenous foamy-like viral element in the coelacanth genome. PLoS Pathog. 2012, 8, e1002790. [Google Scholar] [CrossRef] [PubMed]
- Achong, B.G.; Mansell, P.W.; Epstein, M.A.; Clifford, P. An unusual virus in cultures from a human nasopharyngeal carcinoma. J. Natl. Cancer Inst. 1971, 46, 299–307. [Google Scholar] [PubMed]
- Herchenröder, O.; Renne, R.; Loncar, D.; Cobb, E.K.; Murthy, K.K.; Schneider, J.; Mergia, A.; Luciw, P.A. Isolation, cloning, and sequencing of simian foamy viruses from chimpanzees (sfvcpz): High homology to human foamy virus (hfv). Virology 1994, 201, 187–199. [Google Scholar] [CrossRef]
- Kehl, T.; Tan, J.; Materniak, M. Non-simian foamy viruses: Molecular virology, tropism and prevalence and zoonotic/interspecies transmission. Viruses 2013, 5, 2169–2209. [Google Scholar] [CrossRef]
- Wolfe, N.D.; Switzer, W.M.; Carr, J.K.; Bhullar, V.B.; Shanmugam, V.; Tamoufe, U.; Prosser, A.T.; Torimiro, J.N.; Wright, A.; Mpoudi-Ngole, E.; et al. Naturally acquired simian retrovirus infections in central african hunters. Lancet 2004, 363, 932–937. [Google Scholar] [CrossRef]
- Pinto-Santini, D.M.; Stenbak, C.R.; Linial, M.L. Foamy virus zoonotic infections. Retrovirology 2017, 14, 55. [Google Scholar] [CrossRef]
- Muniz, C.P.; Cavalcante, L.T.F.; Jia, H.; Zheng, H.; Tang, S.; Augusto, A.M.; Pissinatti, A.; Fedullo, L.P.; Santos, A.F.; Soares, M.A.; et al. Zoonotic infection of brazilian primate workers with new world simian foamy virus. PLoS ONE 2017, 12, e0184502. [Google Scholar] [CrossRef]
- Chen, Y.; Wei, X.; Zhang, G.; Holmes, E.C.; Cui, J. Identification and evolution of avian endogenous foamy viruses. Virus Evol. 2019, in press. [Google Scholar] [CrossRef]
- Katzourakis, A.; Gifford, R.J.; Tristem, M.; Gilbert, M.T.; Pybus, O.G. Macroevolution of complex retroviruses. Science 2009, 325, 1512. [Google Scholar] [CrossRef]
- Linial, M. Why aren′t foamy viruses pathogenic? Trends Microbiol. 2000, 8, 284–289. [Google Scholar] [CrossRef]
- Aiewsakun, P.; Simmonds, P.; Katzourakis, A. The first co-opted endogenous foamy viruses and the evolutionary history of reptilian foamy viruses. Viruses 2019, 11, 641. [Google Scholar] [CrossRef] [PubMed]
- Materniak, M.; Hechler, T.; Löchelt, M.; Kuzmak, J. Similar patterns of infection with bovine foamy virus in experimentally inoculated calves and sheep. J. Virol. 2013, 87, 3516–3525. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Materniak-Kornas, M.; Stricker, E.; Heit-Mondrzyk, A.; Pougialis, G.; Hugo, A.; Kubis, P.; Sell, B.; Hotz-Wagenblatt, A.; Kuzmak, J.; et al. Functional analysis of bovine foamy virus-encoded mirnas reveals the prime importance of defined mirna for virus replication and host-virus interaction. Viruses 2019. under review. [Google Scholar]
- Romen, F.; Backes, P.; Materniak, M.; Sting, R.; Vahlenkamp, T.W.; Riebe, R.; Pawlita, M.; Kuzmak, J.; Löchelt, M. Serological detection systems for identification of cows shedding bovine foamy virus via milk. Virology 2007, 364, 123–131. [Google Scholar] [CrossRef]
- Materniak, M.; Sieradzki, Z.; Kuzmak, J. Detection of bovine foamy virus in milk and saliva of bfv seropositive cattle. Bull. Vet. Inst. Pulawy 2010, 54, 461–465. [Google Scholar]
- Liebermann, H.; Riebe, R. Isolation of bovine syncytial virus in east germany. Arch. Exp. Vet. 1981, 35, 917–919. [Google Scholar]
- Liebermann, H.; Riebe, R. Cellular spectrum of bovine syncytial virus. Arch. Exp. Vet. 1981, 35, 945–953. [Google Scholar]
- Holzschu, D.L.; Delaney, M.A.; Renshaw, R.W.; Casey, J.W. The nucleotide sequence and spliced pol mrna levels of the nonprimate spumavirus bovine foamy virus. J. Virol. 1998, 72, 2177–2182. [Google Scholar]
- Rethwilm, A.; Bodem, J. Evolution of foamy viruses: The most ancient of all retroviruses. Viruses 2013, 5, 2349–2374. [Google Scholar] [CrossRef]
- Zhang, S.; Cui, X.; Li, J.; Liang, Z.; Qiao, W.; Tan, J. Lysine residues k66, k109, and k110 in the bovine foamy virus transactivator protein are required for transactivation and viral replication. Virol. Sin. 2016, 31, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Whisnant, A.W.; Kehl, T.; Bao, Q.; Materniak, M.; Kuzmak, J.; Löchelt, M.; Cullen, B.R. Identification of novel, highly expressed retroviral micrornas in cells infected by bovine foamy virus. J. Virol. 2014, 88, 4679–4686. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Heit, A.; Hotz-Wagenblatt, A.; Löchelt, M. Functional characterization of the bovine foamy virus mirna expression cassette and its dumbbell-shaped pri-mirna. Virus Genes 2018, 54, 550–560. [Google Scholar] [CrossRef] [PubMed]
- Bao, Q.; Hipp, M.; Hugo, A.; Lei, J.; Liu, Y.; Kehl, T.; Hechler, T.; Löchelt, M. In vitro evolution of bovine foamy virus variants with enhanced cell-free virus titers and transmission. Viruses 2015, 7, 5855–5874. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, X.; Liang, Z.; Bing, T.; Qiao, W.; Tan, J. The influence of envelope c-terminus amino acid composition on the ratio of cell-free to cell-cell transmission for bovine foamy virus. Viruses 2019, 11, 130. [Google Scholar] [CrossRef]
- Bao, Q.; Hotz-Wagenblatt, A.; Betts, M.J.; Hipp, M.; Hugo, A.; Pougialis, G.; Lei-Rossmann, J.; Löchelt, M. Distinct cell and isolate-specific adaptation strategies of gag and env yield high titer bovine foamy virus variants. Infect. Genet. Evol. 2019. under review. [Google Scholar]
- Tan, J.; Qiao, W.; Wang, J.; Xu, F.; Li, Y.; Zhou, J.; Chen, Q.; Geng, Y. Ifp35 is involved in the antiviral function of interferon by association with the viral tas transactivator of bovine foamy virus. J. Virol. 2008, 82, 4275–4283. [Google Scholar] [CrossRef]
- Materniak-Kornas, M.; Kubis, P.; Kuzmak, J. Investigation of active replication sites of BFV in different tissues of experimentally inoculated calves. In Proceedings of the 12th International Foamy Virus Conference, Paris, France, 9–10 June 2016. [Google Scholar]
- Enders, J.F.; Peebles, T.C. Propagation in tissue cultures of cytopathogenic agents from patients with measles. Proc. Soc. Exp. Biol. Med. 1954, 86, 277–286. [Google Scholar] [CrossRef]
- Malmquist, W.A.; Van der Maaten, M.J.; Boothe, A.D. Isolation, immunodiffusion, immunofluorescence, and electron microscopy of a syncytial virus of lymphosarcomatous and apparently normal cattle. Cancer Res. 1969, 29, 188–200. [Google Scholar]
- Tan, J.; Qiao, W.; Xu, F.; Han, H.; Chen, Q.; Geng, Y. Dimerization of btas is required for the transactivational activity of bovine foamy virus. Virology 2008, 376, 236–241. [Google Scholar] [CrossRef]
- Chang, J.; Lee, K.J.; Jang, K.L.; Lee, E.K.; Baek, G.H.; Sung, Y.C. Human foamy virus bel1 transactivator contains a bipartite nuclear localization determinant which is sensitive to protein context and triple multimerization domains. J. Virol. 1995, 69, 801–808. [Google Scholar] [PubMed]
- Ma, Q.; Tan, J.; Cui, X.; Luo, D.; Yu, M.; Liang, C.; Qiao, W. Residues r(199)h(200) of prototype foamy virus transactivator bel1 contribute to its binding with ltr and ip promoters but not its nuclear localization. Virology 2014, 449, 215–223. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Tan, J.; Wang, J.; Chen, Q.; Geng, Y.; Qiao, W. Analysis of bovine foamy virus btas mrna transcripts during persistent infection. Virus Genes 2010, 40, 84–93. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Tan, J.; Su, Y.; Qiao, W.; Geng, Y.; Chen, Q. Transcription factor ap1 modulates the internal promoter activity of bovine foamy virus. Virus Res. 2010, 147, 139–144. [Google Scholar] [CrossRef]
- Bannert, H.; Muranyi, W.; Ogryzko, V.V.; Nakatani, Y.; Flugel, R.M. Coactivators p300 and pcaf physically and functionally interact with the foamy viral trans-activator. BMC Mol. Biol. 2004, 5, 16. [Google Scholar] [CrossRef] [PubMed]
- Bodem, J.; Krausslich, H.G.; Rethwilm, A. Acetylation of the foamy virus transactivator tas by pcaf augments promoter-binding affinity and virus transcription. J. Gen. Virol. 2007, 88, 259–263. [Google Scholar] [CrossRef]
- Chang, R.; Tan, J.; Xu, F.; Han, H.; Geng, Y.; Li, Y.; Qiao, W. Lysine acetylation sites in bovine foamy virus transactivator btas are important for its DNA binding activity. Virology 2011, 418, 21–26. [Google Scholar] [CrossRef]
- Wang, J.; Tan, J.; Guo, H.; Zhang, Q.; Jia, R.; Xu, X.; Geng, Y.; Qiao, W. Bovine foamy virus transactivator btas interacts with cellular relb to enhance viral transcription. J. Virol. 2010, 84, 11888–11897. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.; Tan, J.; Zhang, X.; Guo, H.; Zhang, Q.; Guo, T.; Geng, Y.; Qiao, W. Bfv activates the nf-kappab pathway through its transactivator (btas) to enhance viral transcription. Virology 2010, 400, 215–223. [Google Scholar] [CrossRef]
- Löchelt, M. Foamy virus transactivation and gene expression. Curr. Top. Microbiol. Immunol. 2003, 277, 27–61. [Google Scholar]
- Hahn, H.; Baunach, G.; Brautigam, S.; Mergia, A.; Neumann-Haefelin, D.; Daniel, M.D.; McClure, M.O.; Rethwilm, A. Reactivity of primate sera to foamy virus gag and bet proteins. J. Gen. Virol. 1994, 75, 2635–2644. [Google Scholar] [CrossRef] [PubMed]
- Löchelt, M.; Romen, F.; Bastone, P.; Muckenfuss, H.; Kirchner, N.; Kim, Y.B.; Truyen, U.; Rosler, U.; Battenberg, M.; Saib, A.; et al. The antiretroviral activity of apobec3 is inhibited by the foamy virus accessory bet protein. Proc. Natl. Acad. Sci. USA 2005, 102, 7982–7987. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Russell, R.A.; Wiegand, H.L.; Moore, M.D.; Schafer, A.; McClure, M.O.; Cullen, B.R. Foamy virus bet proteins function as novel inhibitors of the apobec3 family of innate antiretroviral defense factors. J. Virol. 2005, 79, 8724–8731. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perkovic, M.; Schmidt, S.; Marino, D.; Russell, R.A.; Stauch, B.; Hofmann, H.; Kopietz, F.; Kloke, B.P.; Zielonka, J.; Strover, H.; et al. Species-specific inhibition of apobec3c by the prototype foamy virus protein bet. J. Biol. Chem. 2009, 284, 5819–5826. [Google Scholar] [CrossRef] [Green Version]
- Saib, A.; Koken, M.H.; van der Spek, P.; Peries, J.; de Thé, H. Involvement of a spliced and defective human foamy virus in the establishment of chronic infection. J. Virol. 1995, 69, 5261–5268. [Google Scholar]
- Callahan, M.E.; Switzer, W.M.; Matthews, A.L.; Roberts, B.D.; Heneine, W.; Folks, T.M.; Sandstrom, P.A. Persistent zoonotic infection of a human with simian foamy virus in the absence of an intact orf-2 accessory gene. J. Virol. 1999, 73, 9619–9624. [Google Scholar]
- Meiering, C.D.; Linial, M.L. Reactivation of a complex retrovirus is controlled by a molecular switch and is inhibited by a viral protein. Proc. Natl. Acad. Sci. USA 2002, 99, 15130–15135. [Google Scholar] [CrossRef] [Green Version]
- Lukic, D.S.; Hotz-Wagenblatt, A.; Lei, J.; Räthe, A.M.; Muhle, M.; Denner, J.; Münk, C.; Löchelt, M. Identification of the feline foamy virus bet domain essential for apobec3 counteraction. Retrovirology 2013, 10, 76. [Google Scholar] [CrossRef] [Green Version]
- Bing, T.; Yu, H.; Li, Y.; Sun, L.; Tan, J.; Geng, Y.; Qiao, W. Characterization of a full-length infectious clone of bovine foamy virus 3026. Virol. Sin. 2014, 29, 94–102. [Google Scholar] [CrossRef]
- Materniak, M.; Bicka, L.; Kuzmak, J. Isolation and partial characterization of bovine foamy virus from polish cattle. Pol. J. Vet. Sci. 2006, 9, 207–211. [Google Scholar]
- Hechler, T.; Materniak, M.; Kehl, T.; Kuzmak, J.; Löchelt, M. Complete genome sequences of two novel european clade bovine foamy viruses from germany and poland. J. Virol. 2012, 86, 10905–10906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindemann, D.; Rethwilm, A. Characterization of a human foamy virus 170-kilodalton env-bet fusion protein generated by alternative splicing. J. Virol. 1998, 72, 4088–4094. [Google Scholar]
- Bing, T.; Wu, K.; Cui, X.; Shao, P.; Zhang, Q.; Bai, X.; Tan, J.; Qiao, W. Identification and functional characterization of bet protein as a negative regulator of bfv3026 replication. Virus Genes 2014, 48, 464–473. [Google Scholar] [CrossRef] [PubMed]
- Horton, P.; Park, K.J.; Obayashi, T.; Fujita, N.; Harada, H.; Adams-Collier, C.J.; Nakai, K. Wolf psort: Protein localization predictor. Nucleic Acids Res. 2007, 35, W585–W587. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen Ba, A.N.; Pogoutse, A.; Provart, N.; Moses, A.M. Nlstradamus: A simple hidden markov model for nuclear localization signal prediction. BMC Bioinform. 2009, 10, 202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lecellier, C.H.; Vermeulen, W.; Bachelerie, F.; Giron, M.L.; Saib, A. Intra- and intercellular trafficking of the foamy virus auxiliary bet protein. J. Virol. 2002, 76, 3388–3394. [Google Scholar] [CrossRef] [Green Version]
- Corbett, A.H.; Silver, P.A. Nucleocytoplasmic transport of macromolecules. Microbiol. Mol. Biol. Rev. 1997, 61, 193–211. [Google Scholar]
- Alke, A.; Schwantes, A.; Kido, K.; Flötenmeyer, M.; Flügel, R.M.; Löchelt, M. The bet gene of feline foamy virus is required for virus replication. Virology 2001, 287, 310–320. [Google Scholar] [CrossRef] [Green Version]
- Yu, S.F.; Linial, M.L. Analysis of the role of the bel and bet open reading frames of human foamy virus by using a new quantitative assay. J. Virol. 1993, 67, 6618–6624. [Google Scholar]
- Bock, M.; Heinkelein, M.; Lindemann, D.; Rethwilm, A. Cells expressing the human foamy virus (hfv) accessory bet protein are resistant to productive hfv superinfection. Virology 1998, 250, 194–204. [Google Scholar] [CrossRef] [Green Version]
- Mullers, E. The foamy virus gag proteins: What makes them different? Viruses 2013, 5, 1023–1041. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eastman, S.W.; Linial, M.L. Identification of a conserved residue of foamy virus gag required for intracellular capsid assembly. J. Virol. 2001, 75, 6857–6864. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pfrepper, K.I.; Löchelt, M.; Rackwitz, H.R.; Schnölzer, M.; Heid, H.; Flügel, R.M. Molecular characterization of proteolytic processing of the gag proteins of human spumavirus. J. Virol. 1999, 73, 7907–7911. [Google Scholar] [PubMed]
- Pfrepper, K.I.; Rackwitz, H.R.; Schnölzer, M.; Heid, H.; Löchelt, M.; Flügel, R.M. Molecular characterization of proteolytic processing of the pol proteins of human foamy virus reveals novel features of the viral protease. J. Virol. 1998, 72, 7648–7652. [Google Scholar]
- Wang, J.; Guo, H.Y.; Jia, R.; Xu, X.; Tan, J.; Geng, Y.Q.; Qiao, W.T. Preparation of bfv gag antiserum and preliminary study on cellular distribution of bfv. Virol. Sin. 2010, 25, 115–122. [Google Scholar] [CrossRef]
- Yu, S.F.; Edelmann, K.; Strong, R.K.; Moebes, A.; Rethwilm, A.; Linial, M.L. The carboxyl terminus of the human foamy virus gag protein contains separable nucleic acid binding and nuclear transport domains. J. Virol. 1996, 70, 8255–8262. [Google Scholar]
- Kong, X.H.; Yu, H.; Xuan, C.H.; Wang, J.Z.; Chen, Q.M.; Geng, Y.Q. The requirements and mechanism for capsid assembly and budding of bovine foamy virus. Arch. Virol. 2005, 150, 1677–1684. [Google Scholar] [CrossRef]
- Winkler, I.; Bodem, J.; Haas, L.; Zemba, M.; Delius, H.; Flower, R.; Flügel, R.M.; Löchelt, M. Characterization of the genome of feline foamy virus and its proteins shows distinct features different from those of primate spumaviruses. J. Virol. 1997, 71, 6727–6741. [Google Scholar]
- Wang, I.H.; Burckhardt, C.J.; Yakimovich, A.; Greber, U.F. Imaging, tracking and computational analyses of virus entry and egress with the cytoskeleton. Viruses 2018, 10, 166. [Google Scholar] [CrossRef] [Green Version]
- Naghavi, M.H.; Walsh, D. Microtubule regulation and function during virus infection. J. Virol. 2017, 91, e00538-17. [Google Scholar] [CrossRef] [Green Version]
- Arriagada, G. Retroviruses and microtubule-associated motor proteins. Cell Microbiol 2017, 19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harris, R.S.; Hultquist, J.F.; Evans, D.T. The restriction factors of human immunodeficiency virus. J. Biol. Chem. 2012, 287, 40875–40883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, G.; Lei, K.J.; Jin, W.; Greenwell-Wild, T.; Wahl, S.M. Induction of apobec3 family proteins, a defensive maneuver underlying interferon-induced anti-hiv-1 activity. J. Exp. Med. 2006, 203, 41–46. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stopak, K.S.; Chiu, Y.L.; Kropp, J.; Grant, R.M.; Greene, W.C. Distinct patterns of cytokine regulation of apobec3g expression and activity in primary lymphocytes, macrophages, and dendritic cells. J. Biol. Chem. 2007, 282, 3539–3546. [Google Scholar] [CrossRef] [Green Version]
- Carthagena, L.; Bergamaschi, A.; Luna, J.M.; David, A.; Uchil, P.D.; Margottin-Goguet, F.; Mothes, W.; Hazan, U.; Transy, C.; Pancino, G.; et al. Human trim gene expression in response to interferons. PLoS ONE 2009, 4, e4894. [Google Scholar] [CrossRef] [Green Version]
- Liberatore, R.A.; Bieniasz, P.D. Tetherin is a key effector of the antiretroviral activity of type i interferon in vitro and In Vivo. Proc. Natl. Acad. Sci. USA 2011, 108, 18097–18101. [Google Scholar] [CrossRef] [Green Version]
- Sheehy, A.M.; Gaddis, N.C.; Choi, J.D.; Malim, M.H. Isolation of a human gene that inhibits hiv-1 infection and is suppressed by the viral vif protein. Nature 2002, 418, 646–650. [Google Scholar] [CrossRef]
- Stremlau, M.; Owens, C.M.; Perron, M.J.; Kiessling, M.; Autissier, P.; Sodroski, J. The cytoplasmic body component trim5alpha restricts hiv-1 infection in old world monkeys. Nature 2004, 427, 848–853. [Google Scholar] [CrossRef]
- Neil, S.J.; Zang, T.; Bieniasz, P.D. Tetherin inhibits retrovirus release and is antagonized by hiv-1 vpu. Nature 2008, 451, 425–430. [Google Scholar] [CrossRef] [Green Version]
- Laguette, N.; Sobhian, B.; Casartelli, N.; Ringeard, M.; Chable-Bessia, C.; Segeral, E.; Yatim, A.; Emiliani, S.; Schwartz, O.; Benkirane, M. Samhd1 is the dendritic- and myeloid-cell-specific hiv-1 restriction factor counteracted by vpx. Nature 2011, 474, 654–657. [Google Scholar] [CrossRef]
- Lu, J.; Pan, Q.; Rong, L.; He, W.; Liu, S.L.; Liang, C. The ifitm proteins inhibit hiv-1 infection. J. Virol. 2011, 85, 2126–2137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Pan, Q.; Ding, S.; Qian, J.; Xu, F.; Zhou, J.; Cen, S.; Guo, F.; Liang, C. The interferon-inducible mxb protein inhibits hiv-1 infection. Cell Host Microbe 2013, 14, 398–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Usami, Y.; Wu, Y.; Gottlinger, H.G. Serinc3 and serinc5 restrict hiv-1 infectivity and are counteracted by nef. Nature 2015, 526, 218–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yap, M.W.; Lindemann, D.; Stanke, N.; Reh, J.; Westphal, D.; Hanenberg, H.; Ohkura, S.; Stoye, J.P. Restriction of foamy viruses by primate trim5alpha. J. Virol. 2008, 82, 5429–5439. [Google Scholar] [CrossRef] [Green Version]
- Pacheco, B.; Finzi, A.; McGee-Estrada, K.; Sodroski, J. Species-specific inhibition of foamy viruses from south american monkeys by new world monkey trim5{alpha} proteins. J. Virol. 2010, 84, 4095–4099. [Google Scholar] [CrossRef] [Green Version]
- Delebecque, F.; Suspene, R.; Calattini, S.; Casartelli, N.; Saib, A.; Froment, A.; Wain-Hobson, S.; Gessain, A.; Vartanian, J.P.; Schwartz, O. Restriction of foamy viruses by apobec cytidine deaminases. J. Virol. 2006, 80, 605–614. [Google Scholar] [CrossRef] [Green Version]
- Jouvenet, N.; Neil, S.J.; Zhadina, M.; Zang, T.; Kratovac, Z.; Lee, Y.; McNatt, M.; Hatziioannou, T.; Bieniasz, P.D. Broad-spectrum inhibition of retroviral and filoviral particle release by tetherin. J. Virol. 2009, 83, 1837–1844. [Google Scholar] [CrossRef] [Green Version]
- Xu, F.; Tan, J.; Liu, R.; Xu, D.; Li, Y.; Geng, Y.; Liang, C.; Qiao, W. Tetherin inhibits prototypic foamy virus release. Virol. J. 2011, 8, 198. [Google Scholar] [CrossRef] [Green Version]
- Liang, Z.; Zhang, Y.; Song, J.; Zhang, H.; Zhang, S.; Li, Y.; Tan, J.; Qiao, W. The effect of bovine bst2a1 on the release and cell-to-cell transmission of retroviruses. Virol. J. 2017, 14, 173. [Google Scholar] [CrossRef]
- Regad, T.; Saib, A.; Lallemand-Breitenbach, V.; Pandolfi, P.P.; de Thé, H.; Chelbi-Alix, M.K. Pml mediates the interferon-induced antiviral state against a complex retrovirus via its association with the viral transactivator. EMBO J. 2001, 20, 3495–3505. [Google Scholar] [CrossRef]
- Hu, X.; Yang, W.; Liu, R.; Geng, Y.; Qiao, W.; Tan, J. N-myc interactor inhibits prototype foamy virus by sequestering viral tas protein in the cytoplasm. J. Virol. 2014, 88, 7036–7044. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, L.; Cheng, Q.; Wang, Z.; Yuan, P.; Li, Z.; Sun, Y.; Han, S.; Yin, J.; Peng, B.; He, X.; et al. Human pirh2 is a novel inhibitor of prototype foamy virus replication. Viruses 2015, 7, 1668–1684. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kane, M.; Zang, T.M.; Rihn, S.J.; Zhang, F.; Kueck, T.; Alim, M.; Schoggins, J.; Rice, C.M.; Wilson, S.J.; Bieniasz, P.D. Identification of interferon-stimulated genes with antiretroviral activity. Cell Host Microbe 2016, 20, 392–405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herchenröder, O.; Löchelt, M.; Buseyne, F.; Gessain, A.; Soares, M.A.; Khan, A.S.; Lindemann, D. Twelfth international foamy virus conference-meeting report. Viruses 2019, 11, 134. [Google Scholar] [CrossRef] [Green Version]
- Ledesma-Feliciano, C.; Troyer, R.M.; Zheng, X.; Miller, C.; Cianciolo, R.; Bordicchia, M.; Dannemiller, N.; Gagne, R.; Beatty, J.; Quimby, J.; et al. Feline foamy virus infection: Characterization of experimental infection and prevalence of natural infection in domestic cats with and without chronic kidney disease. Viruses 2019, 11, 662. [Google Scholar] [CrossRef] [Green Version]
- Murray, S.M.; Picker, L.J.; Axthelm, M.K.; Hudkins, K.; Alpers, C.E.; Linial, M.L. Replication in a superficial epithelial cell niche explains the lack of pathogenicity of primate foamy virus infections. J. Virol. 2008, 82, 5981–5985. [Google Scholar] [CrossRef] [Green Version]
- Rhodes-Feuillette, A.; Saal, F.; Lasneret, J.; Santillana-Hayat, M.; Peries, J. Studies on in vitro interferon induction capacity and interferon sensitivity of simian foamy viruses. Arch. Virol. 1987, 97, 77–84. [Google Scholar] [CrossRef]
- Colas, S.; Bourge, J.F.; Wybier, J.; Chelbi-Alix, M.K.; Paul, P.; Emanoil-Ravier, R. Human foamy virus infection activates class i major histocompatibility complex antigen expression. J. Gen. Virol. 1995, 76, 661–667. [Google Scholar] [CrossRef]
- Sabile, A.; Rhodes-Feuillette, A.; Jaoui, F.Z.; Tobaly-Tapiero, J.; Giron, M.L.; Lasneret, J.; Peries, J.; Canivet, M. In vitro studies on interferon-inducing capacity and sensitivity to ifn of human foamy virus. Res. Virol. 1996, 147, 29–37. [Google Scholar] [CrossRef]
- Rua, R.; Lepelley, A.; Gessain, A.; Schwartz, O. Innate sensing of foamy viruses by human hematopoietic cells. J. Virol. 2012, 86, 909–918. [Google Scholar] [CrossRef] [Green Version]
- Meiering, C.D.; Linial, M.L. The promyelocytic leukemia protein does not mediate foamy virus latency in vitro. J. Virol. 2003, 77, 2207–2213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falcone, V.; Schweizer, M.; Toniolo, A.; Neumann-Haefelin, D.; Meyerhans, A. Gamma interferon is a major suppressive factor produced by activated human peripheral blood lymphocytes that is able to inhibit foamy virus-induced cytopathic effects. J. Virol. 1999, 73, 1724–1728. [Google Scholar] [PubMed]
- Rola-Luszczak, M.; Materniak, M.; Pluta, A.; Hulst, M.; Kuzmak, J. Transcriptomic microarray analysis of bomac cells after infection with bovine foamy virus. Arch. Virol. 2014, 159, 1515–1519. [Google Scholar] [CrossRef] [PubMed]
- Bloor, S.; Maelfait, J.; Krumbach, R.; Beyaert, R.; Randow, F. Endoplasmic reticulum chaperone gp96 is essential for infection with vesicular stomatitis virus. Proc. Natl. Acad. Sci. USA 2010, 107, 6970–6975. [Google Scholar] [CrossRef] [Green Version]
- Carrington, M.; O’Brien, S.J. The influence of hla genotype on aids. Annu. Rev. Med. 2003, 54, 535–551. [Google Scholar] [CrossRef] [Green Version]
- Lerner, D.L.; Grant, C.K.; de Parseval, A.; Elder, J.H. Fiv infection of il-2-dependent and -independent feline lymphocyte lines: Host cells range distinctions and specific cytokine upregulation. Vet. Immunol. Immunopathol. 1998, 65, 277–297. [Google Scholar] [CrossRef]
- Rideout, B.A.; Moore, P.F.; Pedersen, N.C. Persistent upregulation of mhc class ii antigen expression on t-lymphocytes from cats experimentally infected with feline immunodeficiency virus. Vet. Immunol. Immunopathol. 1992, 35, 71–81. [Google Scholar] [CrossRef]
- Nogami, S.; Satoh, S.; Tanaka-Nakadate, S.; Yoshida, K.; Nakano, M.; Terano, A.; Shirataki, H. Identification and characterization of taxilin isoforms. Biochem. Biophys. Res. Commun. 2004, 319, 936–943. [Google Scholar] [CrossRef]
- Hoffmann, J.; Boehm, C.; Himmelsbach, K.; Donnerhak, C.; Roettger, H.; Weiss, T.S.; Ploen, D.; Hildt, E. Identification of alpha-taxilin as an essential factor for the life cycle of hepatitis b virus. J. Hepatol. 2013, 59, 934–941. [Google Scholar] [CrossRef]
- Hutter, S.; Zurnic, I.; Lindemann, D. Foamy virus budding and release. Viruses 2013, 5, 1075–1098. [Google Scholar] [CrossRef]
- Kincaid, R.P.; Chen, Y.; Cox, J.E.; Rethwilm, A.; Sullivan, C.S. Noncanonical microrna (mirna) biogenesis gives rise to retroviral mimics of lymphoproliferative and immunosuppressive host mirnas. MBio 2014, 5, e00074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kincaid, R.P.; Burke, J.M.; Sullivan, C.S. Rna virus microrna that mimics a b-cell oncomir. Proc. Natl. Acad. Sci. USA 2012, 109, 3077–3082. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Safari, R.; Hamaidia, M.; de Brogniez, A.; Gillet, N.; Willems, L. Cis-drivers and trans-drivers of bovine leukemia virus oncogenesis. Curr. Opin. Virol. 2017, 26, 15–19. [Google Scholar] [CrossRef] [PubMed]
- Rua, R.; Betsem, E.; Calattini, S.; Saib, A.; Gessain, A. Genetic characterization of simian foamy viruses infecting humans. J. Virol. 2012, 86, 13350–13359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burke, J.M.; Kincaid, R.P.; Aloisio, F.; Welch, N.; Sullivan, C.S. Expression of short hairpin rnas using the compact architecture of retroviral microrna genes. Nucleic Acids Res. 2017, 45, e154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heit, A. In Silico Investigations of Bovine Foamy Virus Micrornas. Master’s Thesis, University of Heidelberg, Heidelberg, Germany, 2017. [Google Scholar]
- Hildebrand, D.; Eberle, M.E.; Wolfle, S.M.; Egler, F.; Sahin, D.; Sahr, A.; Bode, K.A.; Heeg, K. Hsa-mir-99b/let-7e/mir-125a cluster regulates pathogen recognition receptor-stimulated suppressive antigen-presenting cells. Front. Immunol. 2018, 9, 1224. [Google Scholar] [CrossRef]
- Servin-Gonzalez, L.S.; Granados-Lopez, A.J.; Lopez, J.A. Families of micrornas expressed in clusters regulate cell signaling in cervical cancer. Int. J. Mol. Sci. 2015, 16, 12773–12790. [Google Scholar] [CrossRef] [Green Version]
- Shatseva, T.; Lee, D.Y.; Deng, Z.; Yang, B.B. Microrna mir-199a-3p regulates cell proliferation and survival by targeting caveolin-2. J. Cell Sci. 2011, 124, 2826–2836. [Google Scholar] [CrossRef] [Green Version]
- Menning, M.; Kufer, T.A. A role for the ankyrin repeat containing protein ankrd17 in nod1- and nod2-mediated inflammatory responses. FEBS Lett. 2013, 587, 2137–2142. [Google Scholar] [CrossRef]
- Takahashi, Y.; Meyerkord, C.L.; Hori, T.; Runkle, K.; Fox, T.E.; Kester, M.; Loughran, T.P.; Wang, H.G. Bif-1 regulates atg9 trafficking by mediating the fission of golgi membranes during autophagy. Autophagy 2011, 7, 61–73. [Google Scholar] [CrossRef]
- Zhong, P.; Agosto, L.M.; Munro, J.B.; Mothes, W. Cell-to-cell transmission of viruses. Curr. Opin. Virol. 2013, 3, 44–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Tan, M.; Xie, Z.; Dai, L.; Chen, Y.; Zhao, Y. Identification of lysine succinylation as a new post-translational modification. Nat. Chem. Biol. 2011, 7, 58–63. [Google Scholar] [CrossRef] [PubMed]
- Greig, A.S. A syncytium regression test to detect antibodies to bovine syncytial virus. Can. J. Comp. Med. 1979, 43, 112–114. [Google Scholar] [PubMed]
- Jacobs, R.M.; Pollari, F.L.; McNab, W.B.; Jefferson, B. A serological survey of bovine syncytial virus in ontario: Associations with bovine leukemia and immunodeficiency-like viruses, production records, and management practices. Can. J. Vet. Res. 1995, 59, 271–278. [Google Scholar]
- Appleby, R.C. Antibodies to bovine syncytial virus in dairy cattle. Vet. Rec. 1979, 105, 80–81. [Google Scholar] [CrossRef]
- Johnson, R.H.; de la Rosa, J.; Abher, I.; Kertayadnya, I.G.; Entwistle, K.W.; Fordyce, G.; Holroyd, R.G. Epidemiological studies of bovine spumavirus. Vet. Microbiol. 1988, 16, 25–33. [Google Scholar] [CrossRef]
- Materniak-Kornas, M.; Osinski, Z.; Rudzki, M.; Kuzmak, J. Development of a recombinant protein-based elisa for detection of antibodies against bovine foamy virus. J. Vet. Res. 2017, 61, 247–252. [Google Scholar] [CrossRef] [Green Version]
- Jacobs, R.M.; Smith, H.E.; Gregory, B.; Valli, V.E.; Whetstone, C.A. Detection of multiple retroviral infections in cattle and cross-reactivity of bovine immunodeficiency-like virus and human immunodeficiency virus type 1 proteins using bovine and human sera in a western blot assay. Can. J. Vet. Res. 1992, 56, 353–359. [Google Scholar]
- Adjei, A.A.; Adiku, T.K.; Kumi, P.F.; Domfeh, A.B. Human t-lymphotropic type-1 virus specific antibody detected in sera of hiv/aids patients in ghana. Jpn. J. Infect. Dis. 2003, 56, 57–59. [Google Scholar]
- Bandecchi, P.; Matteucci, D.; Baldinotti, F.; Guidi, G.; Abramo, F.; Tozzini, F.; Bendinelli, M. Prevalence of feline immunodeficiency virus and other retroviral infections in sick cats in italy. Vet. Immunol. Immunopathol. 1992, 31, 337–345. [Google Scholar] [CrossRef]
- Lee, I.T.; Levy, J.K.; Gorman, S.P.; Crawford, P.C.; Slater, M.R. Prevalence of feline leukemia virus infection and serum antibodies against feline immunodeficiency virus in unowned free-roaming cats. J. Am. Vet. Med. Assoc. 2002, 220, 620–622. [Google Scholar] [CrossRef] [PubMed]
- Winkler, I.G.; Löchelt, M.; Flower, R.L. Epidemiology of feline foamy virus and feline immunodeficiency virus infections in domestic and feral cats: A seroepidemiological study. J. Clin. Microbiol. 1999, 37, 2848–2851. [Google Scholar] [PubMed]
- Fultz, P.N.; McGinn, T.; Davis, I.C.; Romano, J.W.; Li, Y. Coinfection of macaques with simian immunodeficiency virus and simian t cell leukemia virus type i: Effects on virus burdens and disease progression. J. Infect. Dis. 1999, 179, 600–611. [Google Scholar] [CrossRef] [PubMed]
- Amborski, G.F.; Lo, J.L.; Seger, C.L. Serological detection of multiple retroviral infections in cattle: Bovine leukemia virus, bovine syncytial virus and bovine visna virus. Vet. Microbiol. 1989, 20, 247–253. [Google Scholar] [CrossRef]
- Rola, M. Development of Elisa Test for the Diagnostics of Biv Infections and Determination of the Biv Influence on the Course of Blv Infection. Ph.D. Thesis, NVRI, Pulawy, Poland, 2003. [Google Scholar]
- Cohen, N.D.; Carter, C.N.; Thomas, M.A.; Lester, T.L.; Eugster, A.K. Epizootiologic association between feline immunodeficiency virus infection and feline leukemia virus seropositivity. J. Am. Vet. Med. Assoc. 1990, 197, 220–225. [Google Scholar]
- O’Connor, T.P., Jr.; Tanguay, S.; Steinman, R.; Smith, R.; Barr, M.C.; Yamamoto, J.K.; Pedersen, N.C.; Andersen, P.R.; Tonelli, Q.J. Development and evaluation of immunoassay for detection of antibodies to the feline t-lymphotropic lentivirus (feline immunodeficiency virus). J. Clin. Microbiol. 1989, 27, 474–479. [Google Scholar]
- Briggs, N.C.; Battjes, R.J.; Cantor, K.P.; Blattner, W.A.; Yellin, F.M.; Wilson, S.; Ritz, A.L.; Weiss, S.H.; Goedert, J.J. Seroprevalence of human t cell lymphotropic virus type ii infection, with or without human immunodeficiency virus type 1 coinfection, among us intravenous drug users. J. Infect. Dis. 1995, 172, 51–58. [Google Scholar] [CrossRef]
- Materniak, M. Development of Diagnostic Methods for Bovine Foamy Virus Infections and the Role of Milk and Saliva in Virus Transmission. Ph.D. Thesis, NVRI, Pulawy, Poland, 2008. [Google Scholar]
- Jacobs, R.M.; Song, Z.; Poon, H.; Heeney, J.L.; Taylor, J.A.; Jefferson, B.; Vernau, W.; Valli, V.E. Proviral detection and serology in bovine leukemia virus-exposed normal cattle and cattle with lymphoma. Can. J. Vet. Res. 1992, 56, 339–348. [Google Scholar]
- Hooks, J.J.; Gibbs, C.J., Jr. The foamy viruses. Bacteriol. Rev. 1975, 39, 169–185. [Google Scholar]
- Gillet, N.A.; Hamaidia, M.; de Brogniez, A.; Gutierrez, G.; Renotte, N.; Reichert, M.; Trono, K.; Willems, L. Bovine leukemia virus small noncoding rnas are functional elements that regulate replication and contribute to oncogenesis in vivo. PLoS Pathog. 2016, 12, e1005588. [Google Scholar] [CrossRef]
- Frie, M.C.; Droscha, C.J.; Greenlick, A.E.; Coussens, P.M. Micrornas encoded by bovine leukemia virus (blv) are associated with reduced expression of b cell transcriptional regulators in dairy cattle naturally infected with blv. Front. Vet. Sci. 2017, 4, 245. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kertayadnya, I.G.; Johnson, R.H.; Abher, I.; Burgess, G.W. Detection of immunological tolerance to bovine spumavirus (bsv) with evidence for salivary excretion and spread of bsv from the tolerant animal. Vet. Microbiol. 1988, 16, 35–39. [Google Scholar] [CrossRef]
- Bouillant, A.M.; Ruckerbauer, G.M. Isolation of bovine syncytial virus from lymphocytes recovered from fluids used to flush uterus and oviducts of superovulated cattle. Can. J. Comp. Med. 1984, 48, 332–334. [Google Scholar] [PubMed]
- Switzer, W.M.; Tang, S.; Zheng, H.; Shankar, A.; Sprinkle, P.S.; Sullivan, V.; Granade, T.C.; Heneine, W. Dual simian foamy virus/human immunodeficiency virus type 1 infections in persons from cote d’ivoire. PLoS ONE 2016, 11, e0157709. [Google Scholar] [CrossRef]
- Materniak, M.; Hechler, T.; Löchelt, M.; Kuzmak, J. Seroreactivity of humans and ruminants to bovine foamy virus—Evidence for zoonotic transmission and existence of new reservoirs for foamy viruses. In Proceedings of the 8th International Foamy Virus Conference, Argos, Greece, 7–8 May 2010. [Google Scholar]
- Materniak, M.; Serwacka, A.; Rydzewski, A.; Rudzki, S.; Bocian, L.; Kehl, T.; Löchelt, M.; Kuzmak, J. Seroreactivity to non-primate foamy viruses in patients immunosupressed after kidney transplantation. In Proceedings of the 10th Internationa Foamy Virus Conference, Puławy, Poland, 24–25 June 2014. [Google Scholar]
- Ruckerbauer, G.M.; Sugden, E.A.; Bouillant, A.M. A comparison of the bovine leukemia and bovine syncytial virus status in utero-tubal cells recovered from fluids used to flush the uterus and oviducts of blv-infected, superovulated cattle. Ann. Rech. Vet. 1988, 19, 19–26. [Google Scholar]
- Scott, F.W.; Shively, J.N.; Gaskin, J.; Gillespie, J.H. Bovine syncytial virus isolations. Arch. Gesamte Virusforsch. 1973, 43, 43–52. [Google Scholar] [CrossRef]
- Van der Maaten, M.J.; Hubbert, W.T.; Boothe, A.D.; Bryner, J.H.; Estes, P.C. Isolations of bovine syncytial virus from maternal and fetal blood. Am. J. Vet. Res. 1973, 34, 341–343. [Google Scholar]
- Luther, P.D.; Nuttall, P.A.; Gibbons, R.A. Isolation of viruses from cultures of bovine endometrial cells. J. Infect. Dis. 1978, 138, 660–663. [Google Scholar] [CrossRef]
- Falcone, V.; Leupold, J.; Clotten, J.; Urbanyi, E.; Herchenroder, O.; Spatz, W.; Volk, B.; Bohm, N.; Toniolo, A.; Neumann-Haefelin, D.; et al. Sites of simian foamy virus persistence in naturally infected african green monkeys: Latent provirus is ubiquitous, whereas viral replication is restricted to the oral mucosa. Virology 1999, 257, 7–14. [Google Scholar] [CrossRef] [Green Version]
- Murray, S.M.; Linial, M.L. Foamy virus infection in primates. J. Med. Primatol. 2006, 35, 225–235. [Google Scholar] [CrossRef]
- Murray, S.M.; Picker, L.J.; Axthelm, M.K.; Linial, M.L. Expanded tissue targets for foamy virus replication with simian immunodeficiency virus-induced immunosuppression. J. Virol. 2006, 80, 663–670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flanagan, J.R.; Becker, K.G.; Ennist, D.L.; Gleason, S.L.; Driggers, P.H.; Levi, B.Z.; Appella, E.; Ozato, K. Cloning of a negative transcription factor that binds to the upstream conserved region of moloney murine leukemia virus. Mol. Cell. Biol 1992, 12, 38–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hechler, T.; Khan, A.A.; Löchelt, M.; German Cancer Research Center. Personal communication, 2011.
- Materniak, M.; Kuzmak, J.; Olech, M. Seroreactivity to bovine foamy virus antigens in wild ruminants—Evidence for new reservoirs of foamy viruses? In Proceedings of the XIII DIAGMOL Conference 2012, SGGW, Warsaw, Poland, 24 November 2012. [Google Scholar]
- Liu, W.; Lei, J.; Liu, Y.; Lukic, D.S.; Rathe, A.M.; Bao, Q.; Kehl, T.; Bleiholder, A.; Hechler, T.; Löchelt, M. Feline foamy virus-based vectors: Advantages of an authentic animal model. Viruses 2013, 5, 1702–1718. [Google Scholar] [CrossRef] [PubMed]
- Bauer, T.R., Jr.; Allen, J.M.; Hai, M.; Tuschong, L.M.; Khan, I.F.; Olson, E.M.; Adler, R.L.; Burkholder, T.H.; Gu, Y.C.; Russell, D.W.; et al. Successful treatment of canine leukocyte adhesion deficiency by foamy virus vectors. Nat. Med. 2008, 14, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Ledesma-Feliciano, C.; Hagen, S.; Troyer, R.; Zheng, X.; Musselman, E.; Slavkovic Lukic, D.; Franke, A.M.; Maeda, D.; Zielonka, J.; Münk, C.; et al. Replacement of feline foamy virus bet by feline immunodeficiency virus vif yields replicative virus with novel vaccine candidate potential. Retrovirology 2018, 15, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwantes, A.; Truyen, U.; Weikel, J.; Weiss, C.; Löchelt, M. Application of chimeric feline foamy virus-based retroviral vectors for the induction of antiviral immunity in cats. J. Virol. 2003, 77, 7830–7842. [Google Scholar] [CrossRef] [Green Version]


| Subject/Topic | References |
|---|---|
| BFV as a well-established infection model in life-stock animals (cattle and sheep) | [13,14] |
| BFV as the only known FV in the general human food chain (beef and dairy products) | [15,16] |
| BFV Riems as the only FV passaged exclusively on primary and homologous host cells | [17,18] |
| Integrase domain: disrupted HH-CC zinc finger and unique sequence insertion into the extreme C-terminus | [19] |
| Detailed understanding of gene expression and transactivation of a non-simian FV | [20,21] |
| RNA Pol III miRNAs, unique precursor structure and their functions | [14,22,23] |
| Extremely tight cell association and identification of residues critical for this phenotype | [24,25,26] |
| Detailed understanding of new restriction factors against FVs | [27] |
| Broad tissue tropism and gene expression in BFV-infected calves | [5,28] |
| Virus-Type | Virus Isolate * and Accession Number | Number of Dumbbell-Shaped miRNA Cassettes | Number of AB or BB Boxes |
|---|---|---|---|
| BFV | BFV_Riems [22]; JX307862.1 | 1 | 0 |
| BFV_100; JX307861.1 | 1 | 0 | |
| BFV_11; U94514.1 | 1 | 0 | |
| BFV_3026; AY134750.1 | 1 | 0 | |
| EFV | EFV; AF201902.1 | 1 | 0 |
| FFV | FFV Chatul-3; AJ564746.1 | 4 | 4 |
| FFV F17; U85043.1 | 4 | 4 | |
| FFV FUV; NC_039242.1 | 4 | 4 | |
| FFVPco; KC292054.1 | 3 | 3 | |
| HFV | HFV; U21247.1 | 1 | 0 |
| HSRV1; Y07723.1 | 1 | 0 | |
| HSRV2; Y07724.1 | 1 | 0 | |
| PFV; Y07725.1 | 1 | 0 | |
| SFV | SFV_AG15; JQ867462.1 | 1 | 0 |
| SFVagm; NC_010820.1 [112] | 2 | 1 | |
| SFV_AXX; EU010385.1 | 5 | 3 | |
| SFV_BAD327; JQ867463.1 | 1 | 0 | |
| SFV_BAD468; JQ867465.1 | 1 | 0 | |
| SFV_BAK74; JQ867464.1 | 0 | 0 | |
| SFV_CAE_FV2014; MF582544.1 | 2 | 1 | |
| SFV_CAE_LK3; M74895.1 | 2 | 1 | |
| SFV_CJA; GU356395.1 | 1 | 0 | |
| SFV_CNI; JQ867466.1 | 3 | 1 | |
| SFV_CPZ; U04327.1 | 1 | 0 | |
| SFV_GOR; HM245790.1 | 1 | 0 | |
| SFV_MAC; X54482.1 | 1 | 0 | |
| SFV_MCY; KF026286.1 | 1 | 0 | |
| SFV_MFA; LC094267.1 | 1 | 0 | |
| SFV_MFU; AB923518.1 | 1 | 0 | |
| SFV_MMU; MF280817.1 | 1 | 0 | |
| SFV_OCR; KM233624.1 | 1 | 0 | |
| SFV_ORA; NC_039085.1 | 2 | 1 | |
| SFV_PPY; AJ544579.1 | 3 | 2 | |
| SFV_PSC; KX087159 | 1 | 0 | |
| SFV_PVE; NC_001364.1 | 1 | 0 | |
| SFV_SSC; GU356394.1 | 1 | 0 | |
| SFV_SXA; KP143760.1 | 1 | 0 | |
| SFV-6; L25422 | 1 | 1 |
| miRNA Name | Human and Bovine miRNA with Seed Identity |
|---|---|
| SFVagm -S2-5p | hsa-miR-28-5p, hsa-miR-3139, hsa-miR-708-5p |
| SFVagm -S3-5p | hsa-miR-4739, hsa-miR-4756-5p, hsa-miR-1321 |
| SFVagm -S4-3p | hsa-miR-155-5p |
| SFVagm -S6-3p | hsa-miR-132-3p, hsa-miR-212-3p |
| SFVagm -S7-5p | hsa-miR-3154 |
| BFV Riems miR-BF1-3p | bta-miR-125a, bta-miR-125b, bta-miR-670 |
| BFV Riems miR-BF1-5p | bta-miR-3957 |
| BFV Riems miR-BF2-5p | bta-miR-199a-3p |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Materniak-Kornas, M.; Tan, J.; Heit-Mondrzyk, A.; Hotz-Wagenblatt, A.; Löchelt, M. Bovine Foamy Virus: Shared and Unique Molecular Features In Vitro and In Vivo. Viruses 2019, 11, 1084. https://doi.org/10.3390/v11121084
Materniak-Kornas M, Tan J, Heit-Mondrzyk A, Hotz-Wagenblatt A, Löchelt M. Bovine Foamy Virus: Shared and Unique Molecular Features In Vitro and In Vivo. Viruses. 2019; 11(12):1084. https://doi.org/10.3390/v11121084
Chicago/Turabian StyleMaterniak-Kornas, Magdalena, Juan Tan, Anke Heit-Mondrzyk, Agnes Hotz-Wagenblatt, and Martin Löchelt. 2019. "Bovine Foamy Virus: Shared and Unique Molecular Features In Vitro and In Vivo" Viruses 11, no. 12: 1084. https://doi.org/10.3390/v11121084
APA StyleMaterniak-Kornas, M., Tan, J., Heit-Mondrzyk, A., Hotz-Wagenblatt, A., & Löchelt, M. (2019). Bovine Foamy Virus: Shared and Unique Molecular Features In Vitro and In Vivo. Viruses, 11(12), 1084. https://doi.org/10.3390/v11121084





