Genomic and Proteomic Characterization of Bacteriophage BH1 Spontaneously Released from Probiotic Lactobacillus rhamnosus Pen
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Culture Conditions
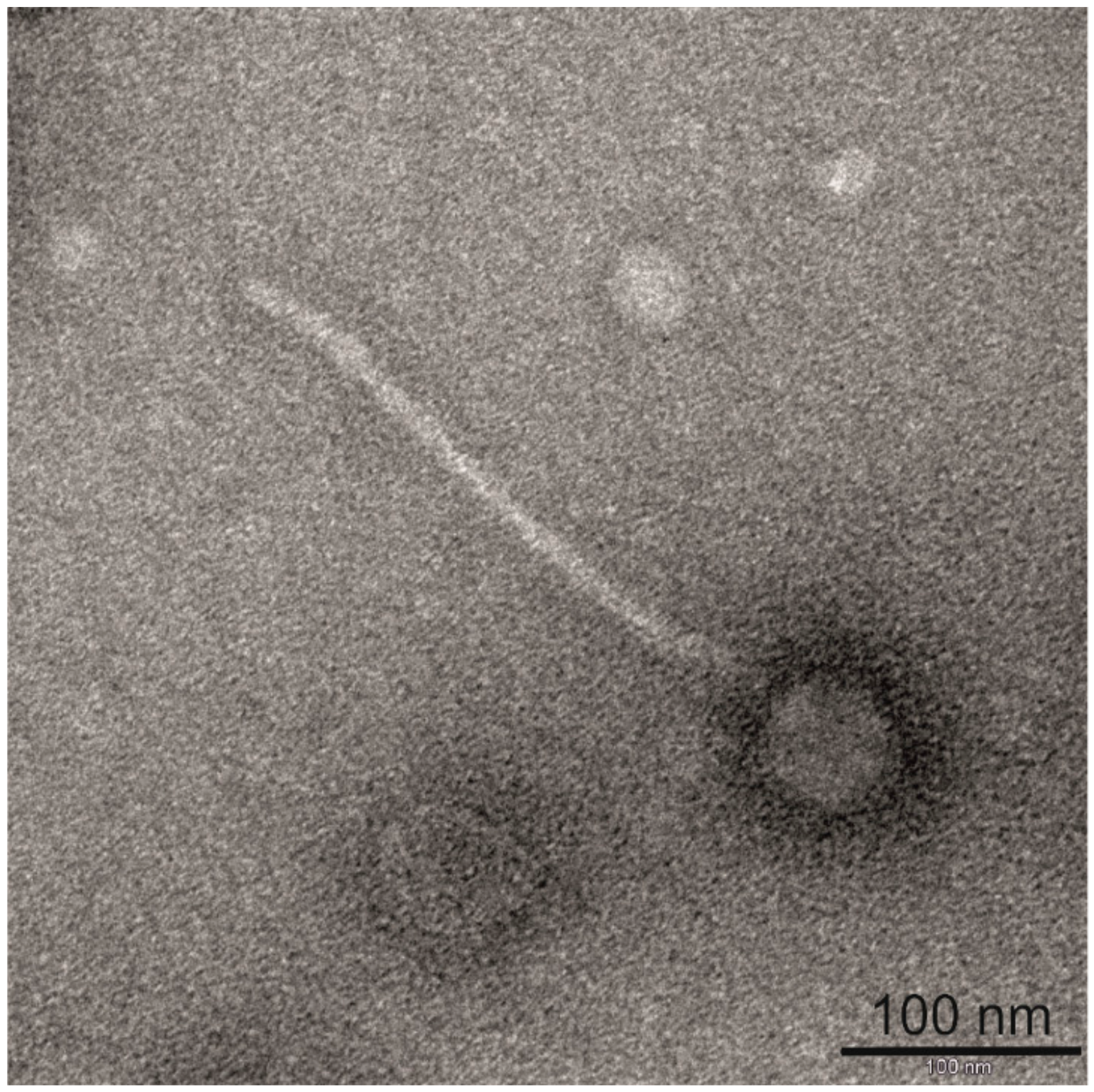
2.2. Electron Microscopy
2.3. Protein Identification
2.4. Genomic Analysis
2.5. Analysis of Prophage Induction—Sample Preparation
2.6. Quantitative PCR and Droplet Digital PCR
2.7. Statistical Analysis
3. Results and Discussion
3.1. Morphological and Proteomic Characterization of Bacteriophage BH1
3.2. Bacteriophage BH1 Genome Analysis
3.3. Genome Organization of Bacteriophage BH1
3.4. Spontaneous Phage Induction Analysis Using QPCR and Digital PCR
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Vijaya Kumar, B.; Vijayendra, S.V.N.; Reddy, O.V.S. Trends in dairy and non-dairy probiotic products - a review. J. Food Sci. Technol. 2015, 52, 6112–6124. [Google Scholar] [CrossRef] [Green Version]
- Putta, S.; Yarla, N.S.; Lakkappa, D.B.; Imandi, S.B.; Malla, R.R.; Chaitanya, A.K.; Chari, B.P.V.; Saka, S.; Vechalapu, R.R.; Kamal, M.A.; et al. Probiotics: Supplements, food, pharmaceutical industry. In Therapeutic, Probiotic, and Unconventional Foods, 1st ed.; Grumezescu, A., Holban, A.M., Eds.; Academic Press, Elsevier: Cambridge, MA, USA, 2018; pp. 15–25. [Google Scholar]
- Ruszczyński, M.; Radzikowski, A.; Szajewska, H. Clinical trial: Effectiveness of Lactobacillus rhamnosus (strains E/N, Oxy and Pen) in the prevention of antibiotic-associated diarrhoea in children. Aliment. Pharmacol. Ther. 2008, 28, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Bermudez-Brito, M.; Plaza-Diaz, J.; Munoz-Quezada, S.; Gomez-Llorente, C.; Gil, A. Probiotic Mechanisms of Action. Ann. Nutr. Metab. 2012, 61, 160–174. [Google Scholar] [CrossRef] [PubMed]
- Plaza-Diaz, J.; Ruiz-Ojeda, F.J.; Gil-Campos, M.; Gil, A. Mechanisms of action of probiotics. In Proceedings of the Advances in Nutrition; Oxford University Press: Oxford, UK, 2019; Volume 10, pp. S49–S185. [Google Scholar]
- Sanders, M.E.; Akkermans, L.M.A.; Haller, D.; Hammerman, C.; Heimbach, J.T.; Hörmannsperger, G.; Huys, G. Safety assessment of probiotics for human use. Gut Microbes 2010, 1, 164–185. [Google Scholar] [CrossRef] [PubMed]
- Daliri, E.B.-M.; Lee, B.H.; Oh, D.H. Safety of probiotics in health and disease. In The Role of Functional Food Security in Global Health, 1st ed.; Watson, W.R., Singh, R., Takahashi, T., Eds.; Academic Press, Elsevier: Cambridge, MA, USA, 2019; pp. 603–622. [Google Scholar]
- Zaburlin, D.; Mercanti, D.J.; Quiberoni, A. A fast PCR-based method for the characterization of prophage profiles in strains of the Lactobacillus casei group. J. Virol. Methods 2017, 248, 226–233. [Google Scholar] [CrossRef]
- Ceapa, C.; Davids, M.; Ritari, J.; Lambert, J.; Wels, M.; Douillard, F.P.; Smokvina, T.; de Vos, W.M.; Knol, J.; Kleerebezem, M. The variable regions of Lactobacillus rhamnosus genomes reveal the dynamic evolution of metabolic and host-adaptation repertoires. Genome Biol. Evol. 2016, 8, 1889–1905. [Google Scholar] [CrossRef] [Green Version]
- Casjens, S. Prophages and bacterial genomics: What have we learned so far? Mol. Microbiol. 2003, 49, 277–300. [Google Scholar] [CrossRef]
- Garneau, J.E.; Moineau, S. Bacteriophages of lactic acid bacteria and their impact on milk fermentations. Microb. Cell Fact. 2011, 10 Suppl. 1, S20. [Google Scholar] [CrossRef] [Green Version]
- Bondy-Denomy, J.; Davidson, A.R. When a virus is not a parasite: The beneficial effects of prophages on bacterial fitness. J. Microbiol. 2014, 52, 235–242. [Google Scholar] [CrossRef]
- Wang, X.; Kim, Y.; Ma, Q.; Hong, S.H.; Pokusaeva, K.; Sturino, J.M.; Wood, T.K. Cryptic prophages help bacteria cope with adverse environments. Nat. Commun. 2010, 1, 147. [Google Scholar] [CrossRef] [Green Version]
- Tuohimaa, A.; Riipinen, K.A.; Brandt, K.; Alatossava, T. The genome of the virulent phage Lc-Nu of probiotic Lactobacillus rhamnosus, and comparative genomics with Lactobacillus casei phages. Arch. Virol. 2006, 151, 947–965. [Google Scholar] [CrossRef] [PubMed]
- Durmaz, E.; Miller, M.J.; Azcarate-Peril, M.A.; Toon, S.P.; Klaenhammer, T.R. Genome sequence and characteristics of Lrm1, a prophage from industrial Lactobacillus rhamnosus strain M1. Appl. Environ. Microbiol. 2008, 74, 4601–4609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jarocki, P.; Podleśny, M.; Pawelec, J.; Malinowska, A.; Kowalczyk, S.; Targoński, Z. Spontaneous release of bacteriophage particles by Lactobacillus rhamnosus Pen. J. Microbiol. Biotechnol. 2013, 23, 357–363. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nanda, A.M.; Thormann, K.; Frunzke, J. Impact of spontaneous prophage induction on the fitness of bacterial populations and host-microbe interactions. J. Bacteriol. 2015, 197, 410–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sambrook, J.; Russell, D. Molecular Cloning: A Laboratory Manual, 3rd ed.; Cold Spring Harbor Laboratory Press: New York, NY, USA, 2001. [Google Scholar]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, 45e. [Google Scholar] [CrossRef] [PubMed]
- Villion, M.; Moineau, S. Bacteriophages of Lactobacillus. Front. Biosci. 2009, 14, 1661–1683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grimaud, R.; Toussaint, A. Assembly of both the head and tail of bacteriophage Mu is blocked in Escherichia coli groEL and groES mutants. J. Bacteriol. 1998, 180, 1148–1153. [Google Scholar]
- Mercanti, D.J.; Rousseau, G.M.; Capra, M.L.; Quiberoni, A.; Tremblay, D.M.; Labrie, S.J.; Moineau, S. Genomic diversity of phages infecting probiotic strains of Lactobacillus paracasei. Appl. Environ. Microbiol. 2016, 82, 95–105. [Google Scholar] [CrossRef] [Green Version]
- Jarocki, P.; Podleśny, M.; Krawczyk, M.; Glibowska, A.; Pawelec, J.; Komoń-Janczara, E.; Kholiavskyi, O.; Dworniczak, M.; Targoński, Z. Complete genome sequence of Lactobacillus rhamnosus Pen, a probiotic component of a medicine used in prevention of antibiotic-associated diarrhoea in children. Gut Pathog. 2018, 10, 5. [Google Scholar] [CrossRef] [Green Version]
- Wuyts, S.; Wittouck, S.; De Boeck, I.; Allonsius, C.N.; Pasolli, E.; Segata, N.; Lebeer, S. Large-scale phylogenomics of the Lactobacillus casei group highlights taxonomic inconsistencies and reveals novel clade-associated features. mSystems 2017, 2, e00061–17. [Google Scholar] [CrossRef] [Green Version]
- Douillard, F.P.; Ribbera, A.; Kant, R.; Pietilä, T.E.; Järvinen, H.M.; Messing, M.; Randazzo, C.L.; Paulin, L.; Laine, P.; Ritari, J.; et al. Comparative genomic and functional analysis of 100 Lactobacillus rhamnosus strains and their comparison with strain GG. PLoS Genet. 2013, 9, e1003683. [Google Scholar] [CrossRef] [PubMed]
- Brandt, K.; Alatossava, T. Specific identification of certain probiotic Lactobacillus rhamnosus strains with PCR primers based on phage-related sequences. Int. J. Food Microbiol. 2003, 84, 189–196. [Google Scholar] [CrossRef]
- Zago, M.; Rossetti, L.; Reinheimer, J.; Carminati, D.; Giraffa, G. Detection and identification of Lactobacillus helveticus bacteriophages by PCR. J. Dairy Res. 2008, 75, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Barrangou, R.; Fremaux, C.; Deveau, H.; Richards, M.; Boyaval, P.; Moineau, S.; Romero, D.A.; Horvath, P. CRISPR provides acquired resistance against viruses in prokaryotes. Science 2007, 315, 1709–1712. [Google Scholar] [CrossRef]
- Lo, T.C.; Shih, T.C.; Lin, C.F.; Chen, H.W.; Lin, T.H. Complete genomic sequence of the temperate bacteriophage ΦAT3 isolated from Lactobacillus casei ATCC 393. Virology 2005, 339, 42–55. [Google Scholar] [CrossRef]
- Kutter, E.; Sulakvelidze, A. Bacteriophages Biology and Applications, 1st ed.; CRC Press: Boca Raton, FL, USA, 2004. [Google Scholar]
- García, P.; Ladero, V.; Suárez, J.E. Analysis of the morphogenetic cluster and genome of the temperate Lactobacillus casei bacteriophage A2. Arch. Virol. 2003, 148, 1051–1070. [Google Scholar] [CrossRef]
- Dieterle, M.E.; Piuri, M.; Bowman, C.; Hatfull, G.; Batthyany, C.; Turjanski, A.; Lanzarotti, E. Exposing the secrets of two well-known Lactobacillus casei phages, J-1 and PL-1, by genomic and structural analysis. Appl. Environ. Microbiol. 2014, 80, 7107–7121. [Google Scholar] [CrossRef] [Green Version]
- Kelly, G.; Prasannan, S.; Daniell, S.; Fleming, K.; Frankel, G.; Dougan, G.; Connerton, I.; Matthews, S. Structure of the cell-adhesion fragment of intimin from enteropathogenic Escherichia coli. Nat. Struct. Biol. 1999, 6, 313–318. [Google Scholar]
- Hatfull, G.F. Dark matter of the biosphere: the amazing world of bacteriophage diversity. J. Virol. 2015, 89, 8107–8110. [Google Scholar] [CrossRef] [Green Version]
- Grose, J.H.; Casjens, S.R. Bacteriophage diversity. In Reference Module in Life Sciences; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar]
- Gründling, A.; Manson, M.D.; Young, R. Holins kill without warning. Proc. Natl. Acad. Sci. USA 2001, 98, 9348–9352. [Google Scholar]
- Kashige, N.; Nakashima, Y.; Miake, F.; Watanabe, K. Cloning, sequence analysis, and expression of Lactobacillus casei phage PL-1 lysis genes. Arch. Virol. 2000, 145, 1521–1534. [Google Scholar] [CrossRef] [PubMed]
- Dieterle, M.E.; Fina Martin, J.; Durán, R.; Nemirovsky, S.I.; Sanchez Rivas, C.; Bowman, C.; Russell, D.; Hatfull, G.F.; Cambillau, C.; Piuri, M. Characterization of prophages containing “evolved” Dit/Tal modules in the genome of Lactobacillus casei BL23. Appl. Microbiol. Biotechnol. 2016, 100, 9201–9215. [Google Scholar] [CrossRef] [PubMed]
- Groth, A.C.; Calos, M.P. Phage integrases: biology and applications. J. Mol. Biol. 2004, 335, 667–678. [Google Scholar] [CrossRef] [PubMed]
- Williams, R.J. Restriction endonucleases: Classification, properties, and applications. Appl. Biochem. Biotechnol. Part B Mol. Biotechnol. 2003, 23, 225–243. [Google Scholar] [CrossRef]
- Obarska-Kosinska, A.; Taylor, J.E.; Callow, P.; Orlowski, J.; Bujnicki, J.M.; Kneale, G.G. HsdR subunit of the type I restriction-modification enzyme EcoR124I: biophysical characterisation and structural modelling. J. Mol. Biol. 2008, 376, 438–452. [Google Scholar] [CrossRef]
- Erill, I.; Campoy, S.; Barbé, J. Aeons of distress: An evolutionary perspective on the bacterial SOS response. FEMS Microbiol. Rev. 2007, 31, 637–656. [Google Scholar] [CrossRef] [Green Version]
- Fornelos, N.; Browning, D.F.; Pavlin, A.; Podlesek, Z.; Hodnik, V.; Salas, M.; Butala, M. Lytic gene expression in the temperate bacteriophage GIL01 is activated by a phage-encoded LexA homologue. Nucleic Acids Res. 2018, 46, 9432–9443. [Google Scholar] [CrossRef]
- Nanda, A.M.; Heyer, A.; Krämer, C.; Grünberger, A.; Kohlheyer, D.; Frunzke, J. Analysis of SOS-induced spontaneous prophage induction in Corynebacterium glutamicum at the single-cell level. J. Bacteriol. 2014, 196, 180–188. [Google Scholar] [CrossRef] [Green Version]
- Ndjonka, D.; Bell, C.E. Structure of a hyper-cleavable monomeric fragment of phage λ repressor containing the cleavage site region. J. Mol. Biol. 2006, 362, 479–489. [Google Scholar] [CrossRef] [Green Version]
- Foley, S.; Lucchini, S.; Zwahlen, M.C.; Brüssow, H. A short noncoding viral DNA element showing characteristics of a replication origin confers bacteriophage resistance to Streptococcus thermophilus. Virology 1998, 250, 377–387. [Google Scholar] [CrossRef] [Green Version]
- Gvakharia, B.O.; Hanson, E.; Koonin, E.K.; Mathews, C.K. Identification of a second functional glutaredoxin encoded by the bacteriophage T4 genome. J. Biol. Chem. 1996, 271, 15307–15310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lunde, M.; Blatny, J.M.; Lillehaug, D.; Aastveit, A.H.; Nes, I.F. Use of real-time quantitative PCR for the analysis of φLC3 prophage stability in lactococci. Appl. Environ. Microbiol. 2003, 69, 41–48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, H.; Cai, Q.; Li, H.; Hu, P. Comparison of droplet digital PCR to real-time PCR for quantification of hepatitis B virus DNA. Biosci. Biotechnol. Biochem. 2016, 80, 2159–2164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maheshwari, Y.; Selvaraj, V.; Hajeri, S.; Yokomi, R. Application of droplet digital PCR for quantitative detection of Spiroplasma citri in comparison with real time PCR. PLoS One 2017, 12, e0184751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alexeeva, S.; Guerra Martínez, J.A.; Spus, M.; Smid, E.J. Spontaneously induced prophages are abundant in a naturally evolved bacterial starter culture and deliver competitive advantage to the host. BMC Microbiol. 2018, 18, 16. [Google Scholar] [CrossRef] [PubMed]
- Ho, C.H.; Stanton-Cook, M.; Beatson, S.A.; Bansal, N.; Turner, M.S. Stability of active prophages in industrial Lactococcus lactis strains in the presence of heat, acid, osmotic, oxidative and antibiotic stressors. Int. J. Food Microbiol. 2016, 220, 26–32. [Google Scholar] [CrossRef] [Green Version]
- Mahony, J.; Lugli, G.A.; van Sinderen, D.; Ventura, M. Impact of gut-associated bifidobacteria and their phages on health: Two sides of the same coin? Appl. Microbiol. Biotechnol. 2018, 102, 2091–2099. [Google Scholar] [CrossRef]
- Baugher, J.L.; Durmaz, E.; Klaenhammer, T.R. Spontaneously induced prophages in Lactobacillus gasseri contribute to horizontal gene transfer. Appl. Environ. Microbiol. 2014, 80, 3508–3517. [Google Scholar] [CrossRef] [Green Version]
- Díaz-Muñoz, S.L.; Koskella, B. Bacteria-phage interactions in natural environments. Adv. Appl. Microbiol. 2014, 89, 135–183. [Google Scholar]
- Lunde, M.; Aastveit, A.H.; Blatny, J.M.; Nes, I.F. Effects of diverse environmental conditions on φLC3 prophage stability in Lactococcus lactis. Appl. Environ. Microbiol. 2005, 71, 721–727. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; McAteer, S.P.; Tree, J.J.; Shaw, D.J.; Wolfson, E.B.K.; Beatson, S.A.; Roe, A.J.; Allison, L.J.; Chase-Topping, M.E.; Mahajan, A.; et al. Lysogeny with Shiga toxin 2-encoding bacteriophages represses type III secretion in enterohemorrhagic Escherichia coli. PLoS Pathog. 2012, 8, e1002672. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parsek, M.R.; Singh, P.K. Bacterial biofilms: an emerging link to disease pathogenesis. Annu. Rev. Microbiol. 2003, 57, 677–701. [Google Scholar] [CrossRef] [PubMed]
- Seo, H.S.; Xiong, Y.Q.; Mitchell, J.; Seepersaud, R.; Bayer, A.S.; Sullam, P.M. Bacteriophage lysin mediates the binding of Streptococcus mitis to human platelets through interaction with fibrinogen. PLoS Pathog. 2010, 6, e1001047. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J.; Siboo, I.R.; Takamatsu, D.; Chambers, H.F.; Sullam, P.M. Mechanism of cell surface expression of the Streptococcus mitis platelet binding proteins PblA and PblB. Mol. Microbiol. 2007, 64, 844–857. [Google Scholar] [CrossRef] [PubMed]
- Presterl, E.; Kneifel, W.; Mayer, H.K.; Zehetgruber, M.; Makristathis, A.; Graninger, W. Endocarditis by Lactobacillus rhamnosus due to yogurt ingestion? Scand. J. Infect. Dis. 2001, 33, 710–714. [Google Scholar] [PubMed]
- Boumis, E.; Capone, A.; Galati, V.; Venditti, C.; Petrosillo, N. Probiotics and infective endocarditis in patients with hereditary hemorrhagic telangiectasia: a clinical case and a review of the literature. BMC Infect. Dis. 2018, 18, 65. [Google Scholar] [CrossRef]
- Aaron, J.G.; Sobieszczyk, M.E.; Weiner, S.D.; Whittier, S.; Lowy, F.D. Lactobacillus rhamnosus endocarditis after upper endoscopy. Open Forum Infect. Dis. 2017, 4, ofx085. [Google Scholar] [CrossRef] [Green Version]
- Kochan, P.; Chmielarczyk, A.; Szymaniak, L.; Brykczynski, M.; Galant, K.; Zych, A.; Pakosz, K.; Giedrys-Kalemba, S.; Lenouvel, E.; Heczko, P.B. Lactobacillus rhamnosus administration causes sepsis in a cardiosurgical patient-is the time right to revise probiotic safety guidelines? Clin. Microbiol. Infect. 2011, 17, 1589–1592. [Google Scholar] [CrossRef] [Green Version]
- Naqvi, S.S.B.; Nagendra, V.; Hofmeyr, A. Probiotic related Lactobacillus rhamnosus endocarditis in a patient with liver cirrhosis. IDCases 2018, 13, e00439. [Google Scholar] [CrossRef]

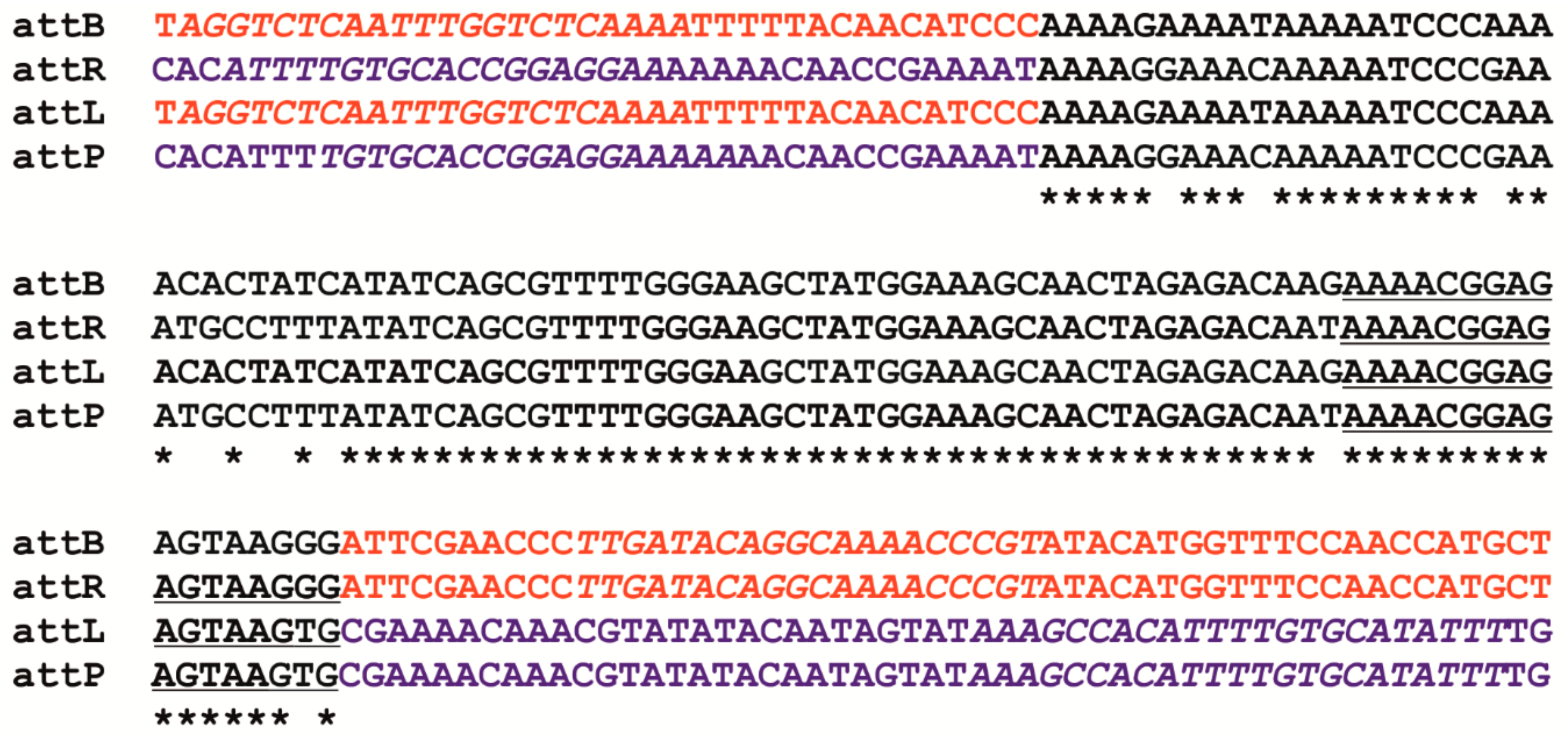



| Primer Set | Amplified Fragment | Primer Sequences (5′–3′) | Position | GenBank Accession Number |
|---|---|---|---|---|
| 1 | attB | AGGTCTCAATTTGGTCTCAAAA | 2237443–2237464 | CP020464.1 |
| ACGGGTTTTGCCTGTATCAA | 2278301–2278320 | CP020464.1 | ||
| 2 | attR | ATTTTGTGCACCGGAGGAA | 21564–21582 2278166–2278184 | MH983004.1 CP020464.1 |
| ACGGGTTTTGCCTGTATCAA | 2278301–2278320 | CP020464.1 | ||
| 3 | attL | AGGTCTCAATTTGGTCTCAAAA | 2237443–2237464 | CP020464.1 |
| AAATATGCACAAAATGTGGCTTT | 21408–21430 2237597–2237619 | MH983004.1 CP020464.1 | ||
| 4 | attP | TGTGCACCGGAGGAAAAA | 21561–21578 2278170–2278187 | MH983004.1 CP020464.1 |
| AAATATGCACAAAATGTGGCTTT | 21408–21430 2237597–2237619 | MH983004.1 CP020464.1 | ||
| 5 | Lrh | TTGACAAGGGACTCAAGGAT | 2208780–2208799 | CP020464.1 |
| TATGATAGCCGGAATCAGCA | 2208910–2208929 | CP020464.1 |
| Protein Description | Mascot Score a | No. of Unique Peptides Matched b | Protein Coverage [% aa] | Protein Molecular Mass | Orf |
|---|---|---|---|---|---|
| phage tail tape measure protein [Lactobacillus rhamnosus] | 1978 | 28 | 19.4 | 173159 | 14 |
| phage tail protein [Lactobacillus rhamnosus] | 2851 | 35 | 37.4 | 107736 | 16 |
| phage tail protein [Lactobacillus rhamnosus] | 1375 | 18 | 32.4 | 71309 | 15 |
| phage portal protein [Lactobacillus rhamnosus] | 1214 | 16 | 47.7 | 46346 | 4 |
| major capsid protein [Lactobacillus rhamnosus] | 2371 | 25 | 78 | 43998 | 6 |
| hypothetical protein [Lactobacillus rhamnosus] | 192 | 2 | 49.4 | 7776 | 7 |
| phage tail protein [Lactobacillus rhamnosus] | 1345 | 16 | 75.1 | 22056 | 12 |
| phage tail protein [Lactobacillus rhamnosus] | 755 | 11 | 95.3 | 14653 | 11 |
| phage head-tail adapter protein [Lactobacillus rhamnosus] | 388 | 7 | 58.7 | 12598 | 9 |
| DNA-packaging protein [Lactobacillus rhamnosus] | 439 | 7 | 47.9 | 13581 | 8 |
| hypothetical protein [Lactobacillus rhamnosus] | 1239 | 17 | 72.7 | 17915 | 36 |
| phage holin [Lactobacillus rhamnosus] | 120 | 2 | 6.6 | 14373 | 20 |
| peptidase U35 [Lactobacillus rhamnosus] | 37 | 1 | 5.7 | 23706 | 5 |
| hypothetical protein [Lactobacillus rhamnosus] | 234 | 4 | 31.2 | 14024 | 10 |
| transcriptional regulator [Lactobacillus rhamnosus] | 49 | 1 | 8.4 | 9473 | 29 |
| hypothetical protein [Lactobacillus rhamnosus] | 43 | 1 | 21.7 | 6948 | 46 |
| hypothetical protein [Lactobacillus rhamnosus] | 252 | 4 | 36.4 | 7358 | 31 |
| Orf Order/Strand | Predicted Start Stop Site | GC (%) | Orf Length (aa) | Predicted Molecular Mass (kDa) | pI | Description | Representative orf (% Coverage/% nt Identity) | Biological Process/Molecular Function a | |
|---|---|---|---|---|---|---|---|---|---|
| Lrm1 NC_011104.1 | Lc-Nu NC_007501.1 | ||||||||
| 1F | 90–545 | 41.01 | 151 | 17.06 | 8.85 | Terminase, small subunit | 100/99.34 | 0/0 | - |
| 2F | 567–2279 | 43.96 | 570 | 65.10 | 5.15 | Terminase, large subunit | 100/93.35 | 0/0 | - |
| 3F | 2291–2482 | 42.19 | 63 | 6.66 | 9.31 | Hypothetical protein | 100/98.44 | 0/0 | - |
| 4F | 2488–3741 | 46.65 | 417 | 46.32 | 5.07 | Portal protein | 100/81.64 | 0/0 | - |
| 5F | 3695–4324 | 47.62 | 209 | 23.72 | 5.45 | Prohead protease | 100/87.78 | 0/0 | - |
| 6F | 4366–5568 | 46.22 | 400 | 44.03 | 5.42 | Major capsid protein | 98.92/ 84.37 | 0/0 | - |
| 7F | 5586–5825 | 50.83 | 79 | 77.63 | 4.53 | Hypothetical protein | 0/0 | 0/0 | GO:0030246 |
| 8F | 5836–6195 | 41.94 | 119 | 13.52 | 4.24 | Putative DNA-packaging protein | 46.67/91.67 | 0/0 | - |
| 9F | 6185–6514 | 47.58 | 109 | 12.61 | 9.57 | Head-tail adaptor | 100/98.48 | 0/0 | - |
| 10F | 6514–6900 | 47.80 | 128 | 14.03 | 5.53 | Putative tail component | 100/96.12 | 0/0 | - |
| 11F | 6900–7286 | 45.22 | 128 | 14.66 | 4.39 | Putative head-to-tail joining protein | 100/95.61 | 0/0 | - |
| 12F | 7320–7937 | 44.82 | 205 | 22.07 | 4.56 | Major tail protein | 96.12/95.79 | 0/0 | - |
| 13F | 8036–8449 | 42.51 | 137 | 15.24 | 6.30 | Hypothetical protein | 100/98.31 | 0/0 | - |
| 14F | 8572–13434 | 45.86 | 1620 | 173.27 | 9.42 | Tail tape measure protein | 97.27/95.48 | 0/0 | - |
| 15F | 13435–15351 | 46.69 | 638 | 71.17 | 5.21 | Tail component protein | 47.99/89.67 | 59.36/82.86 | - |
| 16F | 15352–18306 | 45.69 | 984 | 107.75 | 4.78 | Prophage tail endopeptidase | 36.75/82.60 | 55.40/79.54 | - |
| 17F | 18322–18651 | 45.76 | 109 | 11.90 | 4.38 | Hypothetical protein | 0/0 | 100/99.70 | - |
| 18F | 18648–18791 | 42.36 | 47 | 5.29 | 4.86 | Uncharacterized protein, XkdX family | 0/0 | 100/96.53 | - |
| 19F | 18823–19122 | 45.33 | 99 | 11.30 | 6.63 | Hypothetical protein | 0/0 | 0/0 | - |
| 20F | 19137–19550 | 49.76 | 137 | 14.38 | 4.82 | Holin | 0/0 | 0/0 | - |
| 21F | 19561–20859 | 48.11 | 432 | 47.06 | 8.98 | Glycoside hydrolase, lysin | 100/90.87 | 100/92.33 | GO:0009253 GO:0016998 GO:0003796 |
| 22F | 20904–21128 | 45.78 | 74 | 8.18 | 4.97 | Hypothetical protein | 0/0 | 0/0 | - |
| 23R | 21592–22719 | 42.55 | 375 | 43.28 | 9.71 | Site-specific integrase | 100/98.49 | 0/0 | GO:0006310 GO:0015074 GO:0003677 |
| 24R | 22827–23090 | 35.23 | 87 | 10.24 | 9.80 | Hypothetical protein | 0/0 | 0/0 | - |
| 25F | 23229–23450 | 42.34 | 73 | 8.13 | 4.32 | Hypothetical protein | 0/0 | 0/0 | - |
| 26R | 23560–24741 | 40.78 | 393 | 44.33 | 9.00 | Restriction endonuclease, type I, HsdS | 0/0 | 0/0 | GO:0006304 GO:0003677 |
| 27R | 24832–25050 | 47.03 | 72 | 8.07 | 6.53 | LexA-like peptidase | 0/0 | 0/0 | - |
| 28R | 25122–25895 | 42.77 | 257 | 28.97 | 4.61 | Cro/CI-type transcriptional repressor | 0/0 | 0/0 | GO:0003677 |
| 29F | 26053–26304 | 39.29 | 83 | 9.48 | 10.2 | XRE family transcriptional regulator | 0/0 | 0/0 | GO:0003677 |
| 30F | 26301–26450 | 44.00 | 49 | 5.45 | 6.12 | Hypothetical protein | 0/0 | 0/0 | - |
| 31R | 26447–26647 | 37.81 | 66 | 7.30 | 5.33 | Hypothetical protein | 0/0 | 0/0 | - |
| 32F | 26722–27078 | 42.58 | 118 | 13.92 | 9.15 | DUF771 domain-containing protein | 100/100 | 0/0 | - |
| 33F | 27078–27170 | 47.31 | 30 | 3.36 | 5.96 | Hypothetical protein | 100/97.85 | 0/0 | - |
| 34F | 27163–27315 | 45.10 | 50 | 5.69 | 9.52 | Hypothetical protein | 100/95.42 | 98.69/96.69 | - |
| 35F | 27320–27523 | 45.59 | 67 | 7.51 | 6.00 | Hypothetical protein | 100/86.27 | 100/90.20 | - |
| 36F | 27542–28027 | 46.30 | 161 | 17.92 | 8.73 | Siphovirus-like Gp157 protein | 100/96.91 | 0/0 | - |
| 37F | 28028–28735 | 45.34 | 235 | 26.57 | 6.77 | Nucleotide-binding protein | 100/94.92 | 100/92.95 | - |
| 38F | 28739–29296 | 43.37 | 185 | 20.92 | 5.50 | DUF669 domain-containing protein | 100/95.17 | 97.13/96.13 | - |
| 39F | 29311–30108 | 43.86 | 265 | 31.13 | 9.36 | Putative replication protein | 41.73/85.29 | 0/0 | - |
| 40F | 30095–30877 | 46.87 | 260 | 29.73 | 9.68 | IstB-like ATP binding protein | 100/86.75 | 0/0 | GO:0005524 |
| 41F | 30874–31218 | 43.77 | 114 | 13.09 | 4.51 | Hypothetical protein | 94.49/ 93.56 | 0/0 | - |
| 42F | 31205–31459 | 50.20 | 84 | 9.42 | 9.75 | Hypothetical protein | 0/0 | 100/90.59 | - |
| 43F | 31456–31821 | 43.71 | 121 | 14.39 | 6.92 | Holliday junction resolvase RusA-like | 0/0 | 0/0 | GO:0006281 GO:0006310 GO:0000287 |
| 44F | 31832–32170 | 46.02 | 112 | 12.44 | 5.38 | Putative endonuclease | 0/0 | 0/0 | - |
| 45F | 32182–32883 | 45.44 | 233 | 27.11 | 5.01 | SAM-dependent DNA methyltransferase | 0/0 | 96.15/93.63 | GO:0006306 GO:0003677 GO:0008170 |
| 46F | 32880–33062 | 41.53 | 60 | 6.89 | 4.55 | Hypothetical protein | 55.19/94.06 | 100/94.54 | - |
| 47F | 33059–33601 | 49.35 | 180 | 20.64 | 4.85 | DUF1642 domain-containing protein | 8.47/93.48 | 0/0 | - |
| 48F | 33757–34137 | 45.41 | 126 | 14.21 | 4.40 | Hypothetical protein | 10.24/97.44 | 0/0 | - |
| 49F | 34134–34343 | 40.95 | 69 | 8.34 | 6.73 | Hypothetical protein | 0/0 | 100/ 94.79 | - |
| 50F | 34471–34692 | 42.34 | 73 | 8.42 | 9.52 | HTH-transcriptional regulator | 0/0 | 0/0 | GO:0003677 |
| 51F | 34762–35205 | 47.97 | 147 | 16.85 | 7.88 | Transcriptional regulator, ArpU family | 55.86/ 97.98 | 55.63/97.57 | - |
| 52F | 35599–36672 | 35.85 | 357 | 40.65 | 6.33 | Hypothetical protein | 0/0 | 0/0 | - |
| 53R | 36834–37133 | 42.00 | 99 | 10.81 | 9.37 | Stress response protein CsbD | 0/0 | 0/0 | - |
| 54F | 37552–38769 | 45.07 | 405 | 46.64 | 6.07 | GcrA cell cycle regulator | 0/0 | 100/95.81 | - |
| 55F | 38717–39286 | 44.91 | 189 | 21.44 | 9.28 | HNH endonuclease | 0/0 | 22.81/84.62 | GO:0016788 |
| 56F | 39290–39613 | 46.91 | 107 | 12.69 | 9.01 | Ribonucleoside-diphosphate reductase | 81.48/78.79 | 80.56/89.27 | - |
| 57F | 39816–40610 | 45.28 | 264 | 31.14 | 9.05 | Small terminase subunit/HNH endonuclease | 100/97.99 | 0/0 | GO:0003676 GO:0004519 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jarocki, P.; Komoń-Janczara, E.; Podleśny, M.; Kholiavskyi, O.; Pytka, M.; Kordowska-Wiater, M. Genomic and Proteomic Characterization of Bacteriophage BH1 Spontaneously Released from Probiotic Lactobacillus rhamnosus Pen. Viruses 2019, 11, 1163. https://doi.org/10.3390/v11121163
Jarocki P, Komoń-Janczara E, Podleśny M, Kholiavskyi O, Pytka M, Kordowska-Wiater M. Genomic and Proteomic Characterization of Bacteriophage BH1 Spontaneously Released from Probiotic Lactobacillus rhamnosus Pen. Viruses. 2019; 11(12):1163. https://doi.org/10.3390/v11121163
Chicago/Turabian StyleJarocki, Piotr, Elwira Komoń-Janczara, Marcin Podleśny, Oleksandr Kholiavskyi, Monika Pytka, and Monika Kordowska-Wiater. 2019. "Genomic and Proteomic Characterization of Bacteriophage BH1 Spontaneously Released from Probiotic Lactobacillus rhamnosus Pen" Viruses 11, no. 12: 1163. https://doi.org/10.3390/v11121163
APA StyleJarocki, P., Komoń-Janczara, E., Podleśny, M., Kholiavskyi, O., Pytka, M., & Kordowska-Wiater, M. (2019). Genomic and Proteomic Characterization of Bacteriophage BH1 Spontaneously Released from Probiotic Lactobacillus rhamnosus Pen. Viruses, 11(12), 1163. https://doi.org/10.3390/v11121163





