Porcine Parvovirus Infection Causes Pig Placenta Tissue Damage Involving Nonstructural Protein 1 (NS1)-Induced Intrinsic ROS/Mitochondria-Mediated Apoptosis
Abstract
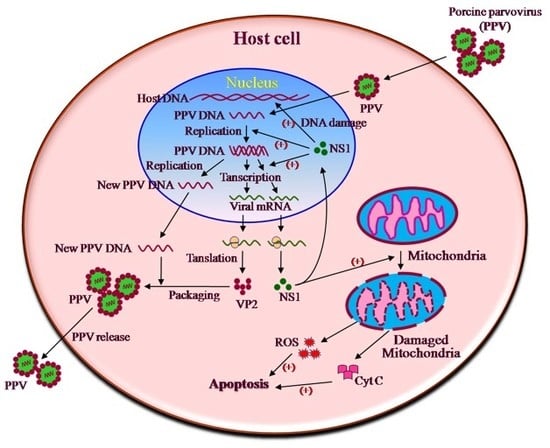
:1. Introduction
2. Materials and Methods
2.1. Plasmid, Virus, Cells, and Tissue
2.2. Reagents
2.3. Preparation and Staining of Pig Placenta Tissue Sections
2.4. Detection of Apoptosis in Placenta Tissues
2.5. Construction of PPV NS1, VP1, and VP2 Expression Vectors
2.6. Infection and Transfection
2.7. Cell Viability Assay
2.8. Mitochondria and Cytosol Fractionation
2.9. Western Blot
2.10. Annexin V/PI Assay
2.11. Detection of Caspase-3, -8, and -9 Activities
2.12. Intracellular ROS and Mitochondrial ROS Detections
2.13. Mitochondrial Damage Measurement
2.14. Mitochondrial Membrane Potential (Δψm) Measurement
2.15. DNA Damage Detection
2.16. Cell Cycle Analysis
2.17. Statistical Analysis
3. Results
3.1. PPV Infection Induces Placenta Tissue Apoptosis and Damage in the Pregnant Sows
3.2. PPV-Induced Apoptosis of PK-15 Cells is Mainly Related to NS1 Protein
3.3. NS1 Induces Intrinsic Apoptosic Pathway in the PK-15 Cells
3.4. NS1 Induce Intracellular and Mitochondrial ROS Accumulations
3.5. ROS Accumulation Is Mainly Involved in NS1-Induced Apoptosis
3.6. PPV- and NS1-Induced ROS Accumulation Induces DNA Damage Leading to Cell Cycle Arrest at the G1 and G2 Phases
3.7. PPV- and NS1-Induced ROS Accumulation Causes Mitochondrial Damage
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mengeling, W.L.; Lager, K.M.; Vorwald, A.C. The effect of porcine parvovirus and porcine reproductive and respiratory syndrome virus on porcine reproductive performance. Anim. Reprod. Sci. 2000, 60–61, 199–210. [Google Scholar] [CrossRef]
- Zeeuw, E.J.; Leinecker, N.; Herwig, V.; Selbitz, H.J.; Truyen, U. Study of the virulence and cross-neutralization capability of recent porcine parvovirus field isolates and vaccine viruses in experimentally infected pregnant gilts. J. Gen. Virol. 2007, 88, 420–427. [Google Scholar] [CrossRef] [PubMed]
- Ji, P.; Liu, Y.; Chen, Y.; Wang, A.; Jiang, D.; Zhao, B.; Wang, J.; Chai, S.; Zhou, E.; Zhang, G. Porcine parvovirus capsid protein expressed in Escherichia coli self-assembles into virus-like particles with high immunogenicity in mice and guinea pigs. Antivir. Res. 2017, 139, 146–152. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Cui, L.; Tian, C.; Zhang, G.; Huo, G.; Tang, L.; Li, Y. Immunogenicity of recombinant classic swine fever virus CD8(+) T lymphocyte epitope and porcine parvovirus VP2 antigen coexpressed by Lactobacillus casei in swine via oral vaccination. Clin. Vaccine. Immunol. 2011, 18, 1979–1986. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Yao, G.; Cui, S. Production and purification of VP2 protein of porcine parvovirus expressed in an insect-baculovirus cell system. Virol. J. 2010, 7. [Google Scholar] [CrossRef]
- Oh, W.T.; Kim, R.Y.; Nguyen, V.G.; Chung, H.C.; Park, B.K. Perspectives on the Evolution of Porcine Parvovirus. Viruses 2017, 9, 196. [Google Scholar] [CrossRef]
- Fernandes, S.; Boisvert, M.; Szelei, J.; Tijssen, P. Differential replication of two porcine parvovirus strains in bovine cell lines ensues from initial DNA processing and NS1 expression. J. Gen. Virol. 2014, 95, 910–921. [Google Scholar] [CrossRef]
- Eichwald, V.; Daeffler, L.; Kelein, M.; Rommelaere, J.; Salomeé, N. The NS2 proteins of parvovirus minute virus of mice are required for efficient nuclear egress of progeny virions in mouse celle. J. Virol. 2002, 76, 10307–10319. [Google Scholar] [CrossRef] [PubMed]
- Kresse, J.I.; Taylor, W.D.; Stewart, W.W.; Eernisse, K.A. Parvovirus infection in pigs with necrotic and vesicle-like lesions. Vet. Microbiol. 1985, 10, 525–531. [Google Scholar] [CrossRef]
- Van Leengoed, L.A.; Vos, J.; Gruys, E.; Rondhuis, P.; Brand, A. Porcine Parvovirus infection: Review and diagnosis in a sow herd with reproductive failure. Vet. Q. 1983, 5, 131–141. [Google Scholar] [CrossRef]
- Mincberg, M.; Gopas, J.; Tal, J. Minute virus of mice (MVMp) infection and NS1 expression induce p53 independent apoptosis in transformed rat fibroblast cells. Virology 2011, 412, 233–243. [Google Scholar] [CrossRef]
- Saxena, L.; Kumar, G.R.; Saxena, S.; Chaturvedi, U.; Sahoo, A.P.; Singh, L.V.; Santra, L.; Palia, S.K.; Desai, G.S.; Tiwari, A.K. Apoptosis induced by NS1 gene of Canine Parvovirus-2 is caspase dependent and p53 independent. Virus. Res. 2013, 173, 426–430. [Google Scholar] [CrossRef]
- Thammasri, K.; Rauhamäki, S.; Wang, L.; Filippou, A.; Kivovich, V.; Marjomäki, V.; Naides, S.J.; Gilbert, L. Human Parvovirus B19 Induced Apoptotic Bodies Contain Altered Self-Antigens that are Phagocytosed by Antigen Presenting Cells. PLoS ONE 2013, 8, e67179. [Google Scholar] [CrossRef]
- Zhang, H.; Huang, Y.; Du, Q.; Luo, X.; Zhang, L.; Zhao, X.; Tong, D. Porcine parvovirus infection induces apoptosis in PK-15 cells through activation of p53 and mitochondria-mediated pathway. Biochem. Biophys. Res. Commun. 2015, 456, 649–655. [Google Scholar] [CrossRef]
- Zhao, X.; Xiang, H.; Bai, X.; Fei, N.; Huang, Y.; Song, X.; Zhang, H.; Zhang, L.; Tong, D. Porcine parvovirus infection activates mitochondria-mediated apoptotic signaling pathway by inducing ROS accumulation. Virol. J. 2016, 13, 26. [Google Scholar] [CrossRef]
- Aranda, A.; Sequedo, L.; Tolosa, L.; Quintas, G.; Burello, E.; Castell, J.V.; Gombau, L. Dichloro-dihydro- fluorescein diacetate (DCFH-DA) assay: A quantitative method for oxidative stress assessment of nanoparticle-treated cells. Toxicol. In Vitro. 2013, 27, 954–963. [Google Scholar] [CrossRef]
- Zhong, Z.; Umemura, A.; Sanchez-Lopez, E.; Liang, S.; Shalapour, S.; Wong, J.; He, F.; Boassa, D.; Perkins, G.; Ali, S.R.; et al. NF-κB Restricts Inflammasome Activation via Elimination of Damaged Mitochondria. Cell 2016, 164, 896–910. [Google Scholar] [CrossRef]
- Ji, J.; Zhang, Y.; Redon, C.E.; Reinhold, W.C.; Chen, A.P.; Fogli, L.K.; Holbeck, S.L.; Parchment, R.E.; Hollingshead, M.; Tomaszewski, J.E.; et al. Phosphorylated fraction of H2AX as a measurement for DNA damage in cancer cells and potential applications of a novel assay. PLoS ONE 2017, 12, e0171582. [Google Scholar] [CrossRef]
- Hristov, G.; Krämer, M.; Li, J.; El-Andaloussi, N.; Mora, R.; Daeffler, L.; Zentgraf, H.; Rommelaere, J.; Marchini, A. Through its nonstructural protein NS1, parvovirus H-1 induces apoptosis via accumulation of reactive oxygen species. J. Virol. 2010, 84, 5909–5922. [Google Scholar] [CrossRef]
- Chen, A.Y.; Zhang, E.Y.; Guan, W.; Cheng, F.; Kleiboeker, S.; Yankee, T.M.; Qiu, J. The small 11 kDa nonstructural protein of human parvovirus B19 plays a key role in inducing apoptosis during B19 virus infection of primary erythroid progenitor cells. Blood 2010, 115, 1070–1080. [Google Scholar] [CrossRef]
- Kinner, A.; Wu, W.; Staudt, C.; Iliakis, G. Gamma-H2AX in recognition and signaling of DNA double-strand breaks in the context of chromatin. Nucleic Acids Res. 2008, 36, 5678–5694. [Google Scholar] [CrossRef]
- Lucantoni, F.; Düssmann, H.; Llorente-Folch, I.; Prehn, J.H.M. BCL2 and BCL(X)L selective inhibitors decrease mitochondrial ATP production in breast cancer cells and are synthetically lethal when combined with 2-deoxy-D-glucose. Oncotarget 2018, 9, 26046–26063. [Google Scholar] [CrossRef]
- Nykky, J.; Vuento, M.; Gilbert, L. Role of mitochondria in parvovirus pathology. PLoS ONE 2014, 9, e86124. [Google Scholar] [CrossRef]
- Narendra, D.P.; Jin, S.M.; Tanaka, A.; Suen, D.F.; Gautier, C.A.; Shen, J.; Cookson, M.R.; Youle, R.J. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol. 2010, 8, e1000298. [Google Scholar] [CrossRef]
- Zhong, Z.; Liang, S.; Sanchez-Lopez, E.; He, F.; Shalapour, S.; Lin, X.J.; Wong, J.; Ding, S.; Seki, E.; Schnabl, B.; et al. New mitochondrial DNA synthesis enables NLRP3 inflammasome activation. Nature 2018, 560, 198–203. [Google Scholar] [CrossRef]
- Meyer, A.; Laverny, G.; Bernardi, L.; Charles, A.L.; Alsaleh, G.; Pottecher, J.; Sibilia, J.; Geny, B. Mitochondria: An Organelle of Bacterial Origin Controlling Inflammation. Front. Immunol. 2018, 9, 536. [Google Scholar] [CrossRef]
- Meyer, J.N.; Hartman, J.H.; Mello, D.F. Mitochondrial Toxicity. Toxicol. Sci. 2018, 162, 15–23. [Google Scholar] [CrossRef]
- Meyer, J.N.; Leuthner, T.C.; Luz, A.L. Mitochondrial fusion, fission, and mitochondrial toxicity. Toxicology 2017, 391, 42–53. [Google Scholar] [CrossRef]
- Youle, R.J.; van der Bliek, A.M. Mitochondrial fission, fusion, and stress. Science 2012, 337, 1062–1065. [Google Scholar] [CrossRef]
- Ornoy, A.; Ergaz, Z. Parvovirus B19 infection during pregnancy and risks to the fetus. Birth. Defects. Res. 2017, 109, 311–323. [Google Scholar] [CrossRef]
- Kilian, E.; Suchodolski, J.S.; Hartmann, K.; Mueller, R.S.; Wess, G.; Unterer, S. Long-term effects of canine parvovirus infection in dogs. PLoS ONE 2018, 13, e0192198. [Google Scholar] [CrossRef] [PubMed]
- Doley, J.; Singh, L.V.; Kumar, G.R.; Sahoo, A.P.; Saxena, L.; Chaturvedi, U.; Saxena, S.; Kumar, R.; Singh, P.K.; Rajmani, R.S.; et al. Canine Parvovirus Type 2a(CPV-2a)-Induced Apoptosis in MDCK Involves Both Extrinsic and Intrinsic Pathways. Appl. Biochem. Biotechnol. 2014, 172, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Tzang, B.S.; Chiang, S.Y.; Chan, H.C.; Liu, C.H.; Hsu, T.C. Human parvovirus B19 antibodies induce altered membrane protein expression and apoptosis of BeWo trophoblasts. Mol. Med. Rep. 2016, 14, 4399–4406. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, Z.; Zhang, J.; Luo, X.; Du, Q.; Chang, L.; Zhao, X.; Huang, Y.; Tong, D. Porcine parvovirus infection impairs progesterone production in luteal cells through mitogen-activated protein kinases, p53, and mitochondria-mediated apoptosis. Biol. Reprod. 2018, 98, 558–569. [Google Scholar] [CrossRef]
- Pan, H.; Zhong, F.; Pan, S.; Li, X.; Zhang, F.; Zhang, K.; Chen, H. Non-structural protein NS1 of canine parvovirus induces the apoptosis of cells. Wei. Sheng. Wu. Xue. Bao. 2012, 52, 367–373. [Google Scholar] [PubMed]










| Gene | Primer Name | Primer Sequence (5′→3′) | Size/bp | Restriction Site |
|---|---|---|---|---|
| NS1 | NS1-F | CGGGGTACCACCATGGCAGCGGGAAACACTTAC | 2007 | Kpn I |
| NS1-Rns | CCGACCGGTTTCAAGGTTTGTTGTGGGTGC | Age I | ||
| VP1 | VP1-F | CGGGGTACCACCATGGCGCCTCCTGCAAAAAGAGCA | 2179 | Kpn I |
| VP1-Rns | CCGACCGGTGTATAATTTTCTTGGTATAAGTTG | Age I | ||
| VP2 | VP2-F | CGGGGTACCACCATGAGTGAAAATGTGGAACAAC | 1758 | Kpn I |
| VP2-Rns | CCGACCGGTGTATAATTTTCTTGGTATAAGTTG | Age I |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, J.; Fan, J.; Li, Y.; Liang, S.; Huo, S.; Wang, X.; Zuo, Y.; Cui, D.; Li, W.; Zhong, Z.; et al. Porcine Parvovirus Infection Causes Pig Placenta Tissue Damage Involving Nonstructural Protein 1 (NS1)-Induced Intrinsic ROS/Mitochondria-Mediated Apoptosis. Viruses 2019, 11, 389. https://doi.org/10.3390/v11040389
Zhang J, Fan J, Li Y, Liang S, Huo S, Wang X, Zuo Y, Cui D, Li W, Zhong Z, et al. Porcine Parvovirus Infection Causes Pig Placenta Tissue Damage Involving Nonstructural Protein 1 (NS1)-Induced Intrinsic ROS/Mitochondria-Mediated Apoptosis. Viruses. 2019; 11(4):389. https://doi.org/10.3390/v11040389
Chicago/Turabian StyleZhang, Jianlou, Jinghui Fan, Yan Li, Shuang Liang, Shanshan Huo, Xing Wang, Yuzhu Zuo, Dan Cui, Wenyan Li, Zhenyu Zhong, and et al. 2019. "Porcine Parvovirus Infection Causes Pig Placenta Tissue Damage Involving Nonstructural Protein 1 (NS1)-Induced Intrinsic ROS/Mitochondria-Mediated Apoptosis" Viruses 11, no. 4: 389. https://doi.org/10.3390/v11040389
APA StyleZhang, J., Fan, J., Li, Y., Liang, S., Huo, S., Wang, X., Zuo, Y., Cui, D., Li, W., Zhong, Z., & Zhong, F. (2019). Porcine Parvovirus Infection Causes Pig Placenta Tissue Damage Involving Nonstructural Protein 1 (NS1)-Induced Intrinsic ROS/Mitochondria-Mediated Apoptosis. Viruses, 11(4), 389. https://doi.org/10.3390/v11040389