Chewing the Fat: The Conserved Ability of DNA Viruses to Hijack Cellular Lipid Metabolism
Abstract
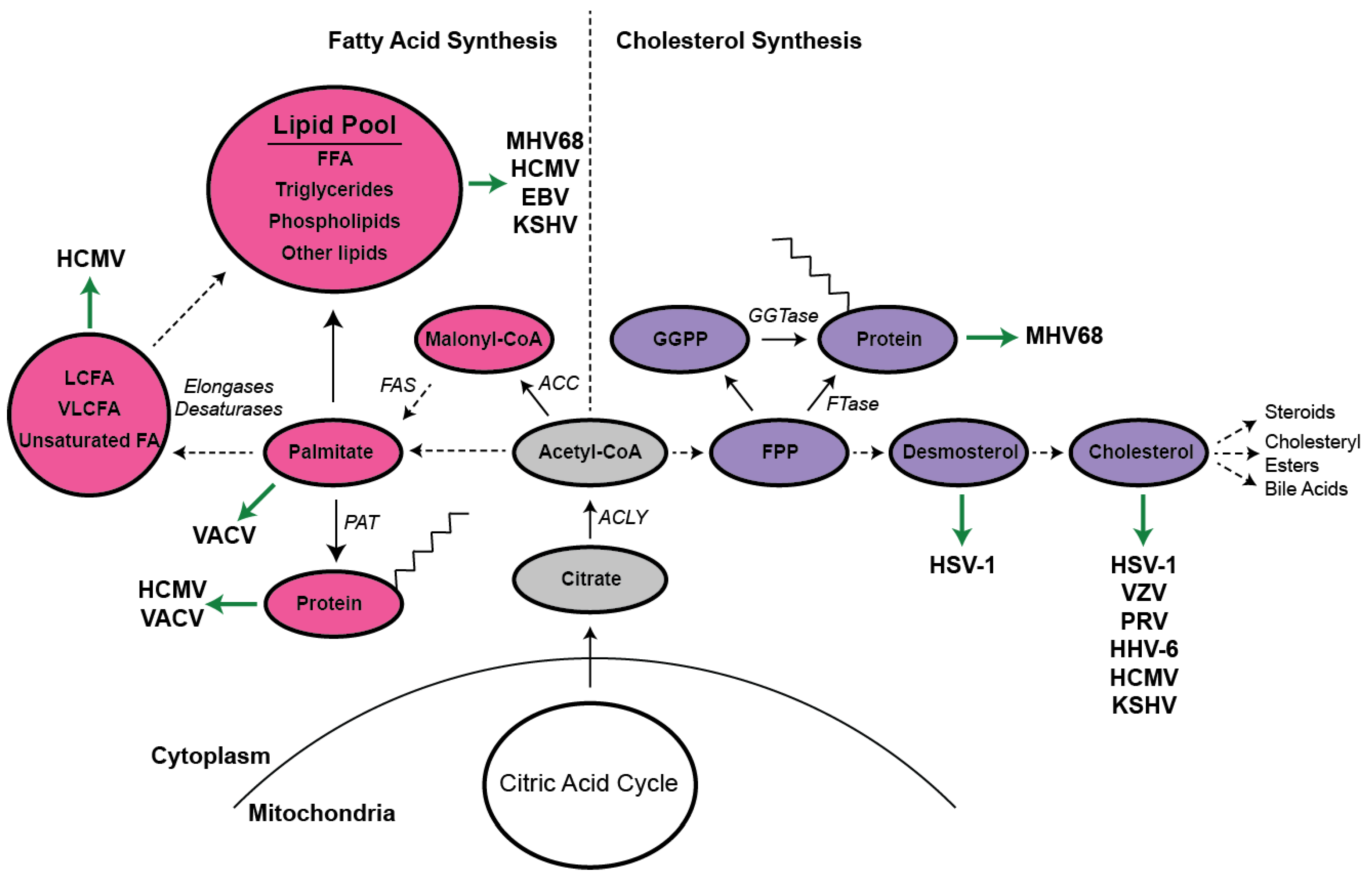
:1. Overview of Lipid Metabolism
2. Transcriptional Regulation of Lipid Metabolism
3. Enzymatic Regulation of Lipid Metabolism
4. Herpesviruses
4.1. Alphaherpesviruses
4.2. Betaherpesviruses
4.3. Gammaherpesviruses
4.4. Murine Herpesvirus 68
4.5. Other DNA Viruses
5. 25-Hydroxycholesterol
6. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- DeBose-Boyd, R.A.; Ye, J. SREBPs in lipid metabolism, insulin signaling, and beyond. Trends Biochem. Sci. 2018, 43, 358–368. [Google Scholar] [CrossRef]
- Horton, J.D.; Goldstein, J.L.; Brown, M.S. SREBPs: Activators of the complete program of cholesterol and fatty acid synthesis in the liver. J. Clin. Investig. 2002, 109, 1125–1131. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.D.; Tontonoz, P. Liver X receptors at the intersection of lipid metabolism and atherogenesis. Atherosclerosis 2015, 242, 29–36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Apfel, R.; Benbrook, D.; Lernhardt, E.; Ortiz, M.A.; Salbert, G.; Pfahl, M. A novel orphan receptor specific for a subset of thyroid hormone-responsive elements and its interaction with the retinoid/thyroid hormone receptor subfamily. Mol. Cell. Biol. 1994, 14, 7025–7035. [Google Scholar] [CrossRef] [PubMed]
- Willy, P.J.; Umesono, K.; Ong, E.S.; Evans, R.M.; Heyman, R.A.; Mangelsdorf, D.J. LXR, a nuclear receptor that defines a distinct retinoid response pathway. Genes Dev. 1995, 9, 1033–1045. [Google Scholar] [CrossRef]
- Auboeuf, D.; Rieusset, J.; Fajas, L.; Vallier, P.; Frering, V.; Riou, J.P.; Staels, B.; Auwerx, J.; Laville, M.; Vidal, H. Tissue distribution and quantification of the expression of mRNAs of peroxisome proliferator–activated receptors and liver X receptor-α in humans: No alteration in adipose tissue of obese and NIDDM patients. Diabetes 1997, 46, 1319–1327. [Google Scholar] [CrossRef] [PubMed]
- Kalaany, N.Y.; Mangelsdorf, D.J. LXRS and FXR: The yin and yang of cholesterol and fat metabolism. Annu. Rev. Physiol. 2006, 68, 159–191. [Google Scholar] [CrossRef]
- Costet, P.; Luo, Y.; Wang, N.; Tall, A.R. Sterol-dependent transactivation of theABC1 promoter by the liver X receptor/retinoid X receptor. J. Biol. Chem. 2000, 275, 28240–28245. [Google Scholar]
- Repa, J.J.; Liang, G.; Ou, J.; Bashmakov, Y.; Lobaccaro, J.M.; Shimomura, I.; Shan, B.; Brown, M.S.; Goldstein, J.L.; Mangelsdorf, D.J. Regulation of mouse sterol regulatory element-binding protein-1c gene (SREBP-1c) by oxysterol receptors, LXRalpha and LXRbeta. Genes Dev 2000, 14, 2819–2830. [Google Scholar] [CrossRef] [Green Version]
- Quehenberger, O.; Armando, A.M.; Brown, A.H.; Milne, S.B.; Myers, D.S.; Merrill, A.H.; Bandyopadhyay, S.; Jones, K.N.; Kelly, S.; Shaner, R.L.; et al. Lipidomics reveals a remarkable diversity of lipids in human plasma. J. Lipid Res. 2010, 51, 3299–3305. [Google Scholar] [CrossRef] [Green Version]
- Parihar, S.P.; Guler, R.; Brombacher, F. Statins: A viable candidate for host-directed therapy against infectious diseases. Nat. Rev. Immunol. 2018. [Google Scholar] [CrossRef]
- Dhar, M.K.; Koul, A.; Kaul, S. Farnesyl pyrophosphate synthase: A key enzyme in isoprenoid biosynthetic pathway and potential molecular target for drug development. New Biotechnol. 2013, 30, 114–123. [Google Scholar] [CrossRef]
- Wang, M.; Casey, P.J. Protein prenylation: Unique fats make their mark on biology. Nat. Rev. Mol. Cell Biol. 2016, 17, 110–122. [Google Scholar] [CrossRef]
- Sinclair, J.; Sissons, P. Latency and reactivation of human cytomegalovirus. J. Gen. Virol. 2006, 87, 1763–1779. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsurumi, T.; Fujita, M.; Kudoh, A. Latent and lytic Epstein-Barr virus replication strategies. Rev. Med. Virol. 2005, 15, 3–15. [Google Scholar] [CrossRef] [PubMed]
- Pantry, S.N.; Medveczky, P.G. Latency, integration, and reactivation of human herpesvirus-6. Viruses 2017, 9, 194. [Google Scholar] [CrossRef] [PubMed]
- Goodrum, F. Human cytomegalovirus latency: Approaching the Gordian knot. Annu. Rev. Virol. 2016, 3, 333–357. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, P.G.; Rovnak, J.; Badani, H.; Cohrs, R.J. A comparison of herpes simplex virus type 1 and varicella-zoster virus latency and reactivation. J. Gen. Virol. 2015, 96, 1581–1602. [Google Scholar] [CrossRef]
- Fields, B.; Knipe, D.; Howley, P. Fields Virology, 6th ed.; Knipepeter, D.M., Howley, M., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013. [Google Scholar]
- Jerkofsky, M.; De Siervo, A.J. Differentiation of strains of varicella-zoster virus by changes in neutral lipid metabolism in infected cells. J. Virol. 1986, 57, 809–815. [Google Scholar]
- Langeland, N.; Haarr, L.; Holmsen, H. Polyphosphoinositide metabolism in baby-hamster kidney cells infected with herpes simplex virus type 1. Biochem. J. 1986, 237, 707–712. [Google Scholar] [CrossRef] [Green Version]
- Langeland, N.; Moore, L.; Holmsen, H.; Haarr, L. Herpes simplex virus-1-specific proteins are involved in alteration of polyphosphoinositide metabolism in baby-hamster kidney cells. Biochem. J. 1989, 261, 683–686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bender, F.C.; Whitbeck, J.C.; de Leon, M.P.; Lou, H.; Eisenberg, R.J.; Cohen, G.H. Specific association of glycoprotein B with lipid rafts during herpes simplex virus entry. J. Virol. 2003, 77, 9542–9552. [Google Scholar] [CrossRef] [PubMed]
- Wudiri, G.A.; Pritchard, S.M.; Li, H.; Liu, J.; Aguilar, H.C.; Gilk, S.D.; Nicola, A.V. Molecular requirement for sterols in herpes simplex virus entry and infectivity. J. Virol. 2014, 88, 13918–13922. [Google Scholar] [CrossRef] [PubMed]
- Hambleton, S.; Steinberg, S.; Gershon, M.; Gershon, A. Cholesterol dependence of varicella-zoster virion entry into target cells. J. Virol. 2007, 81, 7548–7558. [Google Scholar] [CrossRef]
- Desplanques, A.S.; Nauwynck, H.J.; Vercauteren, D.; Geens, T.; Favoreel, H.W. Plasma membrane cholesterol is required for efficient pseudorabies virus entry. Virology 2008, 376, 339–345. [Google Scholar] [CrossRef] [Green Version]
- Gianni, T.; Gatta, V.; Campadelli-Fiume, G. αVβ3-integrin routes herpes simplex virus to an entry pathway dependent on cholesterol-rich lipid rafts and dynamin2. Proc. Natl. Acad. Sci. USA 2010, 107, 22260–22265. [Google Scholar] [CrossRef]
- Wudiri, G.A.; Nicola, A.V. Cellular cholesterol facilitates the post-entry replication cycle of herpes simplex virus 1. J. Virol. 2017. [Google Scholar] [CrossRef]
- Wudiri, G.A.; Schneider, S.M.; Nicola, A.V. Herpes Simplex Virus 1 Envelope Cholesterol Facilitates Membrane Fusion. Front. Microbiol. 2017, 8, 2383. [Google Scholar] [CrossRef] [PubMed]
- Asher, Y.; Heller, M.; Becker, Y. Incorporation of lipids into herpes simplex virus particles. J. Gen. Virol. 1969, 4, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Sutter, E.; de Oliveira, A.P.; Tobler, K.; Schraner, E.M.; Sonda, S.; Kaech, A.; Lucas, M.S.; Ackermann, M.; Wild, P. Herpes simplex virus 1 induces de novo phospholipid synthesis. Virology 2012, 429, 124–135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wild, P.; de Oliveira, A.P.; Sonda, S.; Schraner, E.M.; Ackermann, M.; Tobler, K. The herpes simplex virus 1 Us3 regulates phospholipid synthesis. Virology 2012, 432, 353–360. [Google Scholar] [CrossRef] [PubMed]
- Manicklal, S.; Emery, V.C.; Lazzarotto, T.; Boppana, S.B.; Gupta, R.K. The “silent” global burden of congenital cytomegalovirus. Clin. Microbiol. Rev. 2013, 26, 86–102. [Google Scholar] [CrossRef]
- Bego, M.G.; Jeor, S.S. Human cytomegalovirus infection of cells of hematopoietic origin: HCMV-induced immunosuppression, immune evasion, and latency. Exp. Hematol. 2006, 34, 555–570. [Google Scholar] [CrossRef] [PubMed]
- Söderberg-Nauclér, C.; Streblow, D.N.; Fish, K.N.; Allan-Yorke, J.; Smith, P.P.; Nelson, J.A. Reactivation of latent human cytomegalovirus in CD14+ monocytes is differentiation dependent. J. Virol. 2001, 75, 7543–7554. [Google Scholar] [CrossRef] [PubMed]
- Haspot, F.; Lavault, A.; Sinzger, C.; Sampaio, K.L.; Stierhof, Y.-D.; Pilet, P.; Bressolette-Bodin, C.; Halary, F. Human cytomegalovirus entry into dendritic cells occurs via a macropinocytosis-like pathway in a pH-independent and cholesterol-dependent manner. PLoS ONE 2012, 7, e34795. [Google Scholar] [CrossRef]
- Ryckman, B.J.; Jarvis, M.A.; Drummond, D.D.; Nelson, J.A.; Johnson, D.C. Human cytomegalovirus entry into epithelial and endothelial cells depends on genes UL128 to UL150 and occurs by endocytosis and low-pH fusion. J. Virol. 2006, 80, 710–722. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Li, Y.; Sadaoka, T.; Tang, H.; Yamamoto, T.; Yamanishi, K.; Mori, Y. Human herpesvirus 6 envelope cholesterol is required for virus entry. J. Gen. Virol. 2006, 87, 277–285. [Google Scholar] [CrossRef] [Green Version]
- Tang, H.; Kawabata, A.; Takemoto, M.; Yamanishi, K.; Mori, Y. Human herpesvirus-6 infection induces the reorganization of membrane microdomains in target cells, which are required for virus entry. Virology 2008, 378, 265–271. [Google Scholar] [CrossRef] [Green Version]
- Kawabata, A.; Tang, H.; Huang, H.; Yamanishi, K.; Mori, Y. Human herpesvirus 6 envelope components enriched in lipid rafts: Evidence for virion-associated lipid rafts. Virol. J. 2009, 6, 127. [Google Scholar] [CrossRef]
- Potena, L.; Frascaroli, G.; Grigioni, F.; Lazzarotto, T.; Magnani, G.; Tomasi, L.; Coccolo, F.; Gabrielli, L.; Magelli, C.; Landini, M.P. Hydroxymethyl-glutaryl coenzyme a reductase inhibition limits cytomegalovirus infection in human endothelial cells. Circulation 2004, 109, 532–536. [Google Scholar] [CrossRef]
- Gudleski-O’Regan, N.; Greco, T.M.; Cristea, I.M.; Shenk, T. Increased expression of LDL receptor-related protein 1 during human cytomegalovirus infection reduces virion cholesterol and infectivity. Cell Host Microbe 2012, 12, 86–96. [Google Scholar] [CrossRef]
- Patrone, M.; Coroadinha, A.S.; Teixeira, A.P.; Alves, P.M. Palmitoylation strengthens cholesterol-dependent multimerization and fusion activity of human cytomegalovirus glycoprotein B (gB). J. Biol. Chem. 2016, 291, 4711–4722. [Google Scholar] [CrossRef]
- Landini, M. Early enhanced glucose uptake in human cytomegalovirus-infected cells. J. Gen. Virol. 1984, 65, 1229–1232. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Maguire, T.G.; Alwine, J.C. Human cytomegalovirus infection induces adipocyte-like lipogenesis through activation of sterol regulatory element binding protein 1. J. Virol 2012, 86, 2942–2949. [Google Scholar] [CrossRef]
- Yu, Y.; Pierciey, F.J., Jr.; Maguire, T.G.; Alwine, J.C. PKR-like endoplasmic reticulum kinase is necessary for lipogenic activation during HCMV infection. PLoS Pathog. 2013, 9, e1003266. [Google Scholar] [CrossRef]
- Munger, J.; Bennett, B.D.; Parikh, A.; Feng, X.-J.; McArdle, J.; Rabitz, H.A.; Shenk, T.; Rabinowitz, J.D. Systems-level metabolic flux profiling identifies fatty acid synthesis as a target for antiviral therapy. Nat. Biotechnol. 2008, 26, 1179–1186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spencer, C.M.; Schafer, X.L.; Moorman, N.J.; Munger, J. Human cytomegalovirus induces the activity and expression of acetyl-CoA carboxylase, a fatty acid biosynthetic enzyme whose inhibition attenuates viral replication. J. Virol. 2011, 85, 5814–5824. [Google Scholar] [CrossRef]
- Vysochan, A.; Sengupta, A.; Weljie, A.M.; Alwine, J.C.; Yu, Y. ACSS2-mediated acetyl-CoA synthesis from acetate is necessary for human cytomegalovirus infection. Proc. Natl. Acad. Sci. USA 2017, 114, E1528–E1535. [Google Scholar] [CrossRef] [PubMed]
- Fullerton, M.D.; Galic, S.; Marcinko, K.; Sikkema, S.; Pulinilkunnil, T.; Chen, Z.-P.; O’neill, H.M.; Ford, R.J.; Palanivel, R.; O’brien, M. Single phosphorylation sites in Acc1 and Acc2 regulate lipid homeostasis and the insulin-sensitizing effects of metformin. Nat. Med. 2013, 19, 1649–1654. [Google Scholar] [CrossRef] [Green Version]
- Munday, M.R.; Campbell, D.G.; Carling, D.; Hardie, D.G. Identification by amino acid sequencing of three major regulatory phosphorylation sites on rat acetyl-CoA carboxylase. Eur. J. Biochem. 1988, 175, 331–338. [Google Scholar] [CrossRef] [Green Version]
- Bobrovnikova-Marjon, E.; Hatzivassiliou, G.; Grigoriadou, C.; Romero, M.; Cavener, D.R.; Thompson, C.B.; Diehl, J.A. PERK-dependent regulation of lipogenesis during mouse mammary gland development and adipocyte differentiation. Proc. Natl. Acad. Sci. USA 2008. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.-Y.; Cresswell, P. Viperin regulates cellular lipid metabolism during human cytomegalovirus infection. PLoS Pathog. 2013, 9, e1003497. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Maguire, T.G.; Alwine, J.C. ChREBP, a glucose-responsive transcriptional factor, enhances glucose metabolism to support biosynthesis in human cytomegalovirus-infected cells. Proc. Natl. Acad. Sci. 2014, 111, 1951–1956. [Google Scholar] [CrossRef] [PubMed]
- Seo, J.Y.; Yaneva, R.; Hinson, E.R.; Cresswell, P. Human cytomegalovirus directly induces the antiviral protein viperin to enhance infectivity. Science 2011, 332, 1093–1097. [Google Scholar] [CrossRef]
- Koyuncu, E.; Purdy, J.G.; Rabinowitz, J.D.; Shenk, T. Saturated very long chain fatty acids are required for the production of infectious human cytomegalovirus progeny. PLoS Pathog. 2013, 9, e1003333. [Google Scholar] [CrossRef] [PubMed]
- Naganuma, T.; Sato, Y.; Sassa, T.; Ohno, Y.; Kihara, A. Biochemical characterization of the very long-chain fatty acid elongase ELOVL7. FEBS Lett. 2011, 585, 3337–3341. [Google Scholar] [CrossRef] [Green Version]
- Tamura, K.; Makino, A.; Hullin-Matsuda, F.; Kobayashi, T.; Furihata, M.; Chung, S.; Ashida, S.; Miki, T.; Fujioka, T.; Shuin, T. Novel lipogenic enzyme ELOVL7 is involved in prostate cancer growth through saturated long-chain fatty acid metabolism. Cancer Res. 2009, 69, 8133–8140. [Google Scholar] [CrossRef] [PubMed]
- Purdy, J.G.; Shenk, T.; Rabinowitz, J.D. Fatty acid elongase 7 catalyzes lipidome remodeling essential for human cytomegalovirus replication. Cell Rep. 2015, 10, 1375–1385. [Google Scholar] [CrossRef]
- Clippinger, A.J.; Alwine, J.C. Dynein mediates the localization and activation of mTOR in normal and human cytomegalovirus-infected cells. Genes Dev. 2012, 26, 2015–2026. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kudchodkar, S.B.; Yu, Y.; Maguire, T.G.; Alwine, J.C. Human cytomegalovirus infection alters the substrate specificities and rapamycin sensitivities of raptor-and rictor-containing complexes. Proc. Natl. Acad. Sci. USA 2006, 103, 14182–14187. [Google Scholar] [CrossRef]
- Kudchodkar, S.B.; Yu, Y.; Maguire, T.G.; Alwine, J.C. Human cytomegalovirus infection induces rapamycin-insensitive phosphorylation of downstream effectors of mTOR kinase. J. Virol. 2004, 78, 11030–11039. [Google Scholar] [CrossRef] [PubMed]
- Moorman, N.J.; Shenk, T. Rapamycin-resistant mTORC1 kinase activity is required for herpesvirus replication. J. Virol. 2010, 84, 5260–5269. [Google Scholar] [CrossRef] [PubMed]
- Moorman, N.J.; Cristea, I.M.; Terhune, S.S.; Rout, M.P.; Chait, B.T.; Shenk, T. Human cytomegalovirus protein UL38 inhibits host cell stress responses by antagonizing the tuberous sclerosis protein complex. Cell Host Microbe 2008, 3, 253–262. [Google Scholar] [CrossRef]
- Abrahamsen, L.H.; Clay, M.J.; Lyle, J.M.; Zink, J.M.; Fredrikson, L.J.; DeSiervo, A.J.; Jerkofsky, M. The effects of cytomegalovirus infection on polar lipids and neutral lipids in cultured human cells. Intervirology 1996, 39, 223–229. [Google Scholar] [CrossRef] [PubMed]
- Low, H.; Mukhamedova, N.; Cui, H.L.; McSharry, B.P.; Avdic, S.; Hoang, A.; Ditiatkovski, M.; Liu, Y.; Fu, Y.; Meikle, P.J. Cytomegalovirus restructures lipid rafts via a US28/CDC42-mediated pathway, enhancing cholesterol efflux from host cells. Cell Rep. 2016, 16, 186–200. [Google Scholar] [CrossRef]
- Lillis, A.P.; Van Duyn, L.B.; Murphy-Ullrich, J.E.; Strickland, D.K. LDL receptor-related protein 1: Unique tissue-specific functions revealed by selective gene knockout studies. Physiol. Rev. 2008, 88, 887–918. [Google Scholar] [CrossRef]
- Franchini, M.; Montagnana, M. Low-density lipoprotein receptor-related protein 1: New functions for an old molecule. Clin. Chem. Lab. Med. 2011, 49, 967–970. [Google Scholar] [CrossRef]
- Raghu, H.; Sharma-Walia, N.; Veettil, M.V.; Sadagopan, S.; Caballero, A.; Sivakumar, R.; Varga, L.; Bottero, V.; Chandran, B. Lipid rafts of primary endothelial cells are essential for Kaposi’s sarcoma-associated herpesvirus/human herpesvirus 8-induced phosphatidylinositol 3-kinase and RhoA-GTPases critical for microtubule dynamics and nuclear delivery of viral DNA but dispensable for binding and entry. J. Virol. 2007, 81, 7941–7959. [Google Scholar] [CrossRef]
- Wang, X.; Zhu, N.; Li, W.; Zhu, F.; Wang, Y.; Yuan, Y. Mono-ubiquitylated ORF45 Mediates Association of KSHV Particles with Internal Lipid Rafts for Viral Assembly and Egress. PLoS Pathog. 2015, 11, e1005332. [Google Scholar] [CrossRef]
- Sanchez, E.L.; Pulliam, T.H.; Dimaio, T.A.; Thalhofer, A.B.; Delgado, T.; Lagunoff, M. Glycolysis, Glutaminolysis, and Fatty Acid Synthesis Are Required for Distinct Stages of Kaposi’s Sarcoma-Associated Herpesvirus Lytic Replication. J. Virol. 2017, 91, e02237-16. [Google Scholar] [CrossRef]
- Delgado, T.; Sanchez, E.L.; Camarda, R.; Lagunoff, M. Global metabolic profiling of infection by an oncogenic virus: KSHV induces and requires lipogenesis for survival of latent infection. PLoS Pathog. 2012, 8, e1002866. [Google Scholar] [CrossRef]
- Angius, F.; Uda, S.; Piras, E.; Spolitu, S.; Ingianni, A.; Batetta, B.; Pompei, R. Neutral lipid alterations in human herpesvirus 8-infected HUVEC cells and their possible involvement in neo-angiogenesis. BMC Microbiol. 2015, 15, 74. [Google Scholar] [CrossRef] [PubMed]
- Serquiña, A.K.; Kambach, D.M.; Sarker, O.; Ziegelbauer, J.M. Viral MicroRNAs Repress the Cholesterol Pathway, and 25-Hydroxycholesterol Inhibits Infection. mBio 2017, 8, e00576-17. [Google Scholar] [CrossRef]
- Bhatt, A.P.; Jacobs, S.R.; Freemerman, A.J.; Makowski, L.; Rathmell, J.C.; Dittmer, D.P.; Damania, B. Dysregulation of fatty acid synthesis and glycolysis in non-Hodgkin lymphoma. Proc. Natl. Acad. Sci. USA 2012, 109, 11818–11823. [Google Scholar] [CrossRef] [Green Version]
- Sychev, Z.E.; Hu, A.; DiMaio, T.A.; Gitter, A.; Camp, N.D.; Noble, W.S.; Wolf-Yadlin, A.; Lagunoff, M. Integrated systems biology analysis of KSHV latent infection reveals viral induction and reliance on peroxisome mediated lipid metabolism. PLoS Pathog. 2017, 13, e1006256. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Webster-Cyriaque, J.; Tomlinson, C.C.; Yohe, M.; Kenney, S. Fatty acid synthase expression is induced by the Epstein-Barr virus immediate-early protein BRLF1 and is required for lytic viral gene expression. J. Virol. 2004, 78, 4197–4206. [Google Scholar] [CrossRef]
- Lo, A.K.; Lung, R.W.; Dawson, C.W.; Young, L.S.; Ko, C.W.; Yeung, W.W.; Kang, W.; To, K.F.; Lo, K.W. Activation of sterol regulatory element-binding protein 1 (SREBP1)-mediated lipogenesis by the Epstein-Barr virus-encoded latent membrane protein 1 (LMP1) promotes cell proliferation and progression of nasopharyngeal carcinoma. J. Pathol. 2018, 246, 180–190. [Google Scholar] [CrossRef]
- Barton, E.; Mandal, P.; Speck, S.H. Pathogenesis and host control of gammaherpesviruses: Lessons from the mouse. Annu. Rev. Immunol. 2011, 29, 351–397. [Google Scholar] [CrossRef]
- Virgin, H.W.; Latreille, P.; Wamsley, P.; Hallsworth, K.; Weck, K.E.; Dal Canto, A.J.; Speck, S.H. Complete sequence and genomic analysis of murine gammaherpesvirus 68. J. Virol. 1997, 71, 5894–5904. [Google Scholar] [PubMed]
- Lange, P.T.; Darrah, E.J.; Vonderhaar, E.P.; Mboko, W.P.; Rekow, M.M.; Patel, S.B.; Sidjanin, D.J.; Tarakanova, V.L. Type I interferon counteracts antiviral effects of statins in the context of gammaherpesvirus infection. J. Virol. 2016, 90, 3342–3354. [Google Scholar] [CrossRef]
- Lange, P.; Schorl, C.; Sahoo, D.; Tarakanova, V. Liver X Receptors Suppress Activity of Cholesterol and Fatty Acid Synthesis Pathways To Oppose Gammaherpesvirus Replication. mBio 2018, 9, e01115–e01118. [Google Scholar] [CrossRef]
- Lange, P.; Jondle, C.; Darrah, E.; Johnson, K.; Tarakanova, V.L. LXR alpha Restricts Gammaherpesvirus Reactivation from Latently-Infected Peritoneal Cells. J. Virol. 2019. [Google Scholar] [CrossRef] [PubMed]
- Silva, A.C.; Teixeira, A.P.; Alves, P.M. Impact of Adenovirus infection in host cell metabolism evaluated by 1H-NMR spectroscopy. J. Biotechnol. 2016, 231, 16–23. [Google Scholar] [CrossRef]
- Bravo, I.; Crusius, K.; Alonso, A. The E5 protein of the human papillomavirus type 16 modulates composition and dynamics of membrane lipids in keratinocytes. Arch. Virol. 2005, 150, 231–246. [Google Scholar] [CrossRef] [PubMed]
- Franke, C.A.; Reynolds, P.L.; Hruby, D.E. Fatty acid acylation of vaccinia virus proteins. J. Virol. 1989, 63, 4285–4291. [Google Scholar]
- Child, S.J.; Hruby, D.E. Evidence for multiple species of vaccinia virus-encoded palmitylated proteins. Virology 1992, 191, 262–271. [Google Scholar] [CrossRef]
- Martin, K.H.; Franke, C.A.; Hruby, D.E. Novel acylation of poxvirus A-type inclusion proteins. Virus Res. 1999, 60, 147–157. [Google Scholar] [CrossRef]
- Grosenbach, D.W.; Ulaeto, D.O.; Hruby, D.E. Palmitylation of the vaccinia virus 37-kDa major envelope antigen Identification of a conserved acceptor motif and biological relevance. J. Biol. Chem. 1997, 272, 1956–1964. [Google Scholar] [CrossRef]
- Grosenbach, D.W.; Hruby, D.E. Biology of vaccinia virus acylproteins. Perspective 1998, 4, A33R. [Google Scholar]
- Martin, K.H.; Grosenbach, D.W.; Franke, C.A.; Hruby, D.E. Identification and analysis of three myristylated vaccinia virus late proteins. J. Virol. 1997, 71, 5218–5226. [Google Scholar]
- Schmutz, C.; Rindisbacher, L.; Galmiche, M.C.; Wittek, R. Biochemical analysis of the major vaccinia virus envelope antigen. Virology 1995, 213, 19–27. [Google Scholar] [CrossRef]
- Grosenbach, D.W.; Hansen, S.G.; Hruby, D.E. Identification and analysis of vaccinia virus palmitylproteins. Virology 2000, 275, 193–206. [Google Scholar] [CrossRef]
- Hiller, G.; Weber, K. Golgi-derived membranes that contain an acylated viral polypeptide are used for vaccinia virus envelopment. J. Virol. 1985, 55, 651–659. [Google Scholar]
- Blasco, R.; Moss, B. Extracellular vaccinia virus formation and cell-to-cell virus transmission are prevented by deletion of the gene encoding the 37,000-Dalton outer envelope protein. J. Virol. 1991, 65, 5910–5920. [Google Scholar]
- Payne, L. Polypeptide composition of extracellular enveloped vaccinia virus. J. Virol. 1978, 27, 28–37. [Google Scholar]
- Hiller, G.; Eibl, H.; Weber, K. Characterization of intracellular and extracellular vaccinia virus variants: N1-isonicotinoyl-N2-3-methyl-4-chlorobenzoylhydrazine interferes with cytoplasmic virus dissemination and release. J. Virol. 1981, 39, 903–913. [Google Scholar]
- Grosenbach, D.W.; Hruby, D.E. Analysis of a vaccinia virus mutant expressing a nonpalmitylated form of p37, a mediator of virion envelopment. J. Virol. 1998, 72, 5108–5120. [Google Scholar]
- Chung, C.-S.; Huang, C.-Y.; Chang, W. Vaccinia virus penetration requires cholesterol and results in specific viral envelope proteins associated with lipid rafts. J. Virol. 2005, 79, 1623–1634. [Google Scholar] [CrossRef]
- Orynbayeva, Z.; Kolusheva, S.; Groysman, N.; Gavrielov, N.; Lobel, L.; Jelinek, R. Vaccinia virus interactions with the cell membrane studied by new chromatic vesicle and cell sensor assays. J. Virol. 2007, 81, 1140–1147. [Google Scholar] [CrossRef]
- Izmailyan, R.; Hsao, J.-C.; Chung, C.-S.; Chen, C.-H.; Hsu, P.W.-C.; Liao, C.-L.; Chang, W. Integrin β1 mediates vaccinia virus entry through activation of PI3K/Akt signaling. J. Virol. 2012, 86, 6677–6687. [Google Scholar] [CrossRef]
- Sandgren, K.J.; Wilkinson, J.; Miranda-Saksena, M.; McInerney, G.M.; Byth-Wilson, K.; Robinson, P.J.; Cunningham, A.L. A differential role for macropinocytosis in mediating entry of the two forms of vaccinia virus into dendritic cells. PLoS Pathog. 2010, 6, e1000866. [Google Scholar] [CrossRef]
- Whitbeck, J.C.; Foo, C.-H.; de Leon, M.P.; Eisenberg, R.J.; Cohen, G.H. Vaccinia virus exhibits cell-type-dependent entry characteristics. Virology 2009, 385, 383–391. [Google Scholar] [CrossRef] [Green Version]
- Laliberte, J.P.; Weisberg, A.S.; Moss, B. The membrane fusion step of vaccinia virus entry is cooperatively mediated by multiple viral proteins and host cell components. PLoS Pathog. 2011, 7, e1002446. [Google Scholar] [CrossRef]
- Greseth, M.D.; Traktman, P. De novo fatty acid biosynthesis contributes significantly to establishment of a bioenergetically favorable environment for vaccinia virus infection. PLoS Pathog. 2014, 10, e1004021. [Google Scholar] [CrossRef]
- Lund, E.G.; Kerr, T.A.; Sakai, J.; Li, W.-P.; Russell, D.W. cDNA cloning of mouse and human cholesterol 25-hydroxylases, polytopic membrane proteins that synthesize a potent oxysterol regulator of lipid metabolism. J. Biol. Chem. 1998, 273, 34316–34327. [Google Scholar] [CrossRef]
- Mboko, W.P.; Mounce, B.C.; Emmer, J.; Darrah, E.; Patel, S.B.; Tarakanova, V.L. Interferon regulatory factor-1 restricts gammaherpesvirus replication in primary immune cells. J. Virol. 2014, 88, 6993–7004. [Google Scholar] [CrossRef]
- Blanc, M.; Hsieh, W.Y.; Robertson, K.A.; Kropp, K.A.; Forster, T.; Shui, G.; Lacaze, P.; Watterson, S.; Griffiths, S.J.; Spann, N.J.; et al. The transcription factor STAT-1 couples macrophage synthesis of 25-hydroxycholesterol to the interferon antiviral response. Immunity 2013, 38, 106–118. [Google Scholar] [CrossRef]
- Park, K.; Scott, A.L. Cholesterol 25-hydroxylase production by dendritic cells and macrophages is regulated by type I interferons. J. Leukoc. Biol. 2010, 88, 1081–1087. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.Y.; Aliyari, R.; Chikere, K.; Li, G.; Marsden, M.D.; Smith, J.K.; Pernet, O.; Guo, H.; Nusbaum, R.; Zack, J.A.; et al. Interferon-inducible cholesterol-25-hydroxylase broadly inhibits viral entry by production of 25-hydroxycholesterol. Immunity 2013, 38, 92–105. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, S.; Yi, Z.; Tian, H.; Aliyari, R.; Li, Y.; Chen, G.; Liu, P.; Zhong, J.; Chen, X. Interferon-inducible cholesterol-25-hydroxylase inhibits hepatitis C virus replication via distinct mechanisms. Sci. Rep. 2014, 4, 7242. [Google Scholar] [CrossRef]
- Romero-Brey, I.; Berger, C.; Colpitts, C.C.; Boldanova, T.; Engelmann, M.; Todt, D.; Perin, P.M.; Behrendt, P.; Vondran, F.W.; Xu, S. Interferon-inducible cholesterol-25-hydroxylase restricts hepatitis C virus replication through blockage of membranous web formation. Hepatology 2015, 62, 702–714. [Google Scholar] [Green Version]
- Xiang, Y.; Tang, J.-J.; Tao, W.; Cao, X.; Song, B.-L.; Zhong, J. Identification of cholesterol-25-hydroxylase as a novel host restriction factor as a part of primary innate immune responses against hepatitis C virus infection. J. Virol. 2015, 89, 6805–6816. [Google Scholar] [CrossRef]
- Li, C.; Deng, Y.-Q.; Wang, S.; Ma, F.; Aliyari, R.; Huang, X.-Y.; Zhang, N.-N.; Watanabe, M.; Dong, H.-L.; Liu, P. 25-Hydroxycholesterol protects host against Zika virus infection and its associated microcephaly in a mouse model. Immunity 2017, 46, 446–456. [Google Scholar] [CrossRef]
- Shrivastava-Ranjan, P.; Bergeron, É.; Chakrabarti, A.K.; Albariño, C.G.; Flint, M.; Nichol, S.T.; Spiropoulou, C.F. 25-Hydroxycholesterol inhibition of Lassa virus infection through aberrant GP1 glycosylation. MBio 2016, 7, e01808-16. [Google Scholar] [CrossRef]
- Doms, A.; Sanabria, T.; Hansen, J.N.; Altan-Bonnet, N.; Holm, G.H. 25-Hydroxycholesterol Production by the Cholesterol-25-Hydroxylase Interferon-Stimulated Gene Restricts Mammalian Reovirus Infection. J. Virol. 2018, 92, e01047-18. [Google Scholar] [CrossRef]
- Civra, A.; Cagno, V.; Donalisio, M.; Biasi, F.; Leonarduzzi, G.; Poli, G.; Lembo, D. Inhibition of pathogenic non-enveloped viruses by 25-hydroxycholesterol and 27-hydroxycholesterol. Sci. Rep. 2014, 4, 7487. [Google Scholar] [CrossRef] [Green Version]
- Ke, W.; Fang, L.; Jing, H.; Tao, R.; Wang, T.; Li, Y.; Long, S.; Wang, D.; Xiao, S. Cholesterol 25-Hydroxylase Inhibits Porcine Reproductive and Respiratory Syndrome Virus Replication through Enzyme Activity Dependent and Independent Mechanisms. J. Virol. 2017, 91, e00827-17. [Google Scholar] [CrossRef]
- Song, Z.; Zhang, Q.; Liu, X.; Bai, J.; Zhao, Y.; Wang, X.; Jiang, P. Cholesterol 25-hydroxylase is an interferon-inducible factor that protects against porcine reproductive and respiratory syndrome virus infection. Vet. Microbiol. 2017, 210, 153–161. [Google Scholar] [CrossRef]
- Cagno, V.; Civra, A.; Rossin, D.; Calfapietra, S.; Caccia, C.; Leoni, V.; Dorma, N.; Biasi, F.; Poli, G.; Lembo, D. Inhibition of herpes simplex-1 virus replication by 25-hydroxycholesterol and 27-hydroxycholesterol. Redox Biol. 2017, 12, 522–527. [Google Scholar] [CrossRef]
- Wang, J.; Zeng, L.; Zhang, L.; Guo, Z.-Z.; Lu, S.-F.; Ming, S.-L.; Li, G.-L.; Wan, B.; Tian, K.-G.; Yang, G.-Y. Cholesterol 25-hydroxylase acts as a host restriction factor on pseudorabies virus replication. J. Gen. Virol. 2017, 98, 1467–1476. [Google Scholar] [CrossRef]
- Kandutsch, A.A.; Chen, H.W. Inhibition of sterol synthesis in cultured mouse cells by cholesterol derivatives oxygenated in the side chain. J. Biol. Chem. 1974, 249, 6057–6061. [Google Scholar]
- Breslowa, J.L.; Lothrop, D.A.; Spaulding, D.R.; Kandutsch, A.A. Cholesterol, 7-ketocholesterol and 25-hydroxycholesterol uptake studies and effect on 3-hydroxy-3-methylglutaryl-coenzyme A reductase activity in human fibroblasts. Biochim. Biophys. Acta (BBA)-Lipids Lipid Metab. 1975, 398, 10–17. [Google Scholar] [CrossRef]
- Adams, C.M.; Reitz, J.; De Brabander, J.K.; Feramisco, J.D.; Li, L.; Brown, M.S.; Goldstein, J.L. Cholesterol and 25-hydroxycholesterol inhibit activation of SREBPs by different mechanisms, both involving SCAP and Insigs. J. Biol. Chem. 2004, 279, 55772–55780. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, A.; Ikeda, Y.; Kwon, H.J.; Brown, M.S.; Goldstein, J.L. Sterol-regulated transport of SREBPs from endoplasmic reticulum to Golgi: Oxysterols block transport by binding to Insig. Proc. Natl. Acad. Sci. USA 2007, 104, 6511–6518. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.; Talbot, S.; Robertson, K.A.; Watterson, S.; Forster, T.; Roy, D.; Ghazal, P. Rapid proteasomal elimination of 3-hydroxy-3-methylglutaryl-CoA reductase by interferon-γ in primary macrophages requires endogenous 25-hydroxycholesterol synthesis. Steroids 2015, 99, 219–229. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faust, J.R.; Luskey, K.L.; Chin, D.J.; Goldstein, J.L.; Brown, M.S. Regulation of synthesis and degradation of 3-hydroxy-3-methylglutaryl-coenzyme A reductase by low density lipoprotein and 25-hydroxycholesterol in UT-1 cells. Proc. Natl. Acad. Sci. USA 1982, 79, 5205–5209. [Google Scholar] [CrossRef]
- McGee, T.P.; Cheng, H.H.; Kumagai, H.; Omura, S.; Simoni, R.D. Degradation of 3-hydroxy-3-methylglutaryl-CoA reductase in endoplasmic reticulum membranes is accelerated as a result of increased susceptibility to proteolysis. J. Biol. Chem. 1996, 271, 25630–25638. [Google Scholar] [CrossRef]
- Ravid, T.; Doolman, R.; Avner, R.; Harats, D.; Roitelman, J. The ubiquitin-proteasome pathway mediates the regulated degradation of mammalian 3-hydroxy-3-methylglutaryl-coenzyme A reductase. J. Biol. Chem. 2000, 275, 35840–35847. [Google Scholar] [CrossRef]
- Sever, N.; Yang, T.; Brown, M.S.; Goldstein, J.L.; DeBose-Boyd, R.A. Accelerated degradation of HMG CoA reductase mediated by binding of insig-1 to its sterol-sensing domain. Mol. Cell 2003, 11, 25–33. [Google Scholar] [CrossRef]
- Janowski, B.A.; Willy, P.J.; Devi, T.R.; Falck, J.; Mangelsdorf, D.J. An oxysterol signalling pathway mediated by the nuclear receptor LXRα. Nature 1996, 383, 728–731. [Google Scholar] [CrossRef]
- Lehmann, J.M.; Kliewer, S.A.; Moore, L.B.; Smith-Oliver, T.A.; Oliver, B.B.; Su, J.L.; Sundseth, S.S.; Winegar, D.A.; Blanchard, D.E.; Spencer, T.A.; et al. Activation of the nuclear receptor LXR by oxysterols defines a new hormone response pathway. J. Biol. Chem 1997, 272, 3137–3140. [Google Scholar] [CrossRef] [PubMed]
- Björkhem, I. Are side-chain oxidized oxysterols regulators also in vivo? J. Lipid Res. 2009, 50, S213–S218. [Google Scholar] [CrossRef]
- Diczfalusy, U. On the formation and possible biological role of 25-hydroxycholesterol. Biochimie 2013, 95, 455–460. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, Z.; Zhang, Y.; Ma, X.; Chen, Y.; Yu, M.; Ma, C.; Li, X.; Cao, Y.; Liu, J. Activation of liver X receptor plays a central role in antiviral actions of 25-hydroxycholesterol. J. Lipid Res. 2018, 59, 2287–2296. [Google Scholar] [CrossRef] [PubMed]
- Reboldi, A.; Dang, E.V.; McDonald, J.G.; Liang, G.; Russell, D.W.; Cyster, J.G. 25-Hydroxycholesterol suppresses interleukin-1–driven inflammation downstream of type I interferon. Science 2014, 345, 679–684. [Google Scholar] [CrossRef] [PubMed]
- Gold, E.S.; Diercks, A.H.; Podolsky, I.; Podyminogin, R.L.; Askovich, P.S.; Treuting, P.M.; Aderem, A. 25-Hydroxycholesterol acts as an amplifier of inflammatory signaling. Proc. Natl. Acad. Sci. USA 2014, 111, 10666–10671. [Google Scholar] [CrossRef] [PubMed] [Green Version]
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lange, P.T.; Lagunoff, M.; Tarakanova, V.L. Chewing the Fat: The Conserved Ability of DNA Viruses to Hijack Cellular Lipid Metabolism. Viruses 2019, 11, 119. https://doi.org/10.3390/v11020119
Lange PT, Lagunoff M, Tarakanova VL. Chewing the Fat: The Conserved Ability of DNA Viruses to Hijack Cellular Lipid Metabolism. Viruses. 2019; 11(2):119. https://doi.org/10.3390/v11020119
Chicago/Turabian StyleLange, Philip T., Michael Lagunoff, and Vera L. Tarakanova. 2019. "Chewing the Fat: The Conserved Ability of DNA Viruses to Hijack Cellular Lipid Metabolism" Viruses 11, no. 2: 119. https://doi.org/10.3390/v11020119
APA StyleLange, P. T., Lagunoff, M., & Tarakanova, V. L. (2019). Chewing the Fat: The Conserved Ability of DNA Viruses to Hijack Cellular Lipid Metabolism. Viruses, 11(2), 119. https://doi.org/10.3390/v11020119



