Processing Phage Therapy Requests in a Brussels Military Hospital: Lessons Identified
Abstract
:1. Introduction
2. Demographics
3. Infection Types and Bacterial Pathogens
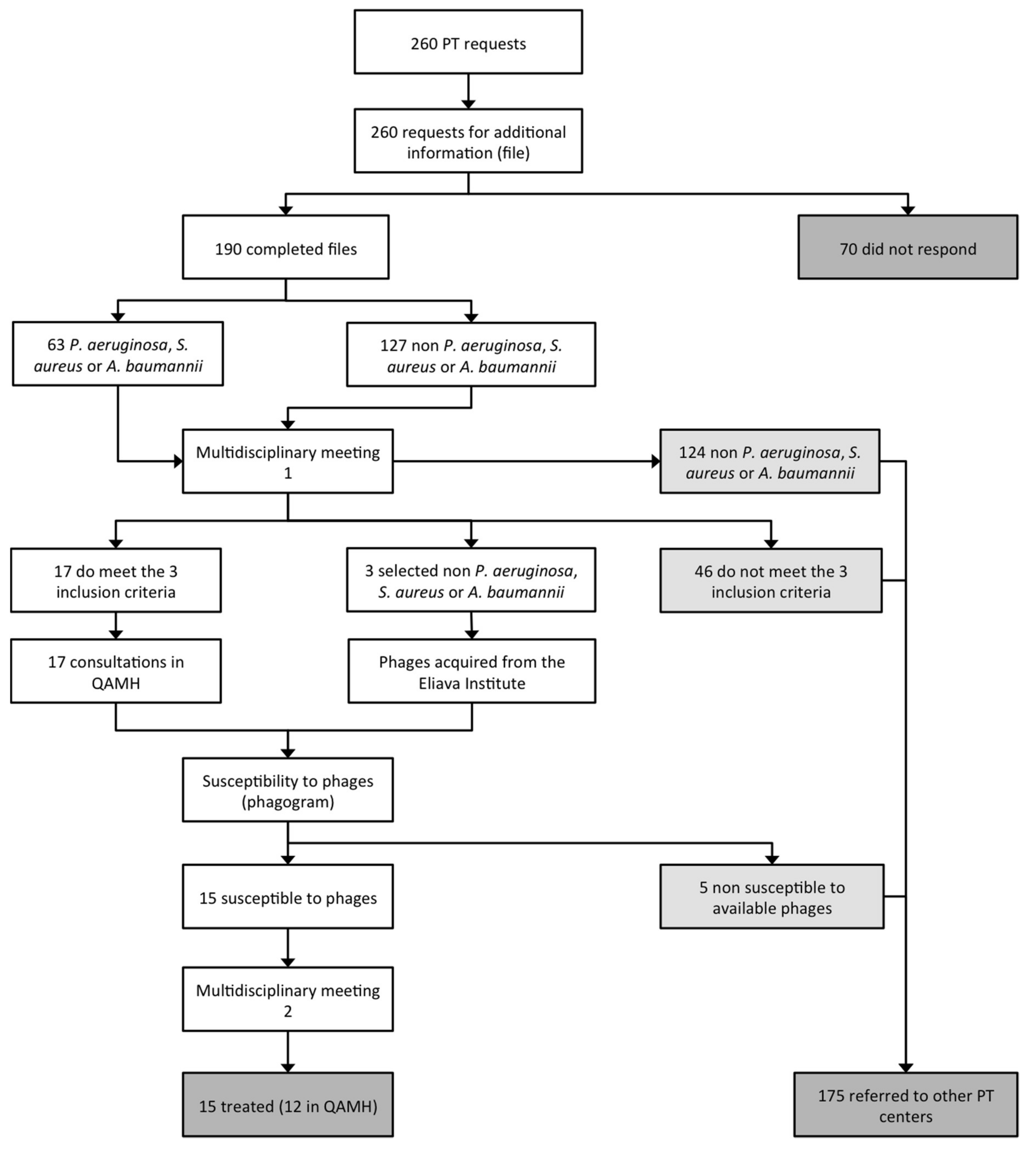
4. Patient Care Workflow
- Bacterial infection associated with antibiotic treatment failure;
- The absence of other therapeutic options.
5. Implications for Future Activities
- 70 applicants (26.9%) did not respond to the email request for more information;
- 124 requests (47.7%) concerned bacterial pathogens against which the QAMH had no potent phages available;
- 46 applications (17.7%) did not meet the other two eligibility criteria (antibiotic treatment failure and/or absence of other therapeutic options);
- 5 (25%) out of the 20 infecting bacterial strains for which a phagogram was performed were found to be non-susceptible to the available phages.
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tackling Drug-Resistant Infections Globally: Final Report and Recommendations. The Review on Antimicrobial Resistance. 2016 Release. Available online: https://amr-review.org/sites/default/files/160525_Final%20paper_with%20cover.pdf (accessed on 10 February 2019).
- United Nations. Draft political declaration of the high-level meeting of the General Assembly on antimicrobial resistance (16-16108 (E)). 2016 Release. Available online: https://www.un.org/pga/71/wp-content/uploads/sites/40/2016/09/DGACM_GAEAD_ESCAB-AMR-Draft-Political-Declaration-1616108E.pdf (accessed on 10 February 2019).
- WHO Publishes List of Bacteria for Which New Antibiotics Are Urgently Needed. Available online: https://www.who.int/news-room/detail/27-02-2017-who-publishes-list-of-bacteria-for-which-new-antibiotics-are-urgently-needed (accessed on 10 February 2019).
- Rice, L.B. Federal funding for the study of antimicrobial resistance in nosocomial pathogens: no ESKAPE. J. Infect. Dis. 2009, 197, 1079–1081. [Google Scholar] [CrossRef] [PubMed]
- Boucher, H.W.; Talbot, G.H.; Bradley, J.S.; Edwards, J.E.; Gilbert, D.; Rice, L.B.; Scheld, M.; Spellberg, B.; Bartlett, J. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 48, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Thiel, K. Old dogma, new tricks—21st Century phage therapy. Nat. Biotechnol. 2004, 22, 31–36. [Google Scholar] [CrossRef] [PubMed]
- World Medical Association Declaration of Helsinki: Ethical principles for medical research involving human subjects. JAMA 2013, 10, 2191–2194.
- Jennes, S.; Merabishvili, M.; Soentjens, P.; Pang, K.W.; Rose, T.; Keersebilck, E.; Soete, O.; François, P.M.; Teodorescu, S.; Verween, G.; Verbeken, G.; De Vos, D.; Pirnay, J.P. Use of bacteriophages in the treatment of colistin-only-sensitive Pseudomonas aeruginosa septicaemia in a patient with acute kidney injury-a case report. Crit. Care. 2017, 21, 129. [Google Scholar] [CrossRef] [PubMed]
- Pirnay, J.P.; Verbeken, G.; Ceyssens, P.J.; Huys, I.; De Vos, D.; Ameloot, C.; Fauconnier, A. The Magistral Phage. Viruses 2018, 10, 64. [Google Scholar] [CrossRef] [PubMed]
- Zorg.nu. Bacteriofagen: Een Alternatief Voor Antibiotica? Available online: https://zorgnu.avrotros.nl/uitzending/21-03-2017/bacteriofagen-een-alternatief-voor-antibiotica/ (accessed on 10 February 2019).
- Dokters van Morgen over bacteriën. Available online: https://zorgnu.avrotros.nl/uitzending/24-10-2017/ (accessed on 10 February 2019).
- Dokters van Morgen: Bacteriofagen. Available online: https://zorgnu.avrotros.nl/uitzending/05-02-2019/ (accessed on 10 February 2019).
- Magiorakos, A.P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merabishvili, M.; Pirnay, J.P.; Verbeken, G.; Chanishvili, N.; Tediashvili, M.; Lashkhi, N.; Glonti, T.; Krylov, V.; Mast, J.; Van Parys, L.; et al. Quality-controlled small-scale production of a well-defined bacteriophage cocktail for use in human clinical trials. PLoS ONE 2009, 4, e4944. [Google Scholar] [CrossRef] [PubMed]
- Merabishvili, M.; Vandenheuvel, D.; Kropinski, A.M.; Mast, J.; De Vos, D.; Verbeken, G.; Noben, J.P.; Lavigne, R.; Vaneechoutte, M.; Pirnay, J.P. Characterization of newly isolated lytic bacteriophages active against Acinetobacter baumannii. PLoS ONE 2014, 9, e104853. [Google Scholar] [CrossRef] [PubMed]
- Kakabadze, E.; Makalatia, K.; Grdzelishvili, N.; Bakuradze, N.; Goderdzishvili, M.; Kusradze, I.; Phoba, M.F.; Lunguya, O.; Lood, C.; Lavigne, R.; et al. Selection of Potential Therapeutic Bacteriophages that Lyse a CTX-M-15 Extended Spectrum β-Lactamase Producing Salmonella enterica Serovar Typhi Strain from the Democratic Republic of the Congo. Viruses 2018, 10, 172. [Google Scholar] [CrossRef] [PubMed]
- Barbu, E.M.; Cady, K.C.; Hubby, B. Phage Therapy in the Era of Synthetic Biology. Cold Spring Harb. Perspect. Biol. 2016, 8, a023879. [Google Scholar] [CrossRef] [PubMed]
- Torres-Barceló, C.; Arias-Sánchez, F.I.; Vasse, M.; Ramsayer, J.; Kaltz, O.; Hochberg, M.E. A window of opportunity to control the bacterial pathogen Pseudomonas aeruginosa combining antibiotics and phages. PLoS ONE 2014, 9, e106628. [Google Scholar] [CrossRef] [PubMed]
- Kirby, A.E. Synergistic Action of Gentamicin and Bacteriophage in a Continuous Culture Population of Staphylococcus aureus. PLoS ONE 2012, 7, e51017. [Google Scholar] [CrossRef] [PubMed]




| Infection Types | LRTI | UTI | SSTI | ENTI | BoneI | OPI | AbdI | ND | Other | Total | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Demographics | |||||||||||
| Number of requesters | 59 | 79 | 21 | 22 | 16 | 14 | 9 | 12 | 28 | 260 | |
| Age | ≤14 | 1 | 3 | 3 | 1 | 1 | 9 | ||||
| 15–29 | 2 | 1 | 1 | 1 | 5 | ||||||
| 30–59 | 5 | 11 | 5 | 3 | 4 | 2 | 1 | 1 | 5 | 37 | |
| ≥60 | 21 | 26 | 9 | 6 | 4 | 10 | 2 | 1 | 2 | 81 | |
| ND | 30 | 38 | 4 | 12 | 7 | 2 | 6 | 8 | 21 | 128 | |
| Gender | Male | 23 | 33 | 12 | 13 | 10 | 11 | 5 | 7 | 20 | 134 |
| Female | 36 | 46 | 9 | 9 | 6 | 3 | 4 | 5 | 8 | 126 | |
| Countries | The Netherlands | 38 | 69 | 5 | 14 | 5 | 7 | 5 | 10 | 21 | 174 |
| Belgium | 9 | 4 | 12 | 2 | 5 | 6 | 3 | 2 | 7 | 50 | |
| France | 5 | 3 | 3 | 4 | 3 | 1 | 19 | ||||
| Germany | 3 | 2 | 1 | 6 | |||||||
| Luxembourg | 1 | 2 | 3 | ||||||||
| Italy | 1 | 1 | |||||||||
| Spain | 1 | 1 | |||||||||
| United States | 2 | 1 | 3 | ||||||||
| Israel | 2 | 2 | |||||||||
| Unknown | 1 | 1 | |||||||||
| Bacterial pathogens | |||||||||||
| Pseudomonas aeruginosa | 36 | 7 | 8 | 9 | 3 | 2 | 1 | 4 | 70 | ||
| Escherichia coli | 3 | 29 | 2 | 3 | 2 | 2 | 2 | 1 | 44 | ||
| Staphylococcus aureus | 11 | 6 | 5 | 2 | 5 | 1 | 1 | 31 | |||
| Klebsiella pneumoniae | 18 | 1 | 2 | 1 | 2 | 24 | |||||
| Enterococcus faecalis | 12 | 1 | 4 | 17 | |||||||
| Proteus mirabilis | 4 | 5 | 1 | 1 | 11 | ||||||
| Enterobacter cloacae | 6 | 6 | |||||||||
| Mycobacterium avium | 3 | 3 | |||||||||
| Streptococcus pyogenes | 1 | 2 | 1 | 1 | 5 | ||||||
| Staphylococcus epidermidis | 3 | 2 | 1 | 6 | |||||||
| Staphylococcus dysgalactiae | 1 | 2 | 3 | ||||||||
| Acinetobacter baumannii | 2 | 1 | 1 | 4 | |||||||
| Serratia marcescens | 1 | 1 | 2 | ||||||||
| Staphylococcus capitis | 1 | 1 | |||||||||
| Staphylococcus warneri | 2 | 2 | |||||||||
| Borrelia burgdorferi | 2 | 2 | |||||||||
| Burkholderia cenocepacia | 1 | 1 | |||||||||
| Burkholderia multivorans | 1 | 1 | |||||||||
| Enterobacter aerogenes | 2 | 2 | |||||||||
| Granulicatella adiacens | 2 | 2 | |||||||||
| Haemophilus influenzae | 1 | 1 | |||||||||
| Morganella morganii | 1 | 1 | 2 | ||||||||
| Moraxella catarrhalis | 1 | 1 | |||||||||
| Cutibacterium acnes | 2 | 2 | |||||||||
| Stenotrophomonas maltophilia | 1 | 1 | |||||||||
| Yersinia enterocolitica | 1 | 1 | |||||||||
| Coxiella burnetii | 1 | 1 | |||||||||
| Clostridium hathewayi | 1 | 1 | |||||||||
| Helicobacter pylori | 1 | 1 | |||||||||
| Corynebacterium amycolatum | 1 | 1 | |||||||||
| ND | 8 | 22 | 3 | 5 | 2 | 2 | 3 | 5 | 12 | 62 | |
| Total | 75 | 101 | 25 | 27 | 19 | 14 | 9 | 12 | 29 | 311 | |
| Polymicrobial (caused by a combination of bacteria) | 10 | 14 | 4 | 4 | 3 | 2 | 37 | ||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Djebara, S.; Maussen, C.; De Vos, D.; Merabishvili, M.; Damanet, B.; Pang, K.W.; De Leenheer, P.; Strachinaru, I.; Soentjens, P.; Pirnay, J.-P. Processing Phage Therapy Requests in a Brussels Military Hospital: Lessons Identified. Viruses 2019, 11, 265. https://doi.org/10.3390/v11030265
Djebara S, Maussen C, De Vos D, Merabishvili M, Damanet B, Pang KW, De Leenheer P, Strachinaru I, Soentjens P, Pirnay J-P. Processing Phage Therapy Requests in a Brussels Military Hospital: Lessons Identified. Viruses. 2019; 11(3):265. https://doi.org/10.3390/v11030265
Chicago/Turabian StyleDjebara, Sarah, Christiane Maussen, Daniel De Vos, Maya Merabishvili, Benjamin Damanet, Kim Win Pang, Peggy De Leenheer, Isabella Strachinaru, Patrick Soentjens, and Jean-Paul Pirnay. 2019. "Processing Phage Therapy Requests in a Brussels Military Hospital: Lessons Identified" Viruses 11, no. 3: 265. https://doi.org/10.3390/v11030265
APA StyleDjebara, S., Maussen, C., De Vos, D., Merabishvili, M., Damanet, B., Pang, K. W., De Leenheer, P., Strachinaru, I., Soentjens, P., & Pirnay, J. -P. (2019). Processing Phage Therapy Requests in a Brussels Military Hospital: Lessons Identified. Viruses, 11(3), 265. https://doi.org/10.3390/v11030265






