Novel Human Astroviruses: Prevalence and Association with Common Enteric Viruses in Undiagnosed Gastroenteritis Cases in Spain
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient and Sample Population
2.2. Viral RNA Extraction and Real Time Polymerase- or Reverse-Transcription Polymerase Chain Reaction (Real-Time PCR or Real-Time RT-PCR) Assays for Novel Astroviruses and other Enteric Viruses
2.3. Statistical Analyses
3. Results
3.1. More than Half of Samples Undiagnosed by Routine Screening are Positive by Real-Time PCR- or RT-PCR Assays
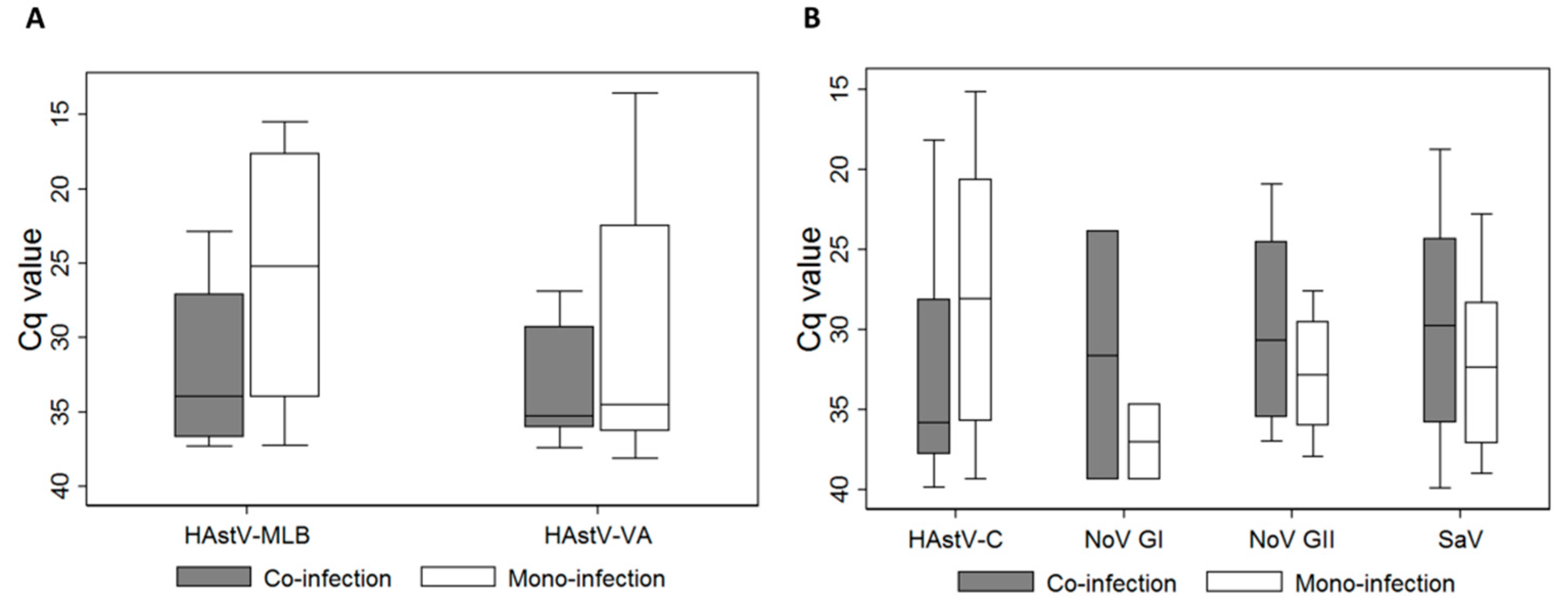
3.2. Diverse Novel HAstVs are Circulating in the Pa]ediatric Population, often in Children under 2 Years Old and in Co-Infection with other Enteric Viruses
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Aliabadi, N.; Tate, J.E.; Haynes, A.K.; Parashar, U.D. Sustained decrease in laboratory detection of rotavirus after implementation of routine vaccination-united states, 2000–2014. MMWR Morb. Mortal. Wkly. Rep. 2015, 64, 337–342. [Google Scholar] [PubMed]
- Appleton, H.; Higgins, P.G. Letter: Viruses and gastroenteritis in infants. Lancet 1975, 1, 1297. [Google Scholar] [CrossRef]
- Bosch, A.; Pinto, R.M.; Guix, S. Human astroviruses. Clin. Microbiol. Rev. 2014, 27, 1048–1074. [Google Scholar] [CrossRef] [PubMed]
- Vu, D.L.; Bosch, A.; Pinto, R.M.; Guix, S. Epidemiology of classic and novel human astrovirus: Gastroenteritis and beyond. Viruses 2017, 9, 33. [Google Scholar] [CrossRef] [PubMed]
- Finkbeiner, S.R.; Holtz, L.R.; Jiang, Y.; Rajendran, P.; Franz, C.J.; Zhao, G.; Kang, G.; Wang, D. Human stool contains a previously unrecognized diversity of novel astroviruses. Virol. J. 2009, 6, 161. [Google Scholar] [CrossRef] [PubMed]
- Phan, T.G.; Nordgren, J.; Ouermi, D.; Simpore, J.; Nitiema, L.W.; Deng, X.; Delwart, E. New astrovirus in human feces from burkina faso. J. Clin. Virol. 2014, 60, 161–164. [Google Scholar] [CrossRef] [PubMed]
- Medici, M.C.; Tummolo, F.; Calderaro, A.; Elia, G.; Banyai, K.; de Conto, F.; Arcangeletti, M.C.; Chezzi, C.; Buonavoglia, C.; Martella, V. Mlb1 astrovirus in children with gastroenteritis, italy. Emerg. Infect. Dis. 2014, 20, 169–170. [Google Scholar] [CrossRef] [PubMed]
- Mitui, M.T.; Bozdayi, G.; Matsumoto, T.; Dalgic, B.; Nishizono, A.; Ahmed, K. Complete genome sequence of an mlb2 astrovirus from a turkish child with diarrhea. Genome Announc. 2013, 1, e00619-13. [Google Scholar] [CrossRef] [PubMed]
- Meyer, C.T.; Bauer, I.K.; Antonio, M.; Adeyemi, M.; Saha, D.; Oundo, J.O.; Ochieng, J.B.; Omore, R.; Stine, O.C.; Wang, D.; et al. Prevalence of classic, mlb-clade and va-clade astroviruses in kenya and the gambia. Virol. J. 2015, 12, 78. [Google Scholar] [CrossRef]
- Holtz, L.R.; Bauer, I.K.; Rajendran, P.; Kang, G.; Wang, D. Astrovirus mlb1 is not associated with diarrhea in a cohort of indian children. PLoS ONE 2011, 6, e28647. [Google Scholar] [CrossRef]
- Kumthip, K.; Khamrin, P.; Ushijima, H.; Maneekarn, N. Molecular epidemiology of classic, mlb and va astroviruses isolated from <5year-old children with gastroenteritis in thailand, 2011-2016. Infect. Genet. Evol. 2018, 65, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Jacobsen, S.; Hohne, M.; Marques, A.M.; Beslmuller, K.; Bock, C.T.; Niendorf, S. Co-circulation of classic and novel astrovirus strains in patients with acute gastroenteritis in germany. J. Infect. 2018, 76, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Zaraket, H.; Abou-El-Hassan, H.; Kreidieh, K.; Soudani, N.; Ali, Z.; Hammadi, M.; Reslan, L.; Ghanem, S.; Hajar, F.; Inati, A.; et al. Characterization of astrovirus-associated gastroenteritis in hospitalized children under five years of age. Infect. Genet. Evol. 2017, 53, 94–99. [Google Scholar] [CrossRef] [PubMed]
- Cordey, S.; Vu, D.-L.; Zanella, M.-C.; Turin, L.; Mamin, A.; Kaiser, L. Novel and classical human astroviruses in stool and cerebrospinal fluid: Comprehensive screening in a tertiary care hospital, switzerland. Emerg. Microbes Infect. 2017, 6, e84. [Google Scholar] [PubMed]
- Khamrin, P.; Thongprachum, A.; Okitsu, S.; Hayakawa, S.; Maneekarn, N.; Ushijima, H. Multiple astrovirus mlb1, mlb2, va2 clades, and classic human astrovirus in children with acute gastroenteritis in japan. J. Med. Virol. 2016, 88, 356–360. [Google Scholar] [CrossRef]
- Xavier, M.d.P.T.P.; Carvalho Costa, F.A.; Rocha, M.S.; Andrade, J.d.S.R.d.; Diniz, F.K.B.; Andrade, T.R.d.; Miagostovich, M.P.; Leite, J.P.G.; Volotão, E.d.M. Surveillance of human astrovirus infection in brazil: The first report of mlb1 astrovirus. PLoS ONE 2015, 10, e0135687. [Google Scholar] [CrossRef] [PubMed]
- Quan, P.L.; Wagner, T.A.; Briese, T.; Torgerson, T.R.; Hornig, M.; Tashmukhamedova, A.; Firth, C.; Palacios, G.; Baisre-De-Leon, A.; Paddock, C.D.; et al. Astrovirus encephalitis in boy with x-linked agammaglobulinemia. Emerg. Infect. Dis. 2010, 16, 918–925. [Google Scholar] [CrossRef]
- Brown, J.R.; Morfopoulou, S.; Hubb, J.; Emmett, W.A.; Ip, W.; Shah, D.; Brooks, T.; Paine, S.M.; Anderson, G.; Virasami, A.; et al. Astrovirus va1/hmo-c: An increasingly recognized neurotropic pathogen in immunocompromised patients. Clin. Infect. Dis. 2015, 60, 881–888. [Google Scholar] [CrossRef]
- Naccache, S.N.; Peggs, K.S.; Mattes, F.M.; Phadke, R.; Garson, J.A.; Grant, P.; Samayoa, E.; Federman, S.; Miller, S.; Lunn, M.P.; et al. Diagnosis of neuroinvasive astrovirus infection in an immunocompromised adult with encephalitis by unbiased next-generation sequencing. Clin. Infect. Dis. 2015, 60, 919–923. [Google Scholar] [CrossRef]
- Lum, S.H.; Turner, A.; Guiver, M.; Bonney, D.; Martland, T.; Davies, E.; Newbould, M.; Brown, J.; Morfopoulou, S.; Breuer, J.; et al. An emerging opportunistic infection: Fatal astrovirus (va1/hmo-c) encephalitis in a pediatric stem cell transplant recipient. Transpl. Infect. Dis. 2016, 18, 960–964. [Google Scholar] [CrossRef]
- Fremond, M.L.; Perot, P.; Muth, E.; Cros, G.; Dumarest, M.; Mahlaoui, N.; Seilhean, D.; Desguerre, I.; Hebert, C.; Corre-Catelin, N.; et al. Next-generation sequencing for diagnosis and tailored therapy: A case report of astrovirus-associated progressive encephalitis. J. Pediatric Infect. Dis. Soc. 2015, 4, e53–e57. [Google Scholar] [CrossRef] [PubMed]
- Cordey, S.; Vu, D.L.; Schibler, M.; L’Huillier, A.G.; Brito, F.; Docquier, M.; Posfay-Barbe, K.M.; Petty, T.J.; Turin, L.; Zdobnov, E.M.; et al. Astrovirus mlb2, a new gastroenteric virus associated with meningitis and disseminated infection. Emerg. Infect. Dis. 2016, 22, 846–853. [Google Scholar] [CrossRef] [PubMed]
- Sato, M.; Kuroda, M.; Kasai, M.; Matsui, H.; Fukuyama, T.; Katano, H.; Tanaka-Taya, K. Acute encephalopathy in an immunocompromised boy with astrovirus-mlb1 infection detected by next generation sequencing. J. Clin. Virol. 2016, 78, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Boga, J.A.; Melon, S.; Nicieza, I.; de Diego, I.; Villar, M.; Parra, F.; De Ona, M. Etiology of sporadic cases of pediatric acute gastroenteritis in asturias, spain, and genotyping and characterization of norovirus strains involved. J. Clin. Microbiol. 2004, 42, 2668–2674. [Google Scholar] [CrossRef] [PubMed]
- Lorrot, M.; Bon, F.; El Hajje, M.J.; Aho, S.; Wolfer, M.; Giraudon, H.; Kaplon, J.; Marc, E.; Raymond, J.; Lebon, P.; et al. Epidemiology and clinical features of gastroenteritis in hospitalised children: Prospective survey during a 2-year period in a parisian hospital, france. Eur. J. Clin. Microbiol. Infect. Dis. 2011, 30, 361–368. [Google Scholar] [CrossRef] [PubMed]
- Martinez Azcona, O.; Vazquez Gomez, L.; Buyo Sanchez, P.; Diaz Soto, R.; Moldes Suarez, L.M. [acute gastroenteritis and enteric viruses: Impact on the detection of norovirus]. An Pediatr (Barc) 2017, 87, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Gupta, S.; Krishnan, A.; Sharma, S.; Kumar, P.; Aneja, S.; Ray, P. Changing pattern of prevalence, genetic diversity, and mixed infections of viruses associated with acute gastroenteritis in pediatric patients in new delhi, india. J. Med. Virol. 2018, 90, 469–476. [Google Scholar] [CrossRef] [PubMed]
- Gosert, R.; Heininger, U.; Hirsch, H.H. Enterovirus detection in patients with acute gastroenteritis in switzerland. J. Med. Virol. 2018, 90, 685–691. [Google Scholar] [CrossRef]
- Andersson, M.E.; Elfving, K.; Shakely, D.; Nilsson, S.; Msellem, M.; Trollfors, B.; Martensson, A.; Bjorkman, A.; Lindh, M. Rapid clearance and frequent reinfection with enteric pathogens among children with acute diarrhea in zanzibar. Clin. Infect. Dis. 2017, 65, 1371–1377. [Google Scholar] [CrossRef]
- Hassan, F.; Kanwar, N.; Harrison, C.J.; Halasa, N.B.; Chappell, J.D.; Englund, J.A.; Klein, E.J.; Weinberg, G.A.; Szilagyi, P.G.; Moffatt, M.E.; et al. Viral etiology of acute gastroenteritis in <2-year-old us children in the post-rotavirus vaccine era. J. Pediatric Infect. Dis. Soc. 2018. [Google Scholar] [CrossRef]
- Diez-Domingo, J.; Garces-Sanchez, M.; Gimenez-Sanchez, F.; Colomina-Rodriguez, J.; Martinon-Torres, F. [what have we learnt about rotavirus in spain in the last 10 years?]. An. Pediatr. (Barc.) 2019. [Google Scholar] [CrossRef]
- Kirkwood, C.D.; Clark, R.; Bogdanovic-Sakran, N.; Bishop, R.F. A 5-year study of the prevalence and genetic diversity of human caliciviruses associated with sporadic cases of acute gastroenteritis in young children admitted to hospital in melbourne, australia (1998–2002). J. Med. Virol. 2005, 77, 96–101. [Google Scholar] [CrossRef] [PubMed]
- Nadan, S.; Taylor, M.B.; Groome, M.J.; Cohen, C.; Madhi, S.A.; Page, N.A. Epidemiology of human astroviruses among children younger than 5 years: Prospective hospital-based sentinel surveillance in south africa, 2009-2014. J. Med Virol. 2019, 91, 225–234. [Google Scholar] [CrossRef] [PubMed]
- Bergallo, M.; Galliano, I.; Dapra, V.; Rassu, M.; Montanari, P.; Tovo, P.A. Molecular detection of human astrovirus in children with gastroenteritis, northern italy. Pediatr. Infect. Dis. J. 2018, 37, 738–742. [Google Scholar] [CrossRef] [PubMed]
- Olortegui, M.P.; Rouhani, S.; Yori, P.P.; Salas, M.S.; Trigoso, D.R.; Mondal, D.; Bodhidatta, L.; Platts-Mills, J.; Samie, A.; Kabir, F.; et al. Astrovirus infection and diarrhea in 8 countries. Pediatrics 2018, 141, e20171326. [Google Scholar] [CrossRef] [PubMed]
- Nijhuis, R.H.T.; Sidorov, I.A.; Chung, P.K.; Wessels, E.; Gulyaeva, A.A.; de Vries, J.J.; Claas, E.C.J.; Gorbalenya, A.E. PCR assays for detection of human astroviruses: In silico evaluation and design, and in vitro application to samples collected from patients in the netherlands. J. Clin. Virol. 2018, 108, 83–89. [Google Scholar] [CrossRef]
- García-Basteiro, A.L.; Bosch, A.; Sicuri, E.; Bayas, J.M.; Trilla, A.; Hayes, E.B. Hospitalizations due to rotavirus gastroenteritis in catalonia, Spain, 2003–2008. BMC Res. Notes 2011, 4, 429. [Google Scholar] [CrossRef]
- Ingle, H.; Lee, S.; Ai, T.; Orvedahl, A.; Rodgers, R.; Zhao, G.; Sullender, M.; Peterson, S.T.; Locke, M.; Liu, T.C.; et al. Viral complementation of immunodeficiency confers protection against enteric pathogens via interferon-lambda. Nat. Microbiol. 2019, 4, 1120–1128. [Google Scholar] [CrossRef]


| Standard Curve (10-Fold Serial Dilutions) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Monoplex | Duplex | |||||||||||
| Assay | Viruses Detected | Target Region (ORF)/Amplicon size (nt) | Fwd primer (5’-3’) 1 | Probe (P) (5’-3’) 1 | Rev Primer (5’-3’) 1 | Final [uM] Fwd/Rev/P | Slope | Intercept | R2 | Slope | Intercept | R2 |
| MLB1 | MLB1 | ORF2/68 | GGTCTTGGAGCYCGAATTC | FAM—TAGRGTTGGTTCAAATCT—MGBNFQ | CGCTGTTTAATGCGCCAAA | 0.6/0.6/0.25 | −4.02 | 43.06 | 0.99 | −3.5 | 40.10 | 0.97 |
| MLB2–3 | MLB2-MLB3 | ORF1b/71 | CCGAGCTCTTAGTGATGCTAGCT | FAM—CGCTTCACTCGGAGAC—MGBNFQ | CACCCCTCCAAATGTACTCCAA | 0.6/0.6/0.2 | −3.34 | 44.29 | 0.99 | −3.19 | 44.69 | 0.99 |
| VA1 | VA1/HMO-C/SG/PS/UK1 | ORF2/66 | CCATCAGCAGTTACYGGGTCTGT | FAM—TTTCCGCATATCCC—MGBNFQ | CGTGGCTCCAGGTGAYTGT | 0.6/0.6/0.2 | −2.72 | 35.24 | 0.96 | −2.83 | 36.03 | 0.97 |
| VA2 | VA2/HMO-A | ORF2/67 | CAGGGCCTGAATTACAAATTTCA | FAM—CATTTATGCATCCTGCTTT—MGBNFQ | GTGCCATCATTTGGCTCTTTC | 0.9/0.9/0.25 | −3.15 | 41.24 | 0.99 | −2.71 | 37.96 | 0.98 |
| VA3 | VA3/HMO-B | ORF1b/67 | TTCCAGGCATTTGAGTTTGCT | FAM—TTGAATCCGGATAAAAC—MGBNFQ | CCCATCCTTCTCTCAGTTCATCA | 0.6/0.6/0.25 | −3.16 | 39.71 | 0.98 | −3.25 | 40.39 | 0.97 |
| VA4 | VA4 | ORF2/62 | GATCCATGTATCGTGCATCGTT | FAM—AACCTTACACAGTCCCCGG—MGBNFQ | GCCCCCCCAAGATGTTG | 0.9/0.9/0.25 | −3.17 | 37.94 | 0.98 | −2.5 | 35.83 | 0.95 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vu, D.-L.; Sabrià, A.; Aregall, N.; Michl, K.; Rodriguez Garrido, V.; Goterris, L.; Bosch, A.; Pintó, R.M.; Guix, S. Novel Human Astroviruses: Prevalence and Association with Common Enteric Viruses in Undiagnosed Gastroenteritis Cases in Spain. Viruses 2019, 11, 585. https://doi.org/10.3390/v11070585
Vu D-L, Sabrià A, Aregall N, Michl K, Rodriguez Garrido V, Goterris L, Bosch A, Pintó RM, Guix S. Novel Human Astroviruses: Prevalence and Association with Common Enteric Viruses in Undiagnosed Gastroenteritis Cases in Spain. Viruses. 2019; 11(7):585. https://doi.org/10.3390/v11070585
Chicago/Turabian StyleVu, Diem-Lan, Aurora Sabrià, Nuria Aregall, Kristina Michl, Virginia Rodriguez Garrido, Lidia Goterris, Albert Bosch, Rosa Maria Pintó, and Susana Guix. 2019. "Novel Human Astroviruses: Prevalence and Association with Common Enteric Viruses in Undiagnosed Gastroenteritis Cases in Spain" Viruses 11, no. 7: 585. https://doi.org/10.3390/v11070585
APA StyleVu, D.-L., Sabrià, A., Aregall, N., Michl, K., Rodriguez Garrido, V., Goterris, L., Bosch, A., Pintó, R. M., & Guix, S. (2019). Novel Human Astroviruses: Prevalence and Association with Common Enteric Viruses in Undiagnosed Gastroenteritis Cases in Spain. Viruses, 11(7), 585. https://doi.org/10.3390/v11070585







