Caliciviridae Other Than Noroviruses
Abstract
:1. Introduction
- NoV structure [14];
- The molecular biology of NoV replication [15];
- NoV replication in the immunocompromised host [16];
- Factors affecting host susceptibility to NoV infection [17];
- In vitro propagation of NoVs [18];
- Innate and acquired immune responses to NoV infections [19];
- Molecular epidemiology of HuNoVs [20];
- Use of animal models of NoV infection [21];
- Treatment and development of antivirals [22];
- Vaccine development [23].
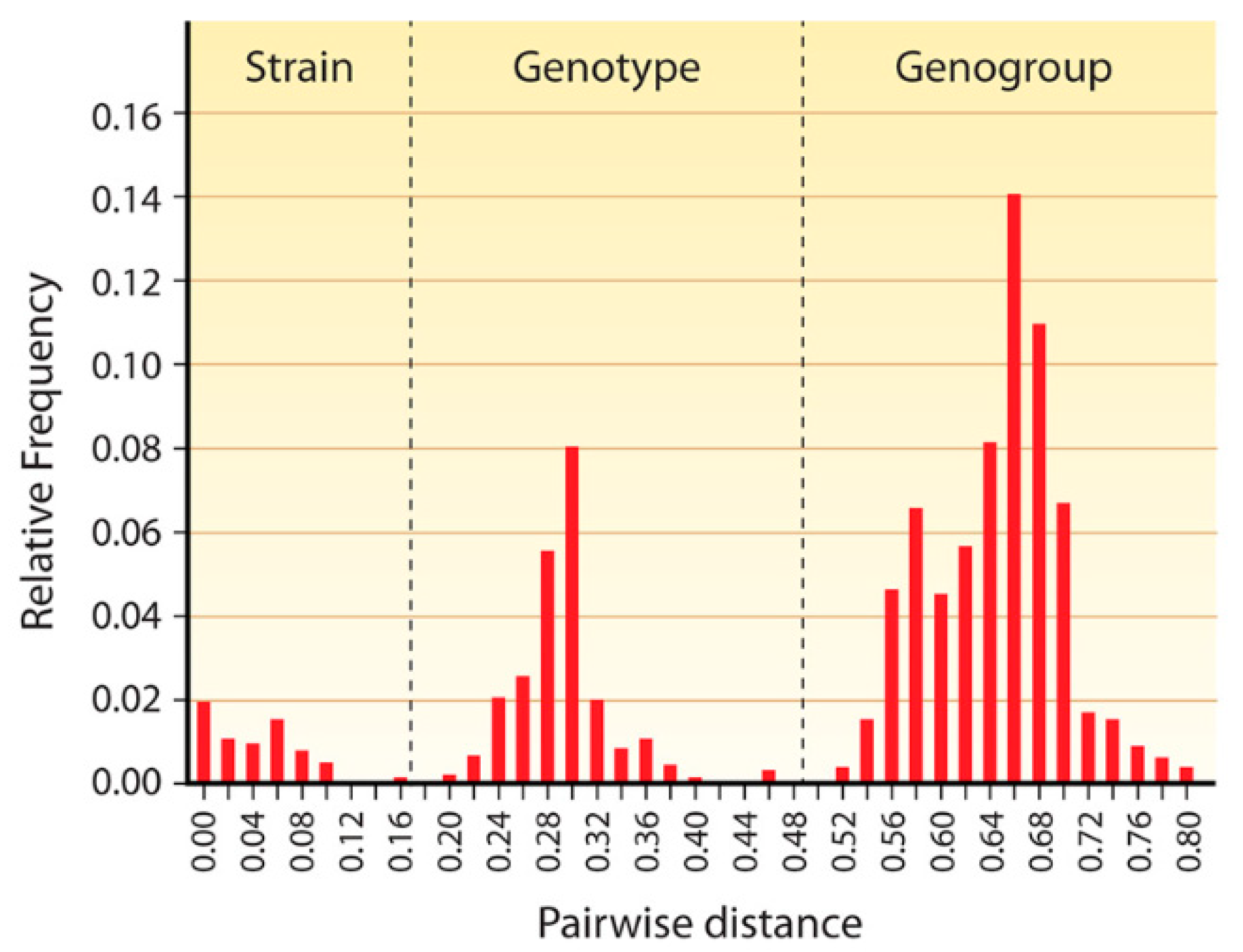
2. Classification
3. Genome Structure
4. Replication
4.1. Sapovirus
4.2. Lagovirus
4.3. Vesivirus
4.4. Nebovirus
4.5. Recovirus
4.6. Valovirus
4.7. Bavovirus and Nacovirus
4.8. Salovirus and Minovirus
5. Concluding Remarks
- The ability to propagate viruses in cell/enteroid culture;
- The availability of helper virus-free (plasmid only-based) reverse genetics systems;
- The availability of suitable animal models.
Funding
Acknowledgments
Conflicts of Interest
References
- Wobus, C.E.; Karst, S.M.; Thackray, L.B.; Chang, K.O.; Sosnovtsev, S.V.; Belliot, G.; Krug, A.; Mackenzie, J.M.; Green, K.Y.; Virgin, H.W. Replication of Norovirus in cell culture reveals a tropism for dendritic cells and macrophages. PLoS Biol. 2004, 2, e432. [Google Scholar] [CrossRef]
- Ward, V.K.; McCormick, C.J.; Clarke, I.N.; Salim, O.; Wobus, C.E.; Thackray, L.B.; Virgin, H.W., 4th; Lambden, P.R. Recovery of infectious murine norovirus using pol II-driven expression of full-length cDNA. Proc. Natl. Acad. Sci. USA 2007, 104, 11050–11055. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaudhry, Y.; Skinner, M.A.; Goodfellow, I.G. Recovery of genetically defined murine norovirus in tissue culture by using a fowlpox virus expressing T7 RNA polymerase. J Gen Virol. 2007, 88 Pt 8, 2091–2100. [Google Scholar] [CrossRef] [Green Version]
- Yunus, M.A.; Chung, L.M.; Chaudhry, Y.; Bailey, D.; Goodfellow, I. Development of an optimized RNA-based murine norovirus reverse genetics system. J. Virol. Methods 2010, 169, 112–118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arias, A.; Bailey, D.; Chaudhry, Y.; Goodfellow, I. Development of a reverse-genetics system for murine norovirus 3: Long-term persistence occurs in the caecum and colon. J. Gen. Virol. 2012, 93 Pt 7, 1432–1441. [Google Scholar] [CrossRef]
- Nice, T.J.; Strong, D.W.; McCune, B.T.; Pohl, C.S.; Virgin, H.W. A single-amino-acid change in murine norovirus NS1/2 is sufficient for colonic tropism and persistence. J. Virol. 2013, 87, 327–334. [Google Scholar] [CrossRef] [PubMed]
- Ohsugi, T.; Matsuura, K.; Kawabe, S.; Nakamura, N.; Kumar, J.M.; Wakamiya, M.; Morikawa, S.; Urano, T. Natural infection of murine norovirus in conventional and specific pathogen-free laboratory mice. Front. Microbiol. 2013, 4, 12. [Google Scholar] [CrossRef] [PubMed]
- Kolawole, A.O.; Gonzalez-Hernandez, M.B.; Turula, H.; Yu, C.; Elftman, M.D.; Wobus, C.E. Oral norovirus infection is blocked in mice lacking Peyer’s patches and mature M cells. J. Virol. 2015, 90, 1499–1506. [Google Scholar] [CrossRef] [PubMed]
- Kolawole, A.O.; Rocha-Pereira, J.; Elftman, M.D.; Neyts, J.; Wobus, C.E. Inhibition of human norovirus by a viral polymerase inhibitor in the B cell culture system and in the mouse model. Antivir. Res. 2016, 132, 46–49. [Google Scholar] [CrossRef] [Green Version]
- Jones, M.K.; Watanabe, M.; Zhu, S.; Graves, C.L.; Keyes, L.R.; Grau, K.R.; Gonzalez-Hernandez, M.B.; Iovine, N.M.; Wobus, C.E.; Vinjé, J.; et al. Enteric bacteria promote human and mouse norovirus infection of B cells. Science 2014, 346, 755–759. [Google Scholar] [CrossRef] [Green Version]
- Ettayebi, K.; Crawford, S.E.; Murakami, K.; Broughman, J.R.; Karandikar, U.; Tenge, V.R.; Neill, F.H.; Blutt, S.E.; Zeng, X.L.; Qu, L.; et al. Replication of human noroviruses in stem cell-derived human enteroids. Science 2016, 353, 1387–1393. [Google Scholar] [CrossRef]
- Taube, S.; Kolawole, A.O.; Höhne, M.; Wilkinson, J.E.; Handley, S.A.; Perry, J.W.; Thackray, L.B.; Akkina, R.; Wobus, C.E. A mouse model for human norovirus. MBio 2013, 4, e00450. [Google Scholar] [CrossRef]
- Cortes-Penfield, N.W.; Ramani, S.; Estes, M.K.; Atmar, R.L. Prospects and challenges in the development of a norovirus vaccine. Clin. Ther. 2017, 39, 1537–1549. [Google Scholar] [CrossRef]
- Smith, H.Q.; Smith, T.J. The Dynamic Capsid Structures of the Noroviruses. Viruses 2019, 11, 235. [Google Scholar] [CrossRef]
- Goodfellow, I.; Vinje, J.; Wilen, C.; Mackenzie, J.; White, P.; Karst, S.; Taube, S.; Hwang, S.; Ward, V. The molecular biology of NoV replication. Viruses, in preparation.
- Debbink, K.; Chong, P. NoV replication in the immunocompromised host. Viruses, in preparation.
- Nordgren, J.; Svensson, L. Genetic Susceptibility to Human Norovirus Infection: An Update. Viruses 2019, 11, 226. [Google Scholar] [CrossRef]
- Jones, M.; Estes, M.K. In vitro propagation of NoVs. Viruses, in preparation.
- Baric, R.; Lindesmith, L.; Mallory, M.; Brewer-Jensen, P. Innate and acquired immune responses to NoV infections. Viruses, in preparation.
- Schultz-Cherry, S.; Mans, J.; Moe, C.; Kirby, A.; Teunis, P.; Lopman, B.A.; Hall, A.J.; Guix, S.; Blazevic, V.; Andrade, J.S.R. Molecular epidemiology of HuNoVs. Viruses, in preparation.
- Todd, K.V.; Tripp, R.A. Human Norovirus: Experimental Models of Infection. Viruses 2019, 11, 151. [Google Scholar] [CrossRef]
- Chang, K.O.; Kim, Y.; Lovell, S.; Rathnayake, A.D.; Groutas, W.C. Antiviral Drug Discovery: Norovirus Proteases and Development of Inhibitors. Viruses 2019, 11, 197. [Google Scholar] [CrossRef]
- Brown, R. Vaccine development. Viruses, in preparation.
- Goodfellow, I.; Taube, S. Calicivirus replication and reverse genetics. In Viral Gastroenteritis; Svensson, L., Desselberger, U., Greenberg, H.B., Estes, M.K., Eds.; Elsevier Academic Press: Amsterdam, The Netherlands, 2016; pp. 355–378. [Google Scholar]
- L’Homme, Y.; Sansregret, R.; Plante-Fortier, E.; Lamontagne, A.M.; Ouardani, M.; Lacroix, G.; Simard, C. Genomic characterization of swine caliciviruses representing a new genus of Caliciviridae. Virus Genes 2009, 39, 66–75. [Google Scholar] [CrossRef]
- Di Martino, B.; Martella, V.; Di Profio, F.; Ceci, C.; Marsilio, F. Detection of St-Valerien-like viruses in swine, Italy. Vet. Microbiol. 2011, 149, 221–224. [Google Scholar] [CrossRef]
- Di Martino, B.; Di Profio, F.; Ceci, C.; Martella, V.; Lavazza, A.; Massirio, I.; Marsilio, F. Seroprevalence of St-Valerien-like caliciviruses in Italian swine. J. Gen. Virol. 2012, 93 Pt 1, 102–105. [Google Scholar] [CrossRef]
- Wolf, S.; Reetz, J.; Hoffmann, K.; Gründel, A.; Schwarz, B.A.; Hänel, I.; Otto, P.H. Discovery and genetic characterization of novel caliciviruses in German and Dutch poultry. Arch. Virol. 2012, 157, 1499–1507. [Google Scholar] [CrossRef] [PubMed]
- Liao, Q.; Wang, X.; Wang, D.; Zhang, D. Complete genome sequence of a novel calicivirus from a goose. Arch. Virol. 2014, 159, 2529–2531. [Google Scholar] [CrossRef] [PubMed]
- Mikalsen, A.B.; Nilsen, P.; Frøystad-Saugen, M.; Lindmo, K.; Eliassen, T.M. Characterization of a novel calicivirus causing systemic infection in atlantic salmon (Salmo salar L.): Proposal for a new genus of Caliciviridae. PLoS ONE 2014, 9, e107132. [Google Scholar] [CrossRef] [PubMed]
- Mor, S.K.; Phelps, N.B.D.; Ng, T.F.F.; Subramaniam, K.; Primus, A.; Armien, A.G.; McCann, R.; Puzach, C.; Waltzek, T.B.; Goyal, S.M. Genomic characterization of a novel calicivirus, FHMCV-2012, from baitfish in the USA. Arch. Virol. 2017, 162, 3619–3627. [Google Scholar] [CrossRef]
- McCune, B.T.; Tang, W.; Lu, J.; Eaglesham, J.B.; Thorne, L.; Mayer, A.E.; Condiff, E.; Nice, T.J.; Goodfellow, I.; Krezel, A.M.; et al. Noroviruses co-opt the function of host proteins VAPA and VAPB for replication via a phenylalanine-phenylalanine-acidic-tract-motif mimic in nonstructural viral protein NS1/2. MBio 2017, 8, e00668-17. [Google Scholar] [CrossRef] [PubMed]
- Doerflinger, S.Y.; Cortese, M.; Romero-Brey, I.; Menne, Z.; Tubiana, T.; Schenk, C.; White, P.A.; Bartenschlager, R.; Bressanelli, S.; Hansman, G.S.; et al. Membrane alterations induced by nonstructural proteins of human norovirus. PLoS Pathog. 2017, 13, e1006705. [Google Scholar] [CrossRef]
- Thorne, L.G.; Goodfellow, I.G. Norovirus gene expression and replication. J. Gen. Virol. 2014, 95 Pt 2, 278–291. [Google Scholar] [CrossRef]
- Bull, R.A.; Tanaka, M.M.; White, P.A. Norovirus recombination. J. Gen. Virol. 2007, 88, 3347–3359. [Google Scholar] [CrossRef] [Green Version]
- Ludwig-Begall, L.F.; Mauroy, A.; Thiry, E. Norovirus recombinants: Recurrent in the field, recalcitrant in the lab—A scoping review of recombination and recombinant types of noroviruses. J. Gen. Virol. 2018, 99, 970–988. [Google Scholar] [CrossRef]
- Oka, T.; Wang, Q.; Katayama, K.; Saif, L.J. Comprehensive review of human sapoviruses. Clin. Microbiol. Rev. 2015, 28, 32–53. [Google Scholar] [CrossRef]
- Oka, T.; Lu, Z.; Phan, T.; Delwart, E.L.; Saif, L.J.; Wang, Q. Genetic characterization and classification of human and animal sapoviruses. PLoS ONE 2016, 11, e0156373. [Google Scholar] [CrossRef] [PubMed]
- Kumthip, K.; Khamrin, P.; Maneekarn, N. Molecular epidemiology and genotype distributions of noroviruses and sapoviruses in Thailand 2000-2016: A review. J. Med. Virol. 2018, 90, 617–624. [Google Scholar] [CrossRef]
- Kuroda, M.; Masuda, T.; Ito, M.; Naoi, Y.; Doan, Y.H.; Haga, K.; Tsuchiaka, S.; Kishimoto, M.; Sano, K.; Omatsu, T.; et al. Genetic diversity and intergenogroup recombination events of sapoviruses detected from feces of pigs in Japan. Infect. Genet. Evol. 2017, 55, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Diez-Valcarce, M.; Castro, C.J.; Marine, R.L.; Halasa, N.; Mayta, H.; Saito, M.; Tsaknaridis, L.; Pan, C.Y.; Bucardo, F.; Becker-Dreps, S.; et al. Genetic diversity of human sapovirus across the Americas. J. Clin. Virol. 2018, 104, 65–72. [Google Scholar] [CrossRef]
- Li, J.; Zhang, W.; Cui, L.; Shen, Q.; Hua, X. Metagenomic identification, genetic characterization and genotyping of porcine sapoviruses. Infect. Genet. Evol. 2018, 62, 244–252. [Google Scholar] [CrossRef] [PubMed]
- Kemenesi, G.; Gellért, Á.; Dallos, B.; Görföl, T.; Boldogh, S.; Estók, P.; Marton, S.; Oldal, M.; Martella, V.; Bányai, K.; et al. Sequencing and molecular modeling identifies candidate members of Caliciviridae family in bats. Infect. Genet. Evol. 2016, 41, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Yinda, C.K.; Conceição-Neto, N.; Zeller, M.; Heylen, E.; Maes, P.; Ghogomu, S.M.; Van Ranst, M.; Matthijnssens, J. Novel highly divergent sapoviruses detected by metagenomics analysis in straw-colored fruit bats in Cameroon. Emerg. Microbes Infect. 2017, 6, e38. [Google Scholar] [CrossRef]
- Barry, A.F.; Durães-Carvalho, R.; Oliveira-Filho, E.F.; Alfieri, A.A.; Van der Poel, W.H.M. High-resolution phylogeny providing insights towards the epidemiology, zoonotic aspects and taxonomy of sapoviruses. Infect. Genet. Evol. 2017, 56, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Lu, Z.; Yokoyama, M.; Chen, N.; Oka, T.; Jung, K.; Chang, K.O.; Annamalai, T.; Wang, Q.; Saif, L.J. Mechanism of cell culture adaptation of an enteric calicivirus, the porcine sapovirus Cowden Strain. J. Virol. 2015, 90, 1345–1358. [Google Scholar] [CrossRef] [PubMed]
- Oka, T.; Stoltzfus, G.T.; Zhu, C.; Jung, K.; Wang, Q.; Saif, L.J. Attempts to grow human noroviruses, a sapovirus, and a bovine norovirus in vitro. PLoS ONE 2018, 13, e0178157. [Google Scholar] [CrossRef]
- Kim, D.S.; Hosmillo, M.; Alfajaro, M.M.; Kim, J.Y.; Park, J.G.; Son, K.Y.; Ryu, E.H.; Sorgeloos, F.; Kwon, H.J.; Park, S.J.; et al. Both α2,3- and α2,6-linked sialic acids on O-linked glycoproteins act as functional receptors for porcine Sapovirus. PLoS Pathog. 2014, 10, e1004172. [Google Scholar] [CrossRef] [PubMed]
- Soliman, M.; Kim, D.S.; Kim, C.; Seo, J.Y.; Kim, J.Y.; Park, J.G.; Alfajaro, M.M.; Baek, Y.B.; Cho, E.H.; Park, S.I.; et al. Porcine sapovirus Cowden strain enters LLC-PK cells via clathrin- and cholesterol-dependent endocytosis with the requirement of dynamin II. Vet. Res. 2018, 49, 92. [Google Scholar] [CrossRef]
- Alfajaro, M.M.; Cho, E.H.; Kim, D.S.; Kim, J.Y.; Park, J.G.; Soliman, M.; Baek, Y.B.; Park, C.H.; Kang, M.I.; Park, S.I.; et al. Early infection of porcine sapovirus disrupts tight junction and uses occludin as a co-receptor. J. Virol. 2018, JVI.01773-18. [Google Scholar] [CrossRef] [PubMed]
- Hosmillo, M.; Chaudhry, Y.; Kim, D.S.; Goodfellow, I.; Cho, K.O. Sapovirus translation requires an interaction between VPg and the cap binding protein eIF4E. J. Virol. 2014, 88, 12213–12221. [Google Scholar] [CrossRef] [PubMed]
- Hosmillo, M.; Sweeney, T.R.; Chaudhry, Y.; Leen, E.; Curry, S.; Goodfellow, I.; Cho, K.O. The RNA helicase eIF4A is required for sapovirus translation. J. Virol. 2016, 90, 5200–5204. [Google Scholar] [CrossRef] [PubMed]
- Soliman, M.; Kim, D.S.; Park, J.G.; Kim, J.Y.; Alfajaro, M.M.; Baek, Y.B.; Cho, E.H.; Park, C.H.; Kang, M.I.; Park, S.I.; et al. PI3K/Akt and MEK/ERK signaling pathways facilitate sapovirus trafficking and late endosomal acidification for viral uncoating in LLC-PK cells. J. Virol. 2018, 92, JVI.01674-18. [Google Scholar] [CrossRef]
- Alfajaro, M.M.; Choi, J.S.; Kim, D.S.; Seo, J.Y.; Kim, J.Y.; Park, J.G.; Soliman, M.; Baek, Y.B.; Cho, E.H.; Kwon, J.; et al. Activation of COX-2/PGE2 promotes sapovirus replication via the inhibition of nitric oxide production. J. Virol. 2017, 91, e01656-16. [Google Scholar] [CrossRef]
- Chang, K.O.; Sosnovtsev, S.V.; Belliot, G.; Wang, Q.; Saif, L.J.; Green, K.Y. Reverse genetics system for porcine enteric calicivirus, a prototype sapovirus in the Caliciviridae. J. Virol. 2005, 79, 1409–1416. [Google Scholar] [CrossRef]
- Magwalivha, M.; Kabue, J.P.; Traore, A.N.; Potgieter, N. Prevalence of human sapovirus in low and middle income countries. Adv. Virol. 2018, 2018, 5986549. [Google Scholar] [CrossRef]
- Van Dycke, J.; Arnoldi, F.; Papa, G.; Vandepoele, J.; Burrone, O.R.; Mastrangelo, E.; Tarantino, D.; Heylen, E.; Neyts, J.; Rocha-Pereira, J. A single nucleoside viral polymerase inhibitor against norovirus, rotavirus, and sapovirus-induced diarrhea. J. Infect. Dis. 2018, 218, 1753–1758. [Google Scholar] [CrossRef]
- Le Pendu, J.; Abrantes, J.; Bertagnoli, S.; Guitton, J.S.; Le Gall-Reculé, G.; Lopes, A.M.; Marchandeau, S.; Alda, F.; Almeida, T.; Célio, A.P.; et al. Proposal for a unified classification system and nomenclature of lagoviruses. J. Gen. Virol. 2017, 98, 1658–1666. [Google Scholar] [CrossRef]
- Lopes, A.M.; Dalton, K.P.; Magalhães, M.J.; Parra, F.; Esteves, P.J.; Holmes, E.C.; Abrantes, J. Full genomic analysis of new variant rabbit hemorrhagic disease virus revealed multiple recombination events. J. Gen. Virol. 2015, 96 Pt 6, 1309–1319. [Google Scholar] [CrossRef]
- Hu, B.; Wang, F.; Fan, Z.; Song, Y.; Abrantes, J.; Zuo, Y.; Esteves, P.J. Recombination between G2 and G6 strains of rabbit hemorrhagic disease virus (RHDV) in China. Arch. Virol. 2017, 162, 269–272. [Google Scholar] [CrossRef]
- Silvério, D.; Lopes, A.M.; Melo-Ferreira, J.; Magalhães, M.J.; Monterroso, P.; Serronha, A.; Maio, E.; Alves, P.C.; Esteves, P.J.; Abrantes, J. Insights into the evolution of the new variant rabbit haemorrhagic disease virus (GI.2) and the identification of novel recombinant strains. Transbound. Emerg. Dis. 2018, 65, 983–992. [Google Scholar] [CrossRef]
- Hall, R.N.; Mahar, J.E.; Read, A.J.; Mourant, R.; Piper, M.; Huang, N.; Strive, T. A strain-specific multiplex RT-PCR for Australian rabbit haemorrhagic disease viruses uncovers a new recombinant virus variant in rabbits and hares. Transbound. Emerg. Dis. 2018, 65, e444–e456. [Google Scholar] [CrossRef]
- Lopes, A.M.; Rouco, C.; Esteves, P.J.; Abrantes, J. GI.1b/GI.1b/GI.2 recombinant rabbit hemorrhagic disease virus 2 (Lagovirus europaeus/GI.2) in Morocco, Africa. Arch. Virol. 2019, 164, 279–283. [Google Scholar] [CrossRef]
- Lopes, A.M.; Breiman, A.; Lora, M.; Le Moullac-Vaidye, B.; Galanina, O.; Nyström, K.; Marchandeau, S.; Le Gall-Reculé, G.; Strive, T.; Neimanis, A.; et al. Host specific glycans are correlated with susceptibility to infection by lagoviruses, but not with their virulence. J. Virol. 2018, 92, e01759-17. [Google Scholar] [CrossRef]
- Song, Y.; Wang, F.; Fan, Z.; Hu, B.; Liu, X.; Wei, H.; Xue, J.; Xu, W.; Qiu, R. Identification of novel rabbit hemorrhagic disease virus B-cell epitopes and their interaction with host histo-blood group antigens. J. Gen. Virol. 2016, 97, 356–365. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.; Fan, Z.; Zuo, Y.; Wei, H.; Hu, B.; Chen, M.; Qiu, R.; Xue, J.; Wang, F. Binding of rabbit hemorrhagic disease virus-like particles to host histo-blood group antigens is blocked by antisera from experimentally vaccinated rabbits. Arch. Virol. 2017, 162, 3425–3430. [Google Scholar] [CrossRef]
- Zhu, J.; Miao, Q.; Tang, J.; Wang, X.; Dong, D.; Liu, T.; Qi, R.; Yang, Z.; Liu, G. Nucleolin mediates the internalization of rabbit hemorrhagic disease virus through clathrin-dependent endocytosis. PLoS Pathog. 2018, 14, e1007383. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, B.; Miao, Q.; Tan, Y.; Li, C.; Chen, Z.; Guo, H.; Liu, G. Viral genome-linked protein (VPg) is essential for translation initiation of rabbit hemorrhagic disease virus (RHDV). PLoS ONE 2015, 10, e0143467. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.; Miao, Q.; Tan, Y.; Guo, H.; Liu, T.; Wang, B.; Chen, Z.; Li, C.; Liu, G. Inclusion of an Arg-Gly-Asp receptor-recognition motif into the capsid protein of rabbit hemorrhagic disease virus enables culture of the virus in vitro. J. Biol. Chem. 2017, 292, 8605–8615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, G.; Ni, Z.; Yun, T.; Yu, B.; Chen, L.; Zhao, W.; Hua, J.; Chen, J. A DNA-launched reverse genetics system for rabbit hemorrhagic disease virus reveals that the VP2 protein is not essential for virus infectivity. J. Gen. Virol. 2008, 89 Pt 12, 3080–3085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abrantes, J.; van der Loo, W.; Le Pendu, J.; Esteves, P.J. Rabbit haemorrhagic disease (RHD) and rabbit haemorrhagic disease virus (RHDV): A review. Vet. Res. 2012, 43, 12. [Google Scholar] [CrossRef] [PubMed]
- Neimanis, A.; Larsson Pettersson, U.; Huang, N.; Gavier-Widén, D.; Strive, T. Elucidation of the pathology and tissue distribution of Lagovirus europaeus GI.2/RHDV2 (rabbit haemorrhagic disease virus 2) in young and adult rabbits (Oryctolagus cuniculus). Vet. Res. 2018, 49, 46. [Google Scholar] [CrossRef] [PubMed]
- Rocha, G.; Alda, F.; Pagés, A.; Merchán, T. Experimental transmission of rabbit haemorrhagic disease virus (RHDV) from rabbit to wild mice (Mus spretus and Apodemus sylvaticus) under laboratory conditions. Infect. Genet. Evol. 2017, 47, 94–98. [Google Scholar] [CrossRef]
- Urakova, N.; Hall, R.; Strive, T.; Frese, M. Restricted host specificity of Rabbit Hemorrhagic Disease Virus is supported by challenge experiments in immune-compromised mice (Mus musculus). J. Wildl. Dis. 2019, 55, 218–222. [Google Scholar] [CrossRef]
- Henning, J.; Meers, J.; Davies, P.R. Exposure of rabbits to ultraviolet light-inactivated rabbit haemorrhagic disease virus (RHDV) and subsequent challenge with virulent virus. Epidemiol. Infect. 2005, 133, 731–735. [Google Scholar] [CrossRef] [Green Version]
- Mahar, J.E.; Hall, R.N.; Peacock, D.; Kovaliski, J.; Piper, M.; Mourant, R.; Huang, N.; Campbell, S.; Gu, X.; Read, A.; et al. Rabbit haemorrhagic disease virus 2 (GI.2) is replacing endemic strains of RHDV in the Australian landscape within 18 months of its arrival. J. Virol. 2018, 92, e01374-17. [Google Scholar]
- Laurent, S.; Vautherot, J.F.; Madelaine, M.F.; Le Gall, G.; Rasschaert, D. Recombinant rabbit hemorrhagic disease virus capsid protein expressed in baculovirus self-assembles into viruslike particles and induces protection. J. Virol. 1994, 68, 6794–6798. [Google Scholar]
- Marín, M.S.; Martín Alonso, J.M.; Pérez Ordoyo García, L.I.; Boga, J.A.; Argüello-Villares, J.L.; Casais, R.; Venugopal, K.; Jiang, W.; Gould, E.A.; Parra, F. Immunogenic properties of rabbit haemorrhagic disease virus structural protein VP60 expressed by a recombinant baculovirus: An efficient vaccine. Virus Res. 1995, 39, 119–128. [Google Scholar] [CrossRef]
- Boga, J.A.; Martín Alonso, J.M.; Casais, R.; Parra, F. A single dose immunization with rabbit haemorrhagic disease virus major capsid protein produced in Saccharomyces cerevisiae induces protection. J. Gen. Virol. 1997, 78 Pt 9, 2315–2318. [Google Scholar] [CrossRef] [PubMed]
- Castañón, S.; Marín, M.S.; Martín-Alonso, J.M.; Boga, J.A.; Casais, R.; Humara, J.M.; Ordás, R.J.; Parra, F. Immunization with potato plants expressing VP60 protein protects against rabbit hemorrhagic disease virus. J. Virol. 1999, 73, 4452–4455. [Google Scholar]
- Di Giallonardo, F.; Holmes, E.C. Viral biocontrol: Grand experiments in disease emergence and evolution. Trends Microbiol. 2015, 23, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Nicholson, L.J.; Mahar, J.E.; Strive, T.; Zheng, T.; Holmes, E.C.; Ward, V.K.; Duckworth, J.A. Benign rabbit calicivirus in New Zealand. Appl. Environ. Microbiol. 2017, 83, e00090-17. [Google Scholar] [CrossRef]
- Cooke, B.D.; Duncan, R.P.; McDonald, I.; Liu, J.; Capucci, L.; Mutze, G.J.; Strive, T. Prior exposure to non-pathogenic calicivirus RCV-A1 reduces both infection rate and mortality from rabbit haemorrhagic disease in a population of wild rabbits in Australia. Transbound. Emerg. Dis. 2018, 65, e470–e477. [Google Scholar] [CrossRef]
- Pedersen, N.C.; Elliott, J.B.; Glasgow, A.; Poland, A.; Keel, K. An isolated epizootic of hemorrhagic-like fever in cats caused by a novel and highly virulent strain of feline calicivirus. Vet. Microbiol. 2000, 73, 281–300. [Google Scholar] [CrossRef]
- Radford, A.D.; Coyne, K.P.; Dawson, S.; Porter, C.J.; Gaskell, R.M. Feline calicivirus. Vet. Res. 2007, 38, 319–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makino, A.; Shimojima, M.; Miyazawa, T.; Kato, K.; Tohya, Y.; Akashi, H. Junctional adhesion molecule 1 is a functional receptor for feline calicivirus. J. Virol. 2016, 80, 4482–4490. [Google Scholar] [CrossRef]
- Pesavento, P.; Liu, H.; Ossiboff, R.J.; Stucker, K.M.; Heymer, A.; Millon, L.; Wood, J.; van der List, D.; Parker, J.S. Characterization of a continuous feline mammary epithelial cell line susceptible to feline epitheliotropic viruses. J. Virol. Methods 2009, 157, 105–110. [Google Scholar] [CrossRef] [PubMed]
- Edwards, J.F.; Yedloutschnig, R.J.; Dardiri, A.H.; Callis, J.J. Vesicular exanthema of swine virus: Isolation and serotyping of field samples. Can. J. Vet. Res. 1987, 51, 358–362. [Google Scholar]
- Neill, J.D.; Meyer, R.F.; Seal, B.S. The capsid protein of vesicular exanthema of swine virus serotype A48: Relationship to the capsid protein of other animal caliciviruses. Virus Res. 1998, 54, 39–50. [Google Scholar] [CrossRef]
- Chen, R.; Neill, J.D.; Prasad, B.V. Crystallization and preliminary crystallographic analysis of San Miguel sea lion virus: An animal calicivirus. J. Struct. Biol. 2003, 141, 143–148. [Google Scholar] [CrossRef]
- Chen, R.; Neill, J.D.; Estes, M.K.; Prasad, B.V. X-ray structure of a native calicivirus: Structural insights into antigenic diversity and host specificity. Proc. Natl. Acad. Sci. USA 2006, 103, 8048–8053. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stuart, A.D.; Brown, T.D. α2,6-linked sialic acid acts as a receptor for feline calicivirus. J. Gen. Virol. 2007, 88 Pt 1, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Conley, M.J.; McElwee, M.; Azmi, L.; Gabrielsen, M.; Byron, O.; Goodfellow, I.G.; Bhella, D. Calicivirus VP2 forms a portal-like assembly following receptor engagement. Nature 2019, 565, 377–381. [Google Scholar] [CrossRef]
- Lu, Z.; Ledgerwood, E.D.; Hinchman, M.M.; Dick, R.; Parker, J.S.L. Conserved surface residues on the feline calicivirus capsid are essential for interaction with its receptor feline junctional adhesion molecule A (fJAM-A). J Virol. 2018, 92, e00035-18. [Google Scholar] [CrossRef] [PubMed]
- Hernández, B.A.; Sandoval-Jaime, C.; Sosnovtsev, S.V.; Green, K.Y.; Gutiérrez-Escolano, A.L. Nucleolin promotes in vitro translation of feline calicivirus genomic RNA. Virology 2016, 489, 51–62. [Google Scholar] [CrossRef]
- Alfajaro, M.M.; Cho, E.H.; Park, J.G.; Kim, J.Y.; Soliman, M.; Baek, Y.B.; Kang, M.I.; Park, S.I.; Cho, K.O. Feline calicivirus- and murine norovirus-induced COX-2/PGE2 signaling pathway has proviral effects. PLoS ONE 2018, 13, e0200726. [Google Scholar] [CrossRef]
- Yumiketa, Y.; Narita, T.; Inoue, Y.; Sato, G.; Kamitani, W.; Oka, T.; Katayama, K.; Sakaguchi, T.; Tohya, Y. Nonstructural protein p39 of feline calicivirus suppresses host innate immune response by preventing IRF-3 activation. Vet. Microbiol. 2016, 185, 62–67. [Google Scholar] [CrossRef]
- Humoud, M.N.; Doyle, N.; Royall, E.; Willcocks, M.M.; Sorgeloos, F.; van Kuppeveld, F.; Roberts, L.O.; Goodfellow, I.G.; Langereis, M.A.; Locker, N. Feline calicivirus infection disrupts assembly of cytoplasmic stress granules and induces G3BP1 cleavage. J. Virol. 2016, 90, 6489–6501. [Google Scholar] [CrossRef]
- Wu, H.; Zu, S.; Sun, X.; Liu, Y.; Tian, J.; Qu, L. N-terminal domain of feline calicivirus (FCV) proteinase-polymerase contributes to the inhibition of host cell transcription. Viruses 2016, 8, 199. [Google Scholar] [CrossRef] [PubMed]
- Oka, T.; Takagi, H.; Tohya, Y. Development of a novel single step reverse genetics system for feline calicivirus. J. Virol. Methods 2014, 207, 178–181. [Google Scholar] [CrossRef] [PubMed]
- Sandoval-Jaime, C.; Green, K.Y.; Sosnovtsev, S.V. Recovery of murine norovirus and feline calicivirus from plasmids encoding EMCV IRES in stable cell lines expressing T7 polymerase. J. Virol. Methods 2015, 217, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Afonso, M.M.; Pinchbeck, G.L.; Smith, S.L.; Daly, J.M.; Gaskell, R.M.; Dawson, S.; Radford, A.D. A multi-national European cross-sectional study of feline calicivirus epidemiology, diversity and vaccine cross-reactivity. Vaccine 2017, 35, 2753–2760. [Google Scholar] [CrossRef] [PubMed]
- Coyne, K.P.; Gaskell, R.M.; Dawson, S.; Porter, C.J.; Radford, A.D. Evolutionary mechanisms of persistence and diversification of a calicivirus within endemically infected natural host populations. J. Virol. 2007, 81, 1961–1971. [Google Scholar] [CrossRef]
- Masubuchi, K.; Wakatsuki, A.; Iwamoto, K.; Takahashi, T.; Kokubu, T.; Shimizu, M. Immunological and genetic characterization of feline caliciviruses used in the development of a new trivalent inactivated vaccine in Japan. J. Vet. Med. Sci. 2010, 72, 1189–1194. [Google Scholar] [CrossRef]
- Wensman, J.J.; Samman, A.; Lindhe, A.; Thibault, J.C.; Berndtsson, L.T.; Hosie, M.J. Ability of vaccine strain induced antibodies to neutralize field isolates of caliciviruses from Swedish cats. Acta Vet. Scand. 2015, 57, 86, Erratum in: Acta Vet. Scand. 2016, 58, 14. [Google Scholar] [CrossRef]
- Sato, H.; Sehata, G.; Okada, N.; Iwamoto, K.; Masubuchi, K.; Kainuma, R.; Noda, T.; Igarashi, T.; Sawada, T.; Noro, T.; et al. Intranasal immunization with inactivated feline calicivirus particles confers robust protection against homologous virus and suppression against heterologous virus in cats. J. Gen. Virol. 2017, 98, 1730–1738. [Google Scholar] [CrossRef]
- Martella, V.; Pinto, P.; Lorusso, E.; Di Martino, B.; Wang, Q.; Larocca, V.; Cavalli, A.; Camero, M.; Decaro, N.; Bányai, K.; et al. Detection and full-length genome characterization of novel canine vesiviruses. Emerg. Infect. Dis. 2015, 21, 1433–1436. [Google Scholar] [CrossRef]
- Renshaw, R.W.; Griffing, J.; Weisman, J.; Crofton, L.M.; Laverack, M.A.; Poston, R.P.; Duhamel, G.E.; Dubovi, E.J. Characterization of a vesivirus associated with an outbreak of acute hemorrhagic gastroenteritis in domestic dogs. J. Clin. Microbiol. 2018, 56, e01951-17. [Google Scholar] [CrossRef]
- Di Martino, B.; Di Profio, F.; Melegari, I.; Sarchese, V.; Massirio, I.; Luciani, A.; Lanave, G.; Marsilio, F.; Martella, V. Serological and molecular investigation of 2117-like vesiviruses in cats. Arch. Virol. 2018, 163, 197–201. [Google Scholar] [CrossRef]
- Plavsic, M.; Shick, K.; Bergmann, K.F.; Mallet, L. Vesivirus 2117: Cell line infectivity range and effectiveness of amplification of a potential adventitious agent in cell culture used for biological production. Biologicals 2016, 44, 540–545. [Google Scholar] [CrossRef]
- Conley, M.; Emmott, E.; Orton, R.; Taylor, D.; Carneiro, D.G.; Murata, K.; Goodfellow, I.G.; Hansman, G.S.; Bhella, D. Vesivirus 2117 capsids more closely resemble sapovirus and lagovirus particles than other known vesivirus structures. J. Gen. Virol. 2017, 98, 68–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sosnovtsev, S.V.; Sandoval-Jaime, C.; Parra, G.I.; Tin, C.M.; Jones, R.W.; Soden, J.; Barnes, D.; Freeth, J.; Smith, A.W.; Green, K.Y. Identification of human junctional adhesion molecule 1 as a functional receptor for the Hom-1 calicivirus on human cells. MBio 2017, 8, e00031-17. [Google Scholar] [CrossRef]
- Smith, A.W.; Iversen, P.L.; Skilling, D.E.; Stein, D.A.; Bok, K.; Matson, D.O. Vesivirus viremia and seroprevalence in humans. J. Med. Virol. 2006, 78, 693–701. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Cho, Y.H.; Park, J.S.; Kim, E.C.; Smith, A.W.; Ko, G. Elevated post-transfusion serum transaminase values associated with a highly significant trend for increasing prevalence of anti-Vesivirus antibody in Korean patients. J. Med. Virol. 2012, 84, 1943–1952. [Google Scholar] [CrossRef] [PubMed]
- Oliver, S.L.; Asobayire, E.; Dastjerdi, A.M.; Bridger, J.C. Genomic characterization of the unclassified bovine enteric virus Newbury agent-1 (Newbury1) endorses a new genus in the family Caliciviridae. Virology 2006, 350, 240–250. [Google Scholar] [CrossRef] [Green Version]
- Cho, E.H.; Soliman, M.; Alfajaro, M.M.; Kim, J.Y.; Seo, J.Y.; Park, J.G.; Kim, D.S.; Baek, Y.B.; Kang, M.I.; Park, S.I.; et al. Bovine nebovirus interacts with a wide spectrum of histo-blood group antigens. J. Virol. 2018, 92, e02160-17. [Google Scholar] [CrossRef]
- Di Martino, B.; Di Profio, F.; Martella, V.; Ceci, C.; Marsilio, F. Evidence for recombination in neboviruses. Vet. Microbiol. 2011, 153, 367–372. [Google Scholar] [CrossRef]
- Guo, Z.; He, Q.; Yue, H.; Zhang, B.; Tang, C. Genomic characterization of a RdRp-recombinant nebovirus strain with a novel VP1 genotype. Virus Res. 2018, 251, 6–13. [Google Scholar] [CrossRef]
- Thomas, C.; Jung, K.; Han, M.G.; Hoet, A.; Scheuer, K.; Wang, Q.; Saif, L.J. Retrospective serosurveillance of bovine norovirus (GIII.2) and nebovirus in cattle from selected feedlots and a veal calf farm in 1999 to 2001 in the United States. Arch. Virol. 2014, 159, 83–90. [Google Scholar] [CrossRef]
- Candido, M.; Alencar, A.L.; Almeida-Queiroz, S.R.; Buzinaro, M.G.; Munin, F.S.; Godoy, S.H.; Livonesi, M.C.; Fernandes, A.M.; Sousa, R.L. First detection and molecular characterization of Nebovirus in Brazil. Epidemiol. Infect. 2016, 144, 1876–1878. [Google Scholar] [CrossRef]
- Turan, T.; Işıdan, H.; Atasoy, M.O.; Irehan, B. Detection and molecular analysis of bovine enteric norovirus and nebovirus in Turkey. J. Vet. Res. 2018, 62, 129–135. [Google Scholar] [CrossRef] [PubMed]
- Pourasgari, F.; Kaplon, J.; Sanchooli, A.; Fremy, C.; Karimi-Naghlani, S.; Otarod, V.; Ambert-Balay, K.; Mojgani, N.; Pothier, P. Molecular prevalence of bovine noroviruses and neboviruses in newborn calves in Iran. Arch. Virol. 2018, 163, 1271–1277. [Google Scholar] [CrossRef]
- Park, S.I.; Jeong, C.; Park, S.J.; Kim, H.H.; Jeong, Y.J.; Hyun, B.H.; Chun, Y.H.; Kang, M.I.; Cho, K.O. Molecular detection and characterization of unclassified bovine enteric caliciviruses in South Korea. Vet. Microbiol. 2008, 130, 371–379. [Google Scholar] [CrossRef]
- Guo, Z.; He, Q.; Yue, H.; Zhang, B.; Tang, C. First detection of nebovirus and norovirus from cattle in China. Arch. Virol. 2018, 163, 475–478. [Google Scholar] [CrossRef]
- Guo, Z.; He, Q.; Zhang, B.; Yue, H.; Tang, C. Detection and molecular characteristics of neboviruses in dairy cows in China. J Gen Virol. 2018. [Google Scholar] [CrossRef]
- Farkas, T.; Sestak, K.; Wei, C.; Jiang, X. Characterization of a rhesus monkey calicivirus representing a new genus of Caliciviridae. J. Virol. 2008, 82, 5408–5416. [Google Scholar] [CrossRef]
- Farkas, T.; Lun, C.W.; Fey, B. Relationship between genotypes and serotypes of genogroup 1 recoviruses: A model for human norovirus antigenic diversity. J. Gen. Virol. 2014, 95 Pt 7, 1469–1478. [Google Scholar] [CrossRef]
- Farkas, T.; Cross, R.W.; Hargitt, E., 3rd; Lerche, N.W.; Morrow, A.L.; Sestak, K. Genetic diversity and histo-blood group antigen interactions of rhesus enteric caliciviruses. J. Virol. 2010, 84, 8617–8625. [Google Scholar] [CrossRef] [PubMed]
- Sestak, K. Role of histo-blood group antigens in primate enteric calicivirus infections. World J. Virol. 2014, 3, 18–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, D.; Huang, P.; Zou, L.; Lowary, T.L.; Tan, M.; Jiang, X. Tulane virus recognizes the A type 3 and B histo-blood group antigens. J. Virol. 2015, 89, 1419–1427. [Google Scholar] [CrossRef] [PubMed]
- Wei, C.; Farkas, T.; Sestak, K.; Jiang, X. Recovery of infectious virus by transfection of in vitro-generated RNA from tulane calicivirus cDNA. J. Virol. 2008, 82, 11429–11436. [Google Scholar] [CrossRef] [PubMed]
- Farkas, T. Rhesus enteric calicivirus surrogate model for human norovirus gastroenteritis. J. Gen. Virol. 2015, 96 Pt 7, 1504–1514. [Google Scholar] [CrossRef]
- Chhabra, P.; Ranjan, P.; Cromeans, T.; Sambhara, S.; Vinjé, J. Critical role of RIG-I and MDA5 in early and late stages of Tulane virus infection. J. Gen. Virol. 2017, 98, 1016–1026. [Google Scholar] [CrossRef] [Green Version]
- Siqueira, J.D.; Dominguez-Bello, M.G.; Contreras, M.; Lander, O.; Caballero-Arias, H.; Xutao, D.; Noya-Alarcon, O.; Delwart, E. Complex virome in feces from Amerindian children in isolated Amazonian villages. Nat. Commun. 2018, 9, 4270. [Google Scholar] [CrossRef]
- Farkas, T.; Wong Ping Lun, C. Prevalence of recovirus-neutralizing antibodies in human serum samples. J. Clin. Microbiol. 2014, 52, 3088–3090. [Google Scholar] [CrossRef]
- Kocher, J.F.; Lindesmith, L.C.; Debbink, K.; Beall, A.; Mallory, M.L.; Yount, B.L.; Graham, R.L.; Huynh, J.; Gates, J.E.; Donaldson, E.F.; et al. Bat caliciviruses and human noroviruses are antigenically similar and have overlapping histo-blood group antigen binding profiles. MBio 2018, 9, e00869-18. [Google Scholar] [CrossRef]

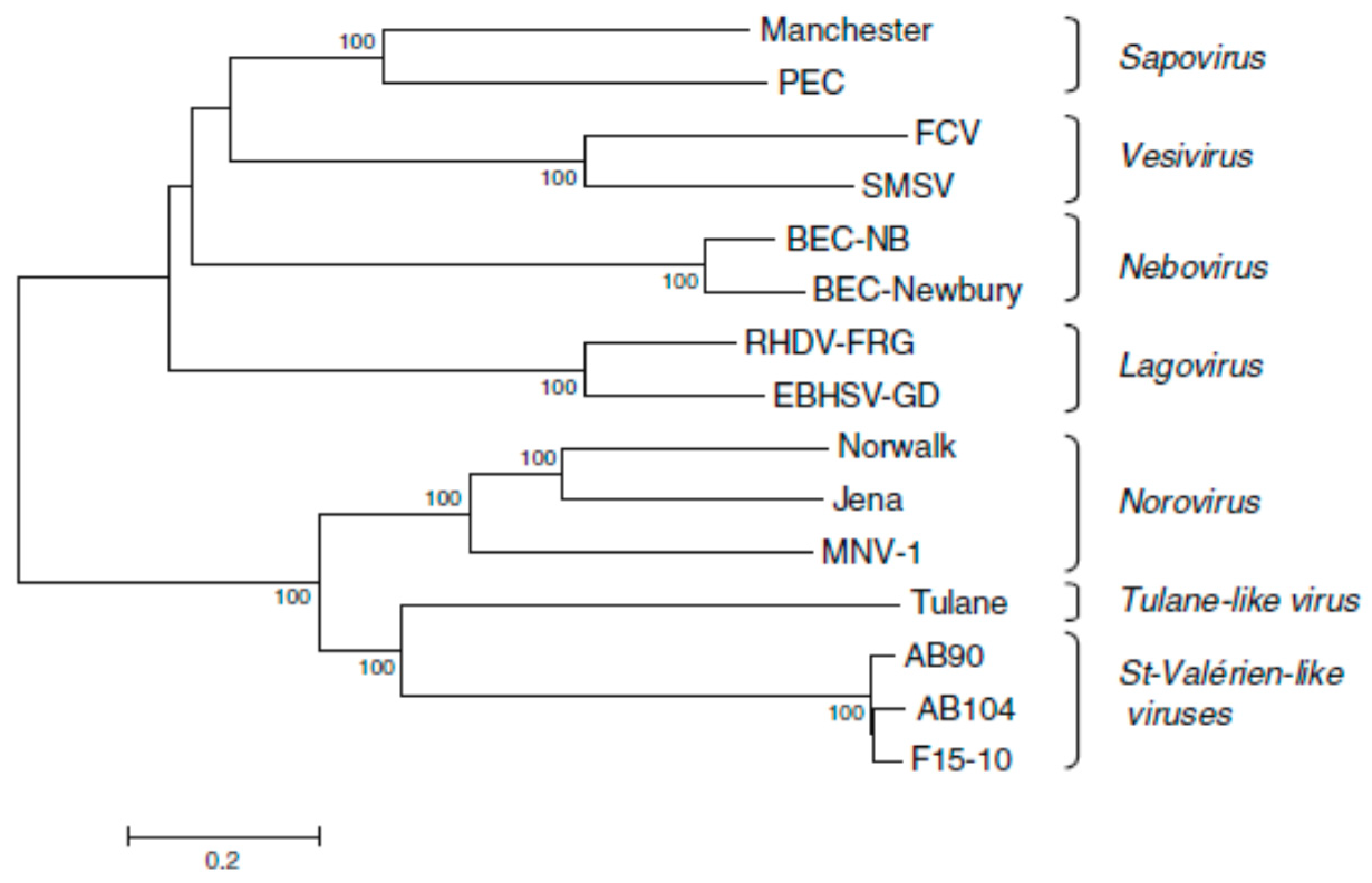

| Genus | Type Species |
|---|---|
| Established: | |
| Norovirus (NoV) | Norwalk virus (NV) |
| Sapovirus (SaV) | Sapporo virus (SV) |
| Lagovirus (LaV) | Rabbit hemorrhagic disease virus (RHDV) |
| European brown hare syndrome virus (EBHSV) | |
| Vesivirus (VeV) | Vesicular exanthema of swine virus (VESV) |
| Feline calicivirus (FCV) | |
| San Miguel sea lion virus (SMSV) | |
| Nebovirus (NeV) | Newbury-1 virus (NBV) |
| Proposed: | |
| Recovirus (ReV) | Tulane virus (simian) |
| Valovirus (VaV) | St Valérian virus (porcine) |
| Bavovirus (BaV) | Bayern virus (avian) |
| Nacovirus (NaV) | Novel avian calicivirus (chicken, turkey) |
| Salovirus (SaV) | Atlantic salmon calicivirus (salmon) |
| Minovirus (MiV) | Fathead minnow calicivirus (minnow) |
| Genus | Cell/Enteroid Culture | Helper Virus-Free RG System | Animal Model |
|---|---|---|---|
| Norovirus | |||
| MuNoV | + [1] | + [2,3,4,5] | + [6,7,8,9] |
| HuNoV | + [10,11] | (+) to be published | (+) [12] |
| Sapovirus | + porcine [46] | + [55] | + [37] |
| - human [47] | |||
| Lagovirus | + [69] | + [70] | + [75] |
| Vesivirus | + [86,87,110] | + [100,101] | + [103] |
| Nebovirus | To be done | To be done | To be done |
| Recovirus | + [128] | + [131] | To be done |
© 2019 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Desselberger, U. Caliciviridae Other Than Noroviruses. Viruses 2019, 11, 286. https://doi.org/10.3390/v11030286
Desselberger U. Caliciviridae Other Than Noroviruses. Viruses. 2019; 11(3):286. https://doi.org/10.3390/v11030286
Chicago/Turabian StyleDesselberger, Ulrich. 2019. "Caliciviridae Other Than Noroviruses" Viruses 11, no. 3: 286. https://doi.org/10.3390/v11030286
APA StyleDesselberger, U. (2019). Caliciviridae Other Than Noroviruses. Viruses, 11(3), 286. https://doi.org/10.3390/v11030286





