Hantaviridae: Current Classification and Future Perspectives
Abstract
:1. Introduction
“Species are usually found in unique ecological niches, i.e., in different primary rodent/insectivore reservoir species. Species exhibit at least 7% difference in aa identity on comparison of the complete glycoprotein precursor and nucleocapsid protein sequences (there are some exceptions presumably caused by historically recent host-switching events). Species show at least four-fold difference in two-way cross neutralization tests. Species do not naturally form reassortants with other species”.[17]
2. Materials and Methods
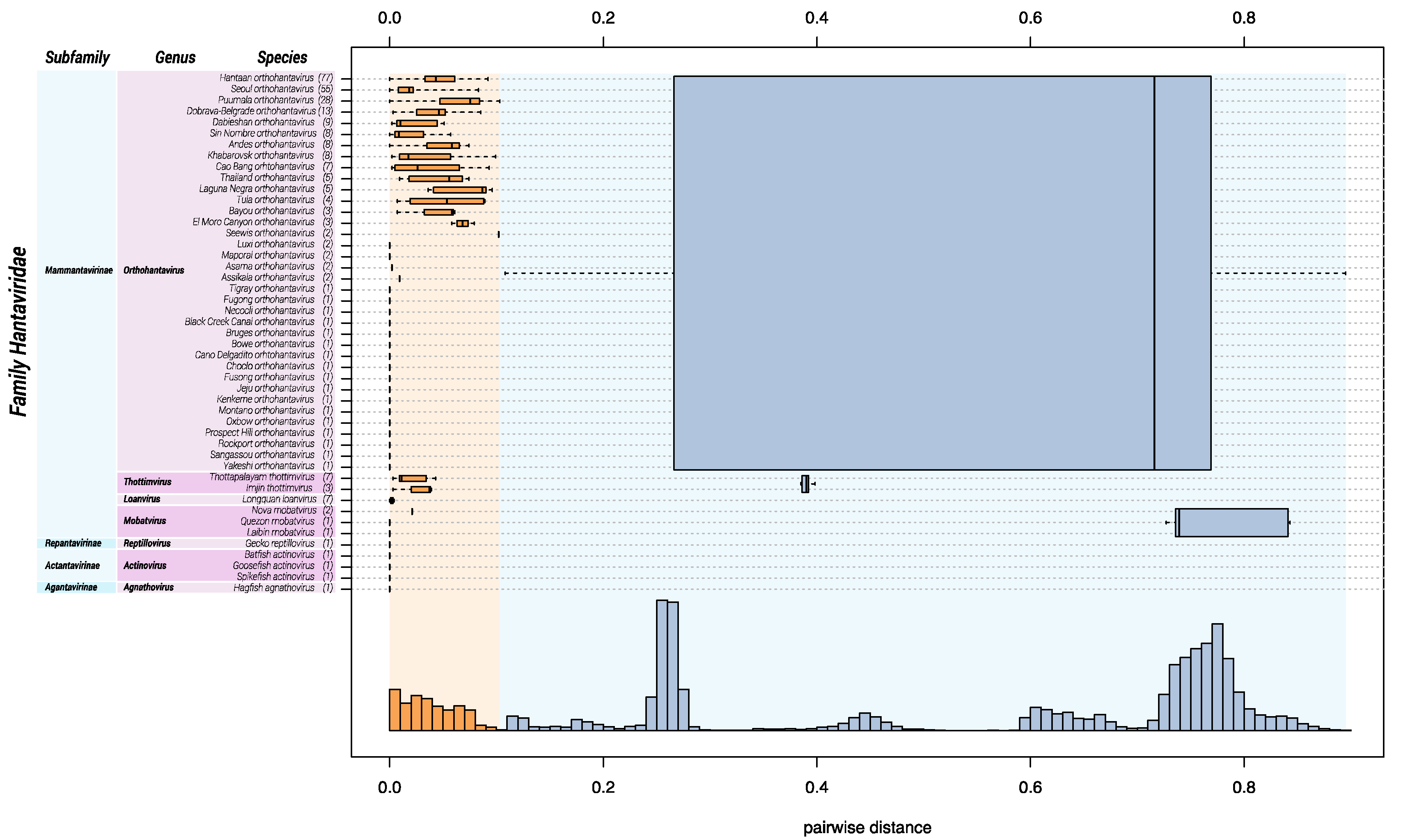
2.1. DEmARC Analysis for Hantaviridae
2.2. Phylogenetic Inference for the Bunyavirales
3. Results
3.1. Change of Demarcation Criteria
3.2. Addition of New Taxa to Hantaviridae
3.3. Abolishment of Hantavirid Taxa and Declassification of Hantavirids
- Amga orthohantavirus: This species was abolished based upon insufficient differentiation from another species in DEmARC analysis. Amga orthohantavirus was established in 2017 for Amga virus (MGAV), which was discovered and sequenced in 2013 [38]. Since then, the coding-complete sequence of the S, M, and L genomic segment of Seewis virus (SWSV), detected in a Eurasian common shrew in 2007 [34], became available. DEmARC analysis demonstrated that Amga virus and Seewis virus belong to the same orthohantavirus species. Based upon the earlier discovery of Seewis virus, the species for both viruses became Seewis orthohantavirus in 2019 (Table 2) [32];
- Isla vista hantavirus: This species was abolished in 2017 based upon incomplete sequence data for the species member, Isla Vista virus (ISLAV) [73]. Only 1 complete S segment sequence, a partial M sequence, and no L segment sequences are available at this time. Our analyses using the incomplete data suggest that ISLAV represents a novel orthohantavirus species;
- Muleshoe hantavirus: This species was abolished in 2017 based upon incomplete sequence data for the species members, Muleshoe virus (MULV) [74]. Only 1 complete S segment sequence is available at this time;
- New York hantavirus: This species was abolished in 2017 based upon insufficient differentiation from another species in DEmARC analysis. The species member, New York virus (NYV), is highly similar to Sin Nombre virus (SNV, Sin Nombre orthohantavirus) in nucleoprotein and glycoprotein amino acid sequence comparisons, indicating that NYV is a SNV variant even though NYV and SNV can be distinguished by seroneutralization [75];
- Rio Mamore hantavirus: This species was omitted in 2017 based upon insufficient differentiation from another species in DEmARC analysis. The species member, Rio Mamoré virus (RIOMV) [76] is highly similar to Laguna Negra virus (LANV; Laguna Negra orthohantavirus) in DEmARC analysis and is now considered a LANV variant;
- Rio Segundo hantavirus: This species was abolished in 2017 based upon incomplete sequence data for the species member, Río Segundo virus (RIOSV) [77]. Only 1 complete S segment sequence is available;
- Saaremaa hantavirus: This species was abolished in 2017 based upon insufficient differentiation from another species in DEmARC analysis. The species member, Saaremaa virus (SAAV) [78], should be considered a member of the species Dobrava-Belgrade orthohantavirus; and
- Topografov hantavirus: This species was abolished in 2017 based upon insufficient differentiation from another species in DEmARC analysis. The species member, Topografov virus (TOPV) [79], is highly similar to Khabarovsk virus (KHAV) and, based upon the DEmARC analysis, should be considered as a KHAV variant.
3.4. Creation of Subfamilies and Genera within Hantaviridae
3.5. Etymology of Taxa included in Hantaviridae
- Actantavirinae: derived from genus name Actinovirus, family name Hantaviridae, and subfamily suffix -virinae;
- Agantavirinae: derived from genus name Agnathovirus, family name Hantaviridae, and subfamily suffix -virinae;
- Mammantavirinae: derived from host class name Mammalia, family name Hantaviridae, and subfamily suffix -virinae;
- Repantavirinae: derived from genus name Reptillovirus, family name Hantaviridae, and subfamily suffix -virinae.
- Actinovirus: derived from host class Actinopterygii and genus suffix -virus;
- Agnathovirus: derived from host superclass Agnatha and genus suffix -virus;
- Loanvirus: derived from Lóngquán virus and genus suffix -virus;
- Mobatvirus: derived from mole and bat hosts and genus suffix -virus;
- Orthohantavirus: derived from Greek ὀρθός [orthós], meaning “straight,” historical genus Hantavirus, and genus suffix -virus;
- Thottimvirus: derived from Thottapalayam virus, Imjin virus, and genus suffix -virus;
- Reptillovirus: derived from host class Reptilia and genus suffix -virus.
3.6. Megataxonomy of Hantaviridae
3.7. Etymology of Megataxa Relating to Hantaviridae
- Riboviria: derived from ribonucleic acid and realm suffix -viria;
- Negarnaviricota: derived from the Latin Nega, meaning “negative” RNA, and phylum suffix -viricota;
- Polyploviricotina: derived from Ancient Greek πολύπλοκος [polýplokos] for “complex” and subphylum suffix -viricotina;
- Ellioviricetes: derived from Richard Elliott, the late pioneer of bunyaviral molecular virology, and class suffix -viricetes;
- Bunyavirales: derived from Bunyamwera virus and order suffix -virales.
4. Future Taxonomic Perspectives
5. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Breitbart, M.; Salamon, P.; Andresen, B.; Mahaffy, J.M.; Segall, A.M.; Mead, D.; Azam, F.; Rohwer, F. Genomic analysis of uncultured marine viral communities. Proc. Natl. Acad. Sci. USA 2002, 99, 14250–14255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coutinho, F.H.; Silveira, C.B.; Gregoracci, G.B.; Thompson, C.C.; Edwards, R.A.; Brussaard, C.P.D.; Dutilh, B.E.; Thompson, F.L. Marine viruses discovered via metagenomics shed light on viral strategies throughout the oceans. Nat. Commun. 2017, 8, 15955. [Google Scholar] [CrossRef] [PubMed]
- Culley, A.I.; Lang, A.S.; Suttle, C.A. Metagenomic analysis of coastal RNA virus communities. Science 2006, 312, 1795–1798. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-X.; Shi, M.; Tian, J.-H.; Lin, X.-D.; Kang, Y.-J.; Chen, L.-J.; Qin, X.-C.; Xu, J.; Holmes, E.C.; Zhang, Y.-Z. Unprecedented genomic diversity of RNA viruses in arthropods reveals the ancestry of negative-sense RNA viruses. Elife 2015, 4, e05378. [Google Scholar] [CrossRef] [PubMed]
- Paez-Espino, D.; Eloe-Fadrosh, E.A.; Pavlopoulos, G.A.; Thomas, A.D.; Huntemann, M.; Mikhailova, N.; Rubin, E.; Ivanova, N.N.; Kyrpides, N.C. Uncovering Earth’s virome. Nature 2016, 536, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Roossinck, M.J.; Martin, D.P.; Roumagnac, P. Plant virus metagenomics: Advances in virus discovery. Phytopathology 2015, 105, 716–727. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Lin, X.-D.; Chen, X.; Tian, J.-H.; Chen, L.-J.; Li, K.; Wang, W.; Eden, J.-S.; Shen, J.-J.; Liu, L.; et al. The evolutionary history of vertebrate RNA viruses. Nature 2018, 556, 197–202. [Google Scholar] [CrossRef]
- Shi, M.; Lin, X.-D.; Tian, J.-H.; Chen, L.-J.; Chen, X.; Li, C.-X.; Qin, X.-C.; Li, J.; Cao, J.-P.; Eden, J.-S.; et al. Redefining the invertebrate RNA virosphere. Nature 2016, 540, 539–543. [Google Scholar] [CrossRef]
- Simmonds, P.; Adams, M.J.; Benkő, M.; Breitbart, M.; Brister, J.R.; Carstens, E.B.; Davison, A.J.; Delwart, E.; Gorbalenya, A.E.; Harrach, B.; et al. Consensus statement: Virus taxonomy in the age of metagenomics. Nat. Rev. Microbiol. 2017, 15, 161–168. [Google Scholar] [CrossRef]
- Simmonds, P. Methods for virus classification and the challenge of incorporating metagenomic sequence data. J. Gen. Virol. 2015, 96, 1193–1206. [Google Scholar] [CrossRef]
- Wolf, Y.I.; Kazlauskas, D.; Iranzo, J.; Lucía-Sanz, A.; Kuhn, J.H.; Krupovic, M.; Dolja, V.V.; Koonin, E.V. Origins and evolution of the global RNA virome. MBio 2018, 9, e02329-18. [Google Scholar] [CrossRef]
- Vaheri, A.; Strandin, T.; Hepojoki, J.; Sironen, T.; Henttonen, H.; Mäkelä, S.; Mustonen, J. Uncovering the mysteries of hantavirus infections. Nat. Rev. Microbiol. 2013, 11, 539–550. [Google Scholar] [CrossRef]
- Carey, D.E.; Reuben, R.; Panicker, K.N.; Shope, R.E.; Myers, R.M. Thottapalayam virus: A presumptive arbovirus isolated from a shrew in India. Indian J. Med. Res. 1971, 59, 1758–1760. [Google Scholar]
- Lee, H.W.; Lee, P.W.; Johnson, K.M. Isolation of the etiologic agent of Korean hemorrhagic fever. J. Infect. Dis. 1978, 137, 298–308. [Google Scholar] [CrossRef]
- Schmaljohn, C.S.; Hasty, S.E.; Dalrymple, J.M.; LeDuc, J.W.; Lee, H.W.; von Bonsdorff, C.-H.; Brummer-Korvenkontio, M.; Vaheri, A.; Tsai, T.F.; Regnery, H.L.; et al. Antigenic and genetic properties of viruses linked to hemorrhagic fever with renal syndrome. Science 1985, 227, 1041–1044. [Google Scholar] [CrossRef]
- Francki, R.I.B.; Fauquet, C.M.; Knudson, D.L.; Brown, F. Classification and Nomenclature of Viruses; Fifth Report of the International Committee on Taxonomy of Viruses; Springer: Vienna, Austria, 1991; Volume 2. [Google Scholar]
- Plyusnin, A.; Beaty, B.J.; Elliott, R.M.; Goldbach, R.; Kormelink, R.; Lundkvist, Å.; Schmaljohn, C.S.; Tesh, R.B. Family Bunyaviridae. In Virus Taxonomy; King, A.M.Q., Adams, M.J., Carstens, E.B., Lefkowitz, E.J., Eds.; Ninth Report of the International Committee on Taxonomy of Viruses; Elsevier/Academic Press: London, UK, 2011; pp. 725–741. [Google Scholar]
- Mielke-Ehret, N.; Mühlbach, H.-P. Emaravirus: A novel genus of multipartite, negative strand RNA plant viruses. Viruses 2012, 4, 1515–1536. [Google Scholar] [CrossRef]
- Akopyants, N.S.; Lye, L.-F.; Dobson, D.E.; Lukeš, J.; Beverley, S.M. A novel bunyavirus-like virus of trypanosomatid protist parasites. Genome Announc. 2016, 4, e00715-16. [Google Scholar] [CrossRef]
- Marklewitz, M.; Zirkel, F.; Rwego, I.B.; Heidemann, H.; Trippner, P.; Kurth, A.; Kallies, R.; Briese, T.; Lipkin, W.I.; Drosten, C.; et al. Discovery of a unique novel clade of mosquito-associated bunyaviruses. J. Virol. 2013, 87, 12850–12865. [Google Scholar] [CrossRef]
- Rodrigues, D.S.; Medeiros, D.B.; Rodrigues, S.G.; Martins, L.C.; de Lima, C.P.; de Oliveira, L.F.; de Vasconcelos, J.M.; Da Silva, D.E.; Cardoso, J.F.; da Silva, S.P.; et al. Pacui virus, Rio Preto da Eva virus, and Tapirape virus, three distinct viruses within the family Bunyaviridae. Genome Announc. 2014, 2, e00923-14. [Google Scholar] [CrossRef]
- Marklewitz, M.; Zirkel, F.; Kurth, A.; Drosten, C.; Junglen, S. Evolutionary and phenotypic analysis of live virus isolates suggests arthropod origin of a pathogenic RNA virus family. Proc. Natl. Acad. Sci. USA 2015, 112, 7536–7541. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.-J.; Liang, M.-F.; Zhang, S.-Y.; Liu, Y.; Li, J.D.; Sun, Y.-L.; Zhang, L.; Zhang, Q.-F.; Popov, V.L.; Li, C.; et al. Fever with thrombocytopenia associated with a novel bunyavirus in China. N. Engl. J. Med. 2011, 364, 1523–1532. [Google Scholar] [CrossRef]
- Rott, M.E.; Kesanakurti, P.; Berwarth, C.; Rast, H.; Boyes, I.; Phelan, J.; Jelkmann, W. Discovery of negative-sense RNA viruses in trees infected with apple rubbery wood disease by next-generation sequencing. Plant Dis. 2018, 102, 1254–1263. [Google Scholar] [CrossRef]
- Navarro, B.; Minutolo, M.; De Stradis, A.; Palmisano, F.; Alioto, D.; Di Serio, F. The first phlebo-like virus infecting plants: A case study on the adaptation of negative-stranded RNA viruses to new hosts. Mol. Plant Pathol. 2018, 19, 1075–1089. [Google Scholar] [CrossRef]
- Lin, Y.-H.; Fujita, M.; Chiba, S.; Hyodo, K.; Andika, I.B.; Suzuki, N.; Kondo, H. Two novel fungal negative-strand RNA viruses related to mymonaviruses and phenuiviruses in the shiitake mushroom (Lentinula edodes). Virology 2019, 533, 125–136. [Google Scholar] [CrossRef]
- Marklewitz, M.; Handrick, S.; Grasse, W.; Kurth, A.; Lukashev, A.; Drosten, C.; Ellerbrok, H.; Leendertz, F.H.; Pauli, G.; Junglen, S. Gouléako virus isolated from West African mosquitoes constitutes a proposed novel genus in the family Bunyaviridae. J. Virol. 2011, 85, 9227–9234. [Google Scholar] [CrossRef]
- Tokarz, R.; Williams, S.H.; Sameroff, S.; Sanchez Leon, M.; Jain, K.; Lipkin, W.I. Virome analysis of Amblyomma americanum, Dermacentor variabilis, and Ixodes scapularis ticks reveals novel highly divergent vertebrate and invertebrate viruses. J. Virol. 2014, 88, 11480–11492. [Google Scholar] [CrossRef]
- Makhsous, N.; Shean, R.C.; Droppers, D.; Guan, J.; Jerome, K.R.; Greninger, A.L. Genome sequences of three novel bunyaviruses, two novel rhabdoviruses, and one novel nyamivirus from Washington State moths. Genome Announc. 2017, 5, e01668-16. [Google Scholar] [CrossRef]
- Yamao, T.; Eshita, Y.; Kihara, Y.; Satho, T.; Kuroda, M.; Sekizuka, T.; Nishimura, M.; Sakai, K.; Watanabe, S.; Akashi, H.; et al. Novel virus discovery in field-collected mosquito larvae using an improved system for rapid determination of viral RNA sequences (RDV ver4.0). Arch. Virol. 2009, 154, 153–158. [Google Scholar] [CrossRef]
- Maes, I.; Alkhovsky, S.V.; Bào, Y.; Beer, M.; Birkhead, M.; Briese, T.; Buchmeier, M.J.; Calisher, C.H.; Charrel, R.N.; Choi, I.R.; et al. Taxonomy of the family Arenaviridae and the order Bunyavirales: Update 2018. Arch. Virol. 2018, 163, 2295–2310. [Google Scholar] [CrossRef]
- Abudurexiti, A.; Adkins, S.; Alioto, D.; Alkhovsky, S.V.; Avšič-Županc, T.; Ballinger, M.J.; Bente, D.A.; Beer, M.; Bergeron, É.; Blair, C.D.; et al. Taxonomy of the order Bunyavirales: Update 2019. Arch. Virol. 2019, 164, 1949–1965. [Google Scholar] [CrossRef]
- Arai, S.; Ohdachi, S.D.; Asakawa, M.; Kang, H.J.; Mocz, G.; Arikawa, J.; Okabe, N.; Yanagihara, R. Molecular phylogeny of a newfound hantavirus in the Japanese shrew mole (Urotrichus talpoides). Proc. Natl. Acad. Sci. USA 2008, 105, 16296–16301. [Google Scholar] [CrossRef]
- Song, J.-W.; Gu, S.H.; Bennett, S.N.; Arai, S.; Puorger, M.; Hilbe, M.; Yanagihara, R. Seewis virus, a genetically distinct hantavirus in the Eurasian common shrew (Sorex araneus). Virol. J. 2007, 4, 114. [Google Scholar] [CrossRef]
- Sumibcay, L.; Kadjo, B.; Gu, S.H.; Kang, H.J.; Lim, B.K.; Cook, J.A.; Song, J.-W.; Yanagihara, R. Divergent lineage of a novel hantavirus in the banana pipistrelle (Neoromicia nanus) in Côte d’Ivoire. Virol. J. 2012, 9, 34. [Google Scholar] [CrossRef]
- Klempa, B.; Fichet-Calvet, E.; Lecompte, E.; Auste, B.; Aniskin, V.; Meisel, H.; Barrière, P.; Koivogui, L.; ter Meulen, J.; Krüger, D.H. Novel hantavirus sequences in shrew, Guinea. Emerg. Infect. Dis. 2007, 13, 520–522. [Google Scholar] [CrossRef]
- Weiss, S.; Witkowski, P.T.; Auste, B.; Nowak, K.; Weber, N.; Fahr, J.; Mombouli, J.-V.; Wolfe, N.D.; Drexler, J.F.; Drosten, C.; et al. Hantavirus in bat, Sierra Leone. Emerg. Infect. Dis. 2012, 18, 159–161. [Google Scholar] [CrossRef]
- Bennett, S.N.; Gu, S.H.; Kang, H.J.; Arai, S.; Yanagihara, R. Reconstructing the evolutionary origins and phylogeography of hantaviruses. Trends Microbiol. 2014, 22, 473–482. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.-Z. Discovery of hantaviruses in bats and insectivores and the evolution of the genus Hantavirus. Virus Res. 2014, 187, 15–21. [Google Scholar] [CrossRef]
- Castel, G.; Tordo, N.; Plyusnin, A. Estimation of main diversification time-points of hantaviruses using phylogenetic analyses of complete genomes. Virus Res. 2017, 233, 60–69. [Google Scholar] [CrossRef]
- Witkowski, P.T.; Drexler, J.F.; Kallies, R.; Ličková, M.; Bokorová, S.; Maganga, G.D.; Szemes, T.; Leroy, E.M.; Krüger, D.H.; Drosten, C.; et al. Phylogenetic analysis of a newfound bat-borne hantavirus supports a laurasiatherian host association for ancestral mammalian hantaviruses. Infect. Genet. Evol. 2016, 41, 113–119. [Google Scholar] [CrossRef]
- Laenen, L.; Dellicour, S.; Vergote, V.; Nauwelaers, I.; de Coster, S.; Verbeeck, I.; Vanmechelen, B.; Lemey, P.; Maes, P. Spatio-temporal analysis of Nova virus, a divergent hantavirus circulating in the European mole in Belgium. Mol. Ecol. 2016, 25, 5994–6008. [Google Scholar] [CrossRef]
- Guo, W.-P.; Lin, X.-D.; Wang, W.; Tian, J.-H.; Cong, M.-L.; Zhang, H.-L.; Wang, M.-R.; Zhou, R.-H.; Wang, J.-B.; Li, M.-H.; et al. Phylogeny and origins of hantaviruses harbored by bats, insectivores, and rodents. PLoS Pathog. 2013, 9, e1003159. [Google Scholar] [CrossRef]
- Laenen, L.; Vergote, V.; Kafetzopoulou, L.E.; Wawina, T.B.; Vassou, D.; Cook, J.A.; Hugot, J.-P.; Deboutte, W.; Kang, H.J.; Witkowski, P.T.; et al. A novel hantavirus of the European mole, Bruges virus, is involved in frequent Nova virus coinfections. Genome Biol. Evol. 2018, 10, 45–55. [Google Scholar] [CrossRef]
- Maes, P.; Klempa, B.; Clement, J.; Matthijnssens, J.; Gajdusek, D.C.; Krüger, D.H.; Van Ranst, M. A proposal for new criteria for the classification of hantaviruses, based on S and M segment protein sequences. Infect. Genet. Evol. 2009, 9, 813–820. [Google Scholar] [CrossRef]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef]
- Suchard, M.A.; Lemey, P.; Baele, G.; Ayres, D.L.; Drummond, A.J.; Rambaut, A. Bayesian phylogenetic and phylodynamic data integration using BEAST 1.10. Virus Evol. 2018, 4, vey016. [Google Scholar] [CrossRef] [Green Version]
- Rambaut, A. FigTree v1. 4. Molecular Evolution, Phylogenetics and Epidemiology; University of Edinburgh, Institute of Evolutionary Biology: Edinburgh, UK, 2012. [Google Scholar]
- Lauber, C.; Gorbalenya, A.E. Partitioning the genetic diversity of a virus family: Approach and evaluation through a case study of picornaviruses. J. Virol. 2012, 86, 3890–3904. [Google Scholar] [CrossRef]
- Lauber, C.; Gorbalenya, A.E. Toward genetics-based virus taxonomy: Comparative analysis of a genetics-based classification and the taxonomy of picornaviruses. J. Virol. 2012, 86, 3905–3915. [Google Scholar] [CrossRef]
- Ladner, J.T.; Beitzel, B.; Chain, P.S.G.; Davenport, M.G.; Donaldson, E.F.; Frieman, M.; Kugelman, J.R.; Kuhn, J.H.; O’Rear, J.; Sabeti, P.C.; et al. Standards for sequencing viral genomes in the era of high-throughput sequencing. MBio 2014, 5, e01360-14. [Google Scholar] [CrossRef]
- Maes, P.; Adkins, S.; Alkhovsky, S.V.; Avšič-Županc, T.; Ballinger, M.J.; Bente, D.A.; Beer, M.; Bergeron, É.; Blair, C.D.; Briese, T.; et al. Taxonomy of the order Bunyavirales: Second update 2018. Arch. Virol. 2019, 164, 927–941. [Google Scholar] [CrossRef]
- Arai, S.; Kang, H.J.; Gu, S.H.; Ohdachi, S.D.; Cook, J.A.; Yashina, L.N.; Tanaka-Taya, K.; Abramov, S.A.; Morikawa, S.; Okabe, N.; et al. Genetic diversity of Artybash virus in the Laxmann’s shrew (Sorex caecutiens). Vector Borne Zoonotic Dis. 2016, 16, 468–475. [Google Scholar] [CrossRef]
- Radosa, L.; Schlegel, M.; Gebauer, P.; Ansorge, H.; Heroldová, M.; Jánová, E.; Stanko, M.; Mošanský, L.; Fričová, J.; Pejčoch, M.; et al. Detection of shrew-borne hantavirus in Eurasian pygmy shrew (Sorex minutus) in Central Europe. Infect. Genet. Evol. 2013, 19, 403–410. [Google Scholar] [CrossRef]
- Gu, S.H.; Nicolas, V.; Lalis, A.; Sathirapongsasuti, N.; Yanagihara, R. Complete genome sequence and molecular phylogeny of a newfound hantavirus harbored by the Doucet’s musk shrew (Crocidura douceti) in Guinea. Infect. Genet. Evol. 2013, 20, 118–123. [Google Scholar] [CrossRef]
- Song, J.-W.; Kang, H.J.; Song, K.-J.; Truong, T.T.; Bennett, S.N.; Arai, S.; Truong, N.U.; Yanagihara, R. Newfound hantavirus in Chinese mole shrew, Vietnam. Emerg. Infect. Dis. 2007, 13, 1784–1787. [Google Scholar] [CrossRef]
- Vincent, M.J.; Quiroz, E.; Gracia, F.; Sanchez, A.J.; Ksiazek, T.G.; Kitsutani, P.T.; Ruedas, L.A.; Tinnin, D.S.; Caceres, L.; Garcia, A.; et al. Hantavirus pulmonary syndrome in Panama: Identification of novel hantaviruses and their likely reservoirs. Virology 2000, 277, 14–19. [Google Scholar] [CrossRef]
- Wang, H.; Yoshimatsu, K.; Ebihara, H.; Ogino, M.; Araki, K.; Kariwa, H.; Wang, Z.; Luo, Z.; Li, D.; Hang, C.; et al. Genetic diversity of hantaviruses isolated in china and characterization of novel hantaviruses isolated from Niviventer confucianus and Rattus rattus. Virology 2000, 278, 332–345. [Google Scholar] [CrossRef]
- Ge, X.-Y.; Yang, W.-H.; Pan, H.; Zhou, J.-H.; Han, X.; Zhu, G.-J.; Desmond, J.S.; Daszak, P.; Shi, Z.-L.; Zhang, Y.-Z. Fugong virus, a novel hantavirus harbored by the small oriental vole (Eothenomys eleusis) in China. Virol. J. 2016, 13, 27. [Google Scholar] [CrossRef]
- Zou, Y.; Wang, J.-B.; Gaowa, H.-S.; Yao, L.-S.; Hu, G.-W.; Li, M.H.; Chen, H.-X.; Plyusnin, A.; Shao, R.; Zhang, Y.-Z. Isolation and genetic characterization of hantaviruses carried by Microtus voles in China. J. Med. Virol. 2008, 80, 680–688. [Google Scholar] [CrossRef]
- Song, J.-W.; Kang, H.J.; Gu, S.H.; Moon, S.S.; Bennett, S.N.; Song, K.-J.; Baek, L.J.; Kim, H.-C.; O’Guinn, M.L.; Chong, S.-T.; et al. Characterization of Imjin virus, a newly isolated hantavirus from the Ussuri white-toothed shrew (Crocidura lasiura). J. Virol. 2009, 83, 6184–6191. [Google Scholar] [CrossRef]
- Arai, S.; Gu, S.H.; Baek, L.J.; Tabara, K.; Bennett, S.N.; Oh, H.-S.; Takada, N.; Kang, H.J.; Tanaka-Taya, K.; Morikawa, S.; et al. Divergent ancestral lineages of newfound hantaviruses harbored by phylogenetically related crocidurine shrew species in Korea. Virology 2012, 424, 99–105. [Google Scholar] [CrossRef] [Green Version]
- Kang, H.J.; Arai, S.; Hope, A.G.; Cook, J.A.; Yanagihara, R. Novel hantavirus in the flat-skulled shrew (Sorex roboratus). Vector Borne Zoonotic Dis. 2010, 10, 593–597. [Google Scholar] [CrossRef]
- Xu, L.; Wu, J.; He, B.; Qin, S.; Xia, L.; Qin, M.; Li, N.; Tu, C. Novel hantavirus identified in black-bearded tomb bats, China. Infect. Genet. Evol. 2015, 31, 158–160. [Google Scholar] [CrossRef]
- Zhang, Y.; Yuan, J.; Yang, X.; Zhou, J.; Yang, W.; Peng, C.; Zhang, H.-L.; Shi, Z. A novel hantavirus detected in Yunnan red-backed vole (Eothenomys miletus) in China. J. Gen. Virol. 2011, 92, 1454–1457. [Google Scholar] [CrossRef]
- Fulhorst, C.F.; Cajimat, M.N.B.; Utrera, A.; Milazzo, M.L.; Duno, G.M. Maporal virus, a hantavirus associated with the fulvous pygmy rice rat (Oligoryzomys fulvescens) in western Venezuela. Virus Res. 2004, 104, 139–144. [Google Scholar] [CrossRef]
- Kariwa, H.; Yoshida, H.; Sánchez-Hernández, C.; de Lourdes Romero-Almaraz, M.; Almazán-Catalán, J.A.; Ramos, C.; Miyashita, D.; Seto, T.; Takano, A.; Totani, M.; et al. Genetic diversity of hantaviruses in Mexico: Identification of three novel hantaviruses from Neotominae rodents. Virus Res. 2012, 163, 486–494. [Google Scholar] [CrossRef]
- Londoño, A.F.; Díaz, F.J.; Agudelo-Flórez, P.; Levis, S.; Rodas, J.D. Genetic evidence of hantavirus infections in wild rodents from northwestern Colombia. Vector Borne Zoonotic Dis. 2011, 11, 701–708. [Google Scholar] [CrossRef]
- Kang, H.J.; Bennett, S.N.; Sumibcay, L.; Arai, S.; Hope, A.G.; Mocz, G.; Song, J.-W.; Cook, J.A.; Yanagihara, R. Evolutionary insights from a genetically divergent hantavirus harbored by the European common mole (Talpa europaea). PLoS ONE 2009, 4, e6149. [Google Scholar] [CrossRef]
- Kang, H.J.; Bennett, S.N.; Dizney, L.; Sumibcay, L.; Arai, S.; Ruedas, L.A.; Song, J.-W.; Yanagihara, R. Host switch during evolution of a genetically distinct hantavirus in the American shrew mole (Neurotrichus gibbsii). Virology 2009, 388, 8–14. [Google Scholar] [CrossRef]
- Kang, H.J.; Bennett, S.N.; Hope, A.G.; Cook, J.A.; Yanagihara, R. Shared ancestry between a newfound mole-borne hantavirus and hantaviruses harbored by cricetid rodents. J. Virol. 2011, 85, 7496–7503. [Google Scholar] [CrossRef]
- Meheretu, Y.; Čížková, D.; Těšíková, J.; Welegerima, K.; Tomas, Z.; Kidane, D.; Girmay, K.; Schmidt-Chanasit, J.; Bryja, J.; Günther, S.; et al. High diversity of RNA viruses in rodents, Ethiopia. Emerg. Infect. Dis. 2012, 18, 2047–2050. [Google Scholar] [CrossRef]
- Song, W.; Torrez-Martinez, N.; Irwin, W.; Harrison, F.J.; Davis, R.; Ascher, M.; Jay, M.; Hjelle, B. Isla Vista virus: A genetically novel hantavirus of the California vole Microtus californicus. J. Gen. Virol. 1995, 76, 3195–3199. [Google Scholar] [CrossRef]
- Rawlings, J.A.; Torrez-Martinez, N.; Neill, S.U.; Moore, G.M.; Hicks, B.N.; Pichuantes, S.; Nguyen, A.; Bharadwaj, M.; Hjelle, B. Cocirculation of multiple hantaviruses in Texas, with characterization of the small (S) genome of a previously undescribed virus of cotton rats (Sigmodon hispidus). Am. J. Trop. Med. Hyg. 1996, 55, 672–679. [Google Scholar] [CrossRef]
- Hjelle, B.; Lee, S.-W.; Song, W.; Torrez-Martinez, N.; Song, J.-W.; Yanagihara, R.; Gavrilovskaya, I.; Mackow, E.R. Molecular linkage of hantavirus pulmonary syndrome to the white-footed mouse, Peromyscus leucopus: Genetic characterization of the M genome of New York virus. J. Virol. 1995, 69, 8137–8141. [Google Scholar]
- Hjelle, B.; Torrez-Martinez, N.; Koster, F.T. Hantavirus pulmonary syndrome-related virus from Bolivia. Lancet 1996, 347, 57. [Google Scholar] [CrossRef]
- Hjelle, B.; Anderson, B.; Torrez-Martinez, N.; Song, W.; Gannon, W.L.; Yates, T.L. Prevalence and geographic genetic variation of hantaviruses of New World harvest mice (Reithrodontomys): Identification of a divergent genotype from a Costa Rican Reithrodontomys mexicanus. Virology 1995, 207, 452–459. [Google Scholar] [CrossRef]
- Nemirov, K.; Vapalahti, O.; Lundkvist, Å.; Vasilenko, V.; Golovljova, I.; Plyusnina, A.; Niemimaa, J.; Laakkonen, J.; Henttonen, H.; Vaheri, A.; et al. Isolation and characterization of Dobrava hantavirus carried by the striped field mouse (Apodemus agrarius) in Estonia. J. Gen. Virol. 1999, 80, 371–379. [Google Scholar] [CrossRef]
- Plyusnin, A.; Vapalahti, O.; Lundkvist, Å.; Henttonen, H.; Vaheri, A. Newly recognised hantavirus in Siberian lemmings. Lancet 1996, 347, 1835. [Google Scholar] [CrossRef]
- Walker, P.J.; Siddell, S.G.; Lefkowitz, E.J.; Mushegian, A.R.; Dempsey, D.M.; Dutilh, B.E.; Harrach, B.; Harrison, R.L.; Hendrickson, R.C.; Junglen, S.; et al. Changes to virus taxonomy and the International Code of Virus Classification and Nomenclature ratified by the International Committee on Taxonomy of Viruses (2019). Arch. Virol. 2019, 164, 2417–2429. [Google Scholar] [CrossRef] [Green Version]
- Kuhn, J.H.; Wolf, Y.I.; Krupovic, M.; Zhang, Y.-Z.; Maes, P.; Dolja, V.V.; Koonin, E.V. Classify viruses—The gain is worth the pain. Nature 2019, 566, 318–320. [Google Scholar] [CrossRef]
- Siddell, S.G.; Walker, P.J.; Lefkowitz, E.J.; Mushegian, A.R.; Adams, M.J.; Dutilh, B.E.; Gorbalenya, A.E.; Harrach, B.; Harrison, R.L.; Junglen, S.; et al. Additional changes to taxonomy ratified in a special vote by the International Committee on Taxonomy of Viruses (October 2018). Arch. Virol. 2019, 164, 943–946. [Google Scholar] [CrossRef] [Green Version]
- Straková, P.; Dufkova, L.; Širmarová, J.; Salát, J.; Bartonička, T.; Klempa, B.; Pfaff, F.; Höper, D.; Hoffmann, B.; Ulrich, R.G.; et al. Novel hantavirus identified in European bat species Nyctalus noctula. Infect. Genet. Evol. 2017, 48, 127–130. [Google Scholar] [CrossRef]
- Arai, S.; Aoki, K.; Sơn, N.T.; Tú, V.T.; Kikuchi, F.; Kinoshita, G.; Fukui, D.; Thành, H.T.; Gu, S.H.; Yoshikawa, Y.; et al. Đakrông virus, a novel mobatvirus (Hantaviridae) harbored by the Stoliczka’s Asian trident bat (Aselliscus stoliczkanus) in Vietnam. Sci. Rep. 2019, 9, 10239. [Google Scholar] [CrossRef]
- Arai, S.; Kikuchi, F.; Bawm, S.; Sơn, N.T.; Lin, K.S.; Tú, V.T.; Aoki, K.; Tsuchiya, K.; Tanaka-Taya, K.; Morikawa, S.; et al. Molecular phylogeny of mobatviruses (Hantaviridae) in Myanmar and Vietnam. Viruses 2019, 11, 228. [Google Scholar] [CrossRef]
- Postler, T.S.; Clawson, A.N.; Amarasinghe, G.K.; Basler, C.F.; Bavari, S.; Benkő, M.; Blasdell, K.R.; Briese, T.; Buchmeier, M.J.; Bukreyev, A.; et al. Possibility and challenges of conversion of current virus species names to Linnaean binomials. Syst. Biol. 2017, 66, 463–473. [Google Scholar] [CrossRef]
- Klempa, B. Reassortment events in the evolution of hantaviruses. Virus Genes 2018, 54, 638–646. [Google Scholar] [CrossRef] [Green Version]


| New Hantavirid Species | Hantavirid | Hantavirid Abbreviation | Reference (s) | Isolate Used for Analysis |
|---|---|---|---|---|
| Amga orthohantavirus1 | Amga virus | MGAV | [38,53] | AH301 |
| Asama orthohantavirus | Asama virus | ASAV | [33] | N10 |
| Asikkala orthohantavirus | Asikkala virus | ASIV | [54] | CZ/Beskydy/412/2010/Sm |
| Bowe orthohantavirus | Bowé virus | BOWV | [55] | VN1512 |
| Bruges orthohantavirus | Bruges virus | BRGV | [44] | BE/Vieux-Genappe/TE/2013/1 |
| Cao Bang orthohantavirus | Cao Bằng virus | CBNV | [56] | 3 |
| Choclo orthohantavirus | Choclo virus | CHOV | [57] | MSB96073 |
| Dabieshan orthohantavirus | Dàbiéshān virus | DBSV | [58] | Yǒngjiā-Nc-58 |
| Fugong orthohantavirus | Fúgòng virus | FUGV | [59] | FG10 |
| Fusong orthohantavirus | Fǔsōng virus | FUSV | [60] | Fǔsōng-Mf-682 |
| Imjin thottimvirus2 | Imjin virus | MJNV | [61] | Cíxī-Cl-23 |
| Jeju orthohantavirus | Jeju virus | JJUV | [62] | 10-11 |
| Kenkeme orthohantavirus | Kenkeme virus | KKMV | [63] | Fǔyuǎn-Sr-326 |
| Laibin mobatvirus2 | Láibīn virus | LAIV | [64] | BT20 |
| Longquan loanvirus2 | Lóngquán virus | LQUV | [43] | Lóngquán-Rs-32 |
| Luxi orthohantavirus | Lúxī virus | LUXV | [65] | LX309 |
| Maporal orthohantavirus | Maporal virus | MAPV | [66] | HV-97021050 |
| Montano orthohantavirus | Montaño virus | MTNV | [67] | 104/2006 |
| Necocli orthohantavirus | Necoclí virus | NECV | [68] | HV-O0020002 |
| Nova mobatvirus2 | Nova virus | NVAV | [69] | 3483 (Te34) |
| Oxbow orthohantavirus | Oxbow virus | OXBV | [70] | Ng1453 |
| Quezon mobatvirus2 | Quezon virus | QZNV | [33] | MT1720/1657 |
| Rockport orthohantavirus | Rockport virus | RKPV | [71] | MSB57412 |
| Yakeshi orthohantavirus | Yákèshí virus | YKSV | [60] | Yákèshí-Si-210 |
| New Hantavirid Species | Hantavirid | Hantavirid Abbreviation | Reference | Isolate Used for Analysis |
|---|---|---|---|---|
| Hagfish agnathovirus | Wēnlǐng hagfish virus | WEHV | [7] | DHMMS23081 |
| Batfish actinovirus | Wēnlǐng minipizza batfish virus | WEMBV | [7] | XQTMS16810 |
| Spikefish actinovirus | Wēnlǐng red spikefish virus | WERSV | [7] | XTXMS70955 |
| Goosefish actinovirus | Wēnlǐng yellow goosefish virus | WEYGV | [7] | XQTMS34106 |
| Seewis orthohantavirus | Seewis virus | SWSV | [34] | EWS25 |
| Tigray orthohantavirus | Tigray virus | TIGV | [72] | ET2121 |
| Gecko reptillovirus | Hǎinán oriental leaf-toed gecko virus | HOLGV | [7] | LPXYC213122 |
| Subfamily | Genus | Number of Genus-Included Species | Number of Genus-Assigned Viruses | Host (s) |
|---|---|---|---|---|
| Actantavirinae | Actinovirus | 3 | 3 | Ray-finned fish |
| Agantavirinae | Agnathovirus | 1 | 1 | Jawless fish |
| Mammantavirinae | Loanvirus | 1 | 1 | Bats |
| Mobatvirus | 3 | 3 | Bats or moles | |
| Orthohantavirus | 36 | 58 | Rodents or shrews | |
| Thottimvirus | 2 | 2 | Shrews | |
| Repantavirinae | Reptillovirus | 1 | 1 | Reptiles |
| Taxonomic Rank | Taxon |
|---|---|
| Realm Kingdom Phylum | Riboviria Unassigned Negarnaviricota |
| Subphylum | Polyploviricotina |
| Class | Ellioviricetes |
| Order | Bunyavirales |
| Subfamily | Genus | Species | Virus (Abbreviation) |
|---|---|---|---|
| Actantavirinae | Actinovirus | Batfish actinovirus * | Wēnlǐng minipizza batfish virus (WEMBV) |
| Goosefish actinovirus | Wēnlǐng yellow goosefish virus (WEYGV) | ||
| Spikefish actinovirus | Wēnlǐng red spikefish virus (WERSV) | ||
| Agantavirinae | Agnathovirus | Hagfish agnathovirus * | Wēnlǐng hagfish virus (WEHV) |
| Mammantavirinae | Loanvirus | Longquan loanvirus * | Lóngquán virus (LQUV) |
| Mobatvirus | Laibin mobatvirus | Láibīn virus (LAIV) | |
| Nova mobatvirus * | Nova virus (NVAV) | ||
| Quezon mobatvirus | Quezon virus (QZNV) | ||
| Orthohantavirus | Andes orthohantavirus | Andes virus (ANDV) | |
| Castelo dos Sonhos virus (CASV) | |||
| Lechiguanas virus (LECV = LECHV) | |||
| Orán virus (ORNV) | |||
| Asama orthohantavirus | Asama virus (ASAV) | ||
| Asikkala orthohantavirus | Asikkala virus (ASIV) | ||
| Bayou orthohantavirus | bayou virus (BAYV) | ||
| Catacamas virus (CATV) | |||
| Black Creek Canal orthohantavirus | Black Creek Canal virus (BCCV) | ||
| Bowe orthohantavirus | Bowé virus (BOWV) | ||
| Bruges orthohantavirus | Bruges virus (BRGV) | ||
| Cano Delgadito orthohantavirus | Caño Delgadito virus (CADV) | ||
| Cao Bang orthohantavirus | Cao Bằng virus (CBNV) | ||
| Liánghé virus (LHEV) | |||
| Choclo orthohantavirus | Choclo virus (CHOV) | ||
| Dabieshan orthohantavirus | Dàbiéshān virus (DBSV) | ||
| Dobrava-Belgrade orthohantavirus | Dobrava virus (DOBV) | ||
| Kurkino virus (KURV) | |||
| Saaremaa virus (SAAV) | |||
| Sochi virus (SOCV) | |||
| El Moro Canyon orthohantavirus | Carrizal virus (CARV) | ||
| El Moro Canyon virus (ELMCV) | |||
| Huitzilac virus (HUIV) | |||
| Fugong orthohantavirus | Fúgòng virus (FUGV) | ||
| Fusong orthohantavirus | Fǔsōng virus (FUSV) | ||
| Hantaan orthohantavirus * | Amur virus (AMRV) | ||
| Hantaan virus (HTNV) | |||
| Soochong virus (SOOV) | |||
| Jeju orthohantavirus | Jeju virus (JJUV) | ||
| Kenkeme orthohantavirus | Kenkeme virus (KKMV) | ||
| Khabarovsk orthohantavirus | Khabarovsk virus (KHAV) | ||
| Topografov virus (TOPV) | |||
| Laguna Negra orthohantavirus | Laguna Negra virus (LANV) | ||
| Maripa virus (MARV) | |||
| Rio Mamoré virus (RIOMV) | |||
| Luxi orthohantavirus | Lúxī virus (LUXV) | ||
| Maporal orthohantavirus | Maporal virus (MAPV) | ||
| Montano orthohantavirus | Montaño virus (MTNV) | ||
| Necocli orthohantavirus | Necoclí virus (NECV) | ||
| Oxbow orthohantavirus | Oxbow virus (OXBV) | ||
| Prospect Hill orthohantavirus | Prospect Hill virus (PHV) | ||
| Puumala orthohantavirus | Hokkaido virus (HOKV) | ||
| Muju virus (MUJV) | |||
| Puumala virus (PUUV) | |||
| Rockport orthohantavirus | Rockport virus (RKPV) | ||
| Sangassou orthohantavirus | Sangassou virus (SANGV) | ||
| Seewis orthohantavirus | Seewis virus (SWSV) | ||
| Seoul orthohantavirus | gōu virus (GOUV) | ||
| Seoul virus (SEOV) | |||
| Sin Nombre orthohantavirus | New York virus (NYV) | ||
| Sin Nombre virus (SNV) | |||
| Thailand orthohantavirus | Anjozorobe virus (ANJZV) | ||
| Serang virus (SERV) | |||
| Thailand virus (THAIV) | |||
| Tigray orthohantavirus | Tigray virus (TIGV) | ||
| Tula orthohantavirus | Adler virus (ADLV) | ||
| Tula virus (TULV) | |||
| Yakeshi orthohantavirus | Yákèshí virus (YKSV) | ||
| Thottimvirus | Imjin thottimvirus | Imjin virus (MJNV) | |
| Thottapalayam thottimvirus * | Thottapalayam virus (TPMV) | ||
| Repantavirinae | Reptillovirus | Gecko reptillovirus * | Hǎinán oriental leaf-toed gecko virus (HOLGV) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Laenen, L.; Vergote, V.; Calisher, C.H.; Klempa, B.; Klingström, J.; Kuhn, J.H.; Maes, P. Hantaviridae: Current Classification and Future Perspectives. Viruses 2019, 11, 788. https://doi.org/10.3390/v11090788
Laenen L, Vergote V, Calisher CH, Klempa B, Klingström J, Kuhn JH, Maes P. Hantaviridae: Current Classification and Future Perspectives. Viruses. 2019; 11(9):788. https://doi.org/10.3390/v11090788
Chicago/Turabian StyleLaenen, Lies, Valentijn Vergote, Charles H. Calisher, Boris Klempa, Jonas Klingström, Jens H. Kuhn, and Piet Maes. 2019. "Hantaviridae: Current Classification and Future Perspectives" Viruses 11, no. 9: 788. https://doi.org/10.3390/v11090788
APA StyleLaenen, L., Vergote, V., Calisher, C. H., Klempa, B., Klingström, J., Kuhn, J. H., & Maes, P. (2019). Hantaviridae: Current Classification and Future Perspectives. Viruses, 11(9), 788. https://doi.org/10.3390/v11090788







