Highly Divergent Genetic Variants of Soricid-Borne Altai Virus (Hantaviridae) in Eurasia Suggest Ancient Host-Switching Events
Abstract
:1. Introduction
2. Materials and Methods
2.1. Trapping and Sample Collection
2.2. RNA Extraction and RT-PCR Analysis
2.3. Genetic Analysis
2.4. Recombination Analysis
2.5. Phylogenetic Analysis
2.6. Mitochondrial DNA (mtDNA) Host Phylogeny
2.7. Virus Isolation
3. Results
3.1. RT-PCR Detection of Hantavirus RNA
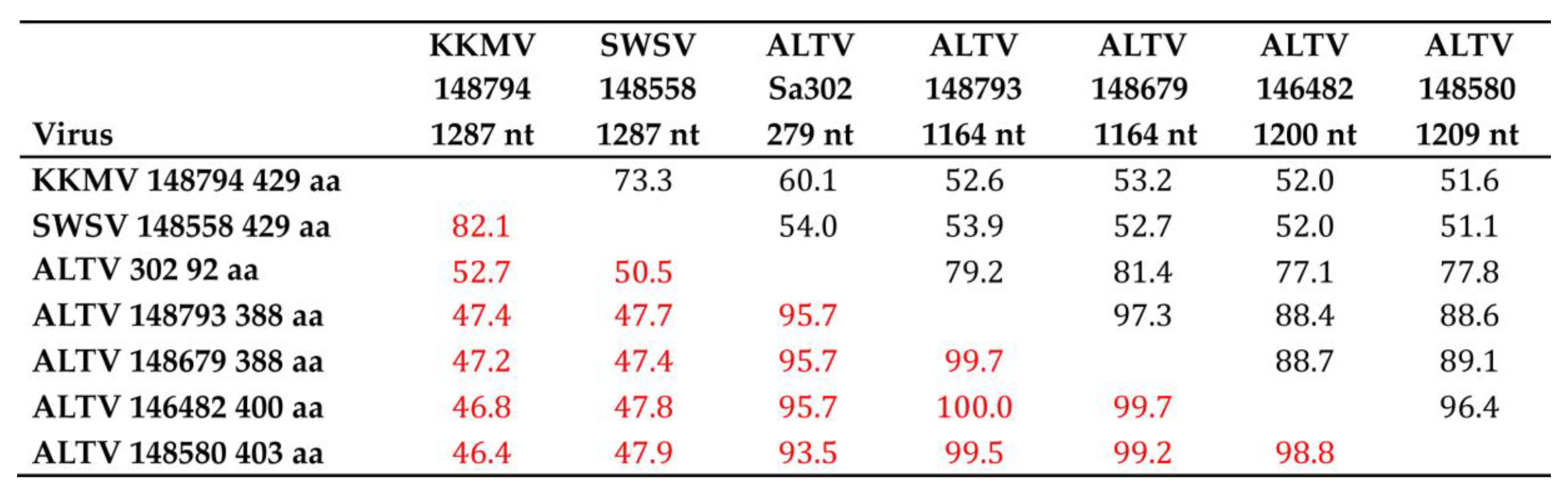
3.2. Genetic Analysis
3.3. Recombination Analysis
3.4. Phylogenetic Analysis
3.5. Host Phylogeny Analysis
3.6. Virus Isolation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Nucleotide Sequences
References
- Carey, D.E.; Reuben, R.; Panicker, K.N.; Shope, R.E.; Myers, R.M. Thottapalayam virus: A presumptive arbovirus isolated from a shrew in India. Indian J. Med. Res. 1971, 59, 1758–1760. [Google Scholar] [PubMed]
- Lee, H.W.; Lee, P.-W.; Johnson, K.M. Isolation of the etiologic agent of Korean hemorrhagic fever. J. Infect. Dis. 1978, 137, 298–308. [Google Scholar] [CrossRef] [PubMed]
- Gavrilovskaya, I.N.; Apekina, N.S.; Myasnikov, Y.A.; Bernshtein, A.D.; Ryltseva, E.V.; Gorbachkova, E.A.; Chumakov, M.P. Features of circulation of hemorrhagic fever with renal syndrome (HFRS) virus among small mammals in the European U.S.S.R. Arch. Virol. 1983, 75, 313–316. [Google Scholar] [CrossRef] [PubMed]
- Tkachenko, E.A.; Ivanov, A.P.; Donets, M.A.; Miasnikov, Y.A.; Ryltseva, E.V.; Gaponova, L.K.; Bashkirtsev, V.N.; Okulova, N.M.; Drozdov, S.G.; Slonova, R.A.; et al. Potential reservoir and vectors of haemorrhagic fever with renal syndrome (HFRS) in the U.S.S.R. Ann. Soc. Belg. Med. Trop. 1983, 63, 267–269. [Google Scholar] [PubMed]
- Gligić, A.; Stojanović, R.; Obradović, M.; Hlača, D.; Dimković, N.; Diglisić, G.; Lukac, V.; Ler, Z.; Bogdanović, R.; Antonijević, B.; et al. Hemorrhagic fever with renal syndrome in Yugoslavia: Epidemiologic and epizootiologic features of a nationwide outbreak in 1989. Eur. J. Epidemiol. 1992, 8, 816–825. [Google Scholar] [CrossRef] [PubMed]
- Song, J.-W.; Kang, H.J.; Gu, S.H.; Moon, S.S.; Bennett, S.N.; Song, K.-J.; Baek, L.J.; Kim, H.C.; O’Guinn, M.L.; Chong, S.T.; et al. Characterization of Imjin virus, a newly isolated hantavirus from the Ussuri white-toothed shrew (Crocidura lasiura). J. Virol. 2009, 83, 6184–6191. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.H.; Kang, H.J.; Baek, L.J.; Noh, J.Y.; Kim, H.C.; Klein, T.A.; Yanagihara, R.; Song, J.-W. Genetic diversity of Imjin virus in the Ussuri white-toothed shrew (Crocidura lasiura) in the Republic of Korea, 2004–2010. Virol. J. 2011, 8, 56. [Google Scholar] [CrossRef] [PubMed]
- Yanagihara, R.; Gu, S.H.; Arai, S.; Kang, H.J.; Song, J.-W. Hantaviruses: Rediscovery and new beginnings. Virus Res. 2014, 187, 6–14. [Google Scholar] [CrossRef] [Green Version]
- Bennett, S.N.; Gu, S.H.; Kang, H.J.; Arai, S.; Yanagihara, R. Reconstructing the evolutionary origins and phylogeography of hantaviruses. Trends Microbiol. 2014, 22, 473–482. [Google Scholar] [CrossRef] [Green Version]
- Arai, S.; Song, J.-W.; Sumibcay, L.; Bennett, S.N.; Nerurkar, V.R.; Parmenter, C.; Cook, J.A.; Yates, T.L.; Yanagihara, R. Hantavirus in northern short-tailed shrew, United States. Emerg. Infect. Dis. 2007, 13, 1420–1423. [Google Scholar] [CrossRef]
- Song, J.-W.; Kang, H.J.; Song, K.-J.; Truong, T.T.; Bennett, S.N.; Arai, S.; Truong, N.U.; Yanagihara, R. Newfound hantavirus in Chinese mole hantavirus in Chinese mole shrew, Vietnam. Emerg. Infect. Dis. 2007, 13, 1784–1787. [Google Scholar] [CrossRef] [PubMed]
- Song, J.-W.; Gu, S.H.; Bennett, S.N.; Arai, S.; Puorger, M.; Hilbe, M.; Yanagihara, R. Seewis virus, a genetically distinct hantavirus in the Eurasian common shrew (Sorex araneus). Virol. J. 2007, 4, 114. [Google Scholar] [CrossRef] [PubMed]
- Arai, S.; Bennett, S.N.; Sumibcay, L.; Cook, J.A.; Song, J.-W.; Hope, A.; Parmenter, C.; Nerurkar, V.R.; Yates, T.L.; Yanagihara, R. Phylogenetically distinct hantaviruses in the masked hrew (Sorex cinereus) and dusky shrew (Sorex monticolus) in the United States. Am. J. Trop. Med. Hyg. 2008, 78, 348–351. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.J.; Arai, S.; Hope, A.G.; Cook, J.A.; Yanagihara, R. Novel hantavirus in the flat-skulled shrew (Sorex roboratus). Vector Borne Zoonotic Dis. 2010, 10, 593–597. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.J.; Kadjo, B.; Dubey, S.; Jacquet, F.; Yanagihara, R. Molecular evolution of Azagny virus, a newfound hantavirus harbored by the West African pygmy shrew (Crocidura obscurior) in Côte d’Ivoire. Virol. J. 2011, 8, 373. [Google Scholar] [CrossRef]
- Arai, S.; Gu, S.H.; Baek, L.J.; Tabara, K.; Bennett, S.N.; Oh, H.S.; Takada, N.; Kang, H.J.; Tanaka-Taya, K.; Morikawa, S.; et al. Divergent ancestral lineages of newfound hantaviruses harbored by phylogenetically related crocidurine shrew species in Korea. Virology 2012, 424, 99–105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gu, S.H.; Markowski, J.; Kang, H.J.; Hejduk, J.; Sikorska, B.; Liberski, P.P.; Yanagihara, R. Boginia virus, a newfound hantavirus harbored by the Eurasian water shrew (Neomys fodiens) in Poland. Virol. J. 2013, 10, 160. [Google Scholar] [CrossRef]
- Gu, S.H.; Nicolas, V.; Lalis, A.; Sathirapongsasuti, N.; Yanagihara, R. Complete genome sequence analysis and molecular phylogeny of a newfound hantavirus harbored by the Doucet’s musk shrew (Crocidura douceti) in Guinea. Infect. Genet. Evol. 2013, 20, 118–123. [Google Scholar] [CrossRef]
- Kang, H.J.; Stanley, W.T.; Esselstyn, J.A.; Gu, S.H.; Yanagihara, R. Expanded host diversity and geographic distribution of hantaviruses in sub-Saharan Africa. J. Virol. 2014, 88, 7663–7667. [Google Scholar] [CrossRef]
- Arai, S.; Kang, H.J.; Gu, S.H.; Ohdachi, S.D.; Cook, J.A.; Yashina, L.N.; Tanaka-Taya, K.; Abramov, S.A.; Morikawa, S.; Okabe, N.; et al. Genetic diversity of Artybash virus in the Laxmann’s shrew (Sorex caecutiens). Vector Borne Zoonotic Dis. 2016, 16, 468–475. [Google Scholar] [CrossRef]
- Gu, S.H.; Arai, S.; Yu, H.-T.; Lim, B.K.; Kang, H.J.; Song, J.-W.; Yanagihara, R. Genetic variants of Cao Bang hantavirus in the Chinese mole shrew (Anourosorex squamipes) and Taiwanese mole shrew (Anourosorex yamashinai). Infect. Genet. Evol. 2016, 40, 113–118. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, M.; Radosa, L.; Rosenfeld, U.M.; Schmidt, S.; Triebenbacher, C.; Löhr, P.W.; Fuchs, D.; Heroldová, M.; Jánová, E.; Stanko, M.; et al. Broad geographical distribution and high genetic diversity of shrew-borne Seewis hantavirus in Central Europe. Virus Genes 2012, 45, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.J.; Arai, S.; Hope, A.G.; Song, J.-W.; Cook, J.A.; Yanagihara, R. Genetic diversity and phylogeography of Seewis virus in the Eurasian common shrew in Finland and Hungary. Virol. J. 2009, 6, 208. [Google Scholar] [CrossRef] [PubMed]
- Ling, J.; Sironen, T.; Voutilainen, L.; Hepojoki, S.; Niemimaa, J.; Isoviita, V.M.; Vaheri, A.; Henttonen, H.; Vapalahti, O. Hantaviruses in Finnish soricomorphs: Evidence for two distinct hantaviruses carried by Sorex araneus suggesting ancient host-switch. Infect. Genet. Evol. 2014, 27, 51–61. [Google Scholar] [CrossRef] [PubMed]
- Ling, J.; Smura, T.; Tamarit, D.; Huitu, O.; Voutilainen, L.; Henttonen, H.; Vaheri, A.; Vapalahti, O.; Sironen, T. Evolution and postglacial colonization of Seewis hantavirus with Sorex araneus in Finland. Infect. Genet. Evol. 2018, 57, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.H.; Hejduk, J.; Markowski, J.; Kang, H.J.; Markowski, M.; Połatyńska, M.; Sikorska, B.; Liberski, P.P.; Yanagihara, R. Co-circulation of soricid- and talpid-borne hantaviruses in Poland. Infect. Genet. Evol. 2014, 28, 296–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yashina, L.; Abramov, S.; Gutorov, V.; Dupal, T.; Krivopalov, A.; Panov, V.; Danchinova, G.; Vinogradov, V.; Luchnikova, E.; Hay, J.; et al. Seewis virus: Phylogeography of a shrew-borne hantavirus in Siberia, Russia. Vector-Borne Zoonotic Dis. 2010, 10, 585–591. [Google Scholar] [CrossRef] [PubMed]
- Korva, M.; Knap, N.; Rus, K.R.; Fajs, L.; Grubelnik, G.; Bremec, M.; Knapič, T.; Trilar, T.; Županc, T.A. Phylogeographic diversity of pathogenic and non-pathogenic hantaviruses in Slovenia. Viruses 2013, 5, 3071–3087. [Google Scholar] [CrossRef] [PubMed]
- Resman, K.; Korva, M.; Fajs, L.; Zidarič, T.; Trilar, T.; Županc, T.A. Molecular evidence and high genetic diversity of shrew-borne Seewis virus in Slovenia. Virus Res. 2013, 177, 113–117. [Google Scholar] [CrossRef] [PubMed]
- Yashina, L.N.; Kartashov, M.Y.; Wang, W.; Li, K.; Zdanovskaya, N.I.; Ivanov, L.I.; Zhang, Y.Z. Co-circulation of distinct shrew-borne hantaviruses in the far east of Russia. Virus Res. 2019, 272, 197717. [Google Scholar] [CrossRef] [PubMed]
- Laenen, L.; Vergote, V.; Calisher, C.H.; Klempa, B.; Klingström, J.; Kuhn, J.H.; Maes, P. Hantaviridae: Current classification and future perspectives. Viruses 2019, 11, 788. [Google Scholar] [CrossRef] [PubMed]
- Cook, J.A.; Galbreath, K.E.; Bell, K.C.; Campbell, M.L.; Carrière, S.; Colella, J.P.; Dawson, N.G.; Dunnum, J.L.; Eckerlin, R.P.; Greiman, S.E.; et al. The Beringian Coevolution Project: Holistic collections of mammals and associated parasites reveal novel perspectives on evolutionary and environmental change in the North. Arctic Sci. 2017, 3, 585–617. [Google Scholar] [CrossRef]
- Sikes, R.S.; Animal Care and Use Committee of the American Society of Mammalogists. 2016 Guidelines of the American Society of Mammalogists for the use of wild mammals in research and education. J. Mammal. 2016, 97, 663–688. [Google Scholar] [CrossRef] [PubMed]
- Dunnum, J.L.; Yanagihara, R.; Johnson, K.M.; Armien, B.; Batsaikhan, N.; Morgan, L.; Cook, J.A. Biospecimen repositories and integrated databases as critical infrastructure for pathogen discovery and pathobiology research. PLoS Negl. Trop. Dis. 2017, 11, e0005133. [Google Scholar] [CrossRef] [PubMed]
- Abascal, F.; Zardoya, R.; Telford, M.J. TranslatorX: Multiple alignment of nucleotide sequences guided by amino acid translations. Nucleic Acids Res. 2010, 38, W7–W13. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. (Oxf.) 1999, 41, 95–98. [Google Scholar]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef]
- Lupas, A.; Van Dyke, M.; Stock, J. Predicting coiled coils from protein sequences. Science 1991, 252, 1162–1164. [Google Scholar] [CrossRef]
- Pond, S.L.; Frost, S.D.W.; Muse, S.V. HyPhy: Hypothesis testing using phylogenies. Bioinformatics 2005, 21, 676–679. [Google Scholar] [CrossRef]
- Stamatakis, A.; Hoover, P.; Rougemont, J. A rapid bootstrap algorithm for the RAxML Web servers. Syst. Biol. 2008, 57, 758–771. [Google Scholar] [CrossRef]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Posada, D.; Crandall, K.A. MODELTEST: Testing the model of DNA substitution. Bioinformatics 1998, 14, 817–818. [Google Scholar] [CrossRef] [PubMed]
- Posada, D. jModelTest: Phylogenetic model averaging. Mol. Biol. Evol. 2008, 25, 1253–1256. [Google Scholar] [CrossRef] [PubMed]
- Irwin, D.M.; Kocher, T.D.; Wilson, A.C. Evolution of the cytochrome b gene of mammals. J. Mol. Evol. 1991, 32, 128–144. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.J.; Bennett, S.N.; Dizney, L.; Sumibcay, L.; Arai, S.; Ruedas, L.A.; Song, J.-W.; Yanagihara, R. Host switch during evolution of a genetically distinct hantavirus in the American shrew mole (Neurotrichus gibbsii). Virology 2009, 388, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Kang, H.J.; Bennett, S.N.; Sumibcay, L.; Arai, S.; Hope, A.G.; Mocz, G.; Song, J.-W.; Cook, J.A.; Yanagihara, R. Evolutionary insights from a genetically divergent hantavirus harbored by the European common mole (Talpa europaea). PLoS ONE 2009, 4, e6149. [Google Scholar] [CrossRef] [PubMed]
- Gu, S.H.; Kumar, M.; Sikorska, B.; Hejduk, J.; Markowski, J.; Markowski, M.; Liberski, P.P.; Yanagihara, R. Isolation and partial characterization of a highly divergent lineage of hantavirus from the European mole (Talpa europaea). Sci. Rep. 2016, 6, 21119. [Google Scholar] [CrossRef]
- Radosa, L.; Schlegel, M.; Gebauer, P.; Ansorge, H.; Heroldová, M.; Jánová, E.; Stanko, M.; Mošanský, L.; Fričová, J.; Pejčoch, M.; et al. Detection of shrew-borne hantavirus in Eurasian pygmy shrew (Sorex minutus) in Central Europe. Infect. Genet. Evol. 2013, 19, 403–410. [Google Scholar] [CrossRef]
- Hinson, E.R.; Shone, S.M.; Zink, M.C.; Glass, G.E.; Klein, S.L. Wounding: The primary mode of Seoul virus transmission among male Norway rats. Am. J. Trop. Med. Hyg. 2004, 70, 310–317. [Google Scholar] [CrossRef]
- Calisher, C.H.; Root, J.J.; Mills, J.N.; Rowe, J.E.; Reeder, S.A.; Jentes, E.S.; Wagoner, K.; Beaty, B.J. Epizootiology of Sin Nombre and El Moro Canyon hantaviruses, southeastern Colorado, 1995–2000. J. Wildl. Dis. 2005, 41, 1–11. [Google Scholar] [CrossRef]
- Douglass, R.J.; Wilson, T.; Semmens, W.J.; Zanto, S.N.; Bond, C.W.; Van Horn, R.C.; Mills, J.N. Longitudinal studies of Sin Nombre virus in deer mouse dominated ecosystems of Montana. Am. J. Trop. Med. Hyg. 2001, 65, 33–41. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, N.E.; Chu, Y.K.; Owen, R.D.; Abuzeineh, A.; De la Sancha, N.; Dick, C.W.; Holsomback, T.; Nisbett, R.A.; Jonsson, C. A longitudinal study of Bayou virus, hosts, and habitat. Am. J. Trop. Med. Hyg. 2005, 73, 1043–1049. [Google Scholar] [CrossRef] [PubMed]
- Yanagihara, R.; Gu, S.H.; Song, J.-W. Expanded host diversity and global distribution of hantaviruses: Implications for identifying and investigating previously unrecognized hantaviral diseases. In Global Virology—Identifying and Investigating Viral Diseases; Shapshak, P., Sinnott, J.T., Somboonwit, C., Kuhn, J., Eds.; Springer Publishing Company: New York, NY, USA, 2015; pp. 161–198. [Google Scholar]
- Arai, S.; Yanagihara, R. Genetic diversity and geographic distribution of bat-borne hantaviruses. In Bat-Borne Viruses; Corrales-Aguilar, E., Schwemmle, M., Eds.; Caister Academic Press: Poole, UK, 2019; pp. 59–86. [Google Scholar]
- Chu, Y.K.; Owen, R.D.; Jonsson, C.B. Phylogenetic exploration of hantaviruses in Paraguay reveals reassortment and host switching in South America. Virol. J. 2011, 8, 399. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.A.; Kim, W.K.; No, J.S.; Lee, S.H.; Lee, S.Y.; Kim, J.H.; Kho, J.H.; Lee, D.; Song, D.H.; Gu, S.H.; et al. Genetic diversity and reassortment of Hantaan virus tripartite RNA genomes in nature, the Republic of Korea. PLoS Negl. Trop. Dis. 2016, 10, e0004650. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Kim, W.K.; No, J.S.; Kim, J.A.; Kim, J.I.; Gu, S.H.; Kim, H.C.; Klein, T.A.; Park, M.S.; Song, J.-W. Dynamic circulation and genetic exchange of a shrew-borne hantavirus, Imjin virus, in the Republic of Korea. Sci. Rep. 2017, 7, 44369. [Google Scholar] [CrossRef]
- Laenen, L.; Vergote, V.; Kafetzopoulou, L.E.; Wawina, T.B.; Vassou, D.; Cook, J.A.; Hugot, J.P.; Deboutte, W.; Kang, H.J.; Witkowski, P.T.; et al. A novel hantavirus of the European mole, Bruges virus, is involved in frequent Nova virus coinfections. Genome Biol. Evol. 2018, 10, 45–55. [Google Scholar] [CrossRef] [PubMed]
- Klempa, B. Reassortment events in the evolution of hantaviruses. Virus Genes 2018, 54, 638–646. [Google Scholar] [CrossRef] [Green Version]



| Country | Collection Site | Species | Year | No. Tested | HantavirusRNA Positive | Divergent Sequence |
|---|---|---|---|---|---|---|
| Finland | Etelä-Suomen Lääni | Sorex araneus | 1982 | 10 | 4 | 0 |
| Lappi | Sorex araneus | 1982 | 3 | 1 | 0 | |
| Oulun Lääni | Sorex araneus | 1982 | 9 | 7 | 0 | |
| Hungary | Györ-Sopron-Moson | Sorex araneus | 1997 | 19 | 11 | 1 |
| Nógrád | Sorex araneus | 1997 | 3 | 1 | 1 | |
| Zala | Sorex araneus | 2000 | 44 | 3 | 0 | |
| Poland | Chmiel | Sorex araneus | 2010 | 11 | 4 | 0 |
| Sorex minutus | 2010 | 7 | 1 | 1 | ||
| Huta Dłutowska | Sorex araneus | 2011 | 9 | 2 | 0 | |
| Kurowice | Sorex araneus | 2013 | 13 | 5 | 0 | |
| Russia | Teletskoye Lake | Sorex araneus | 2007 | 9 | 6 | 1 |
| Irkutsk City | Sorex daphaenodon | 2007 | 2 | 2 | 0 | |
| Amga River | Sorex caecutiens | 2006 | 19 | 10 | 5 | |
| Sorex minutissimus | 2006 | 5 | 1 | 1 | ||
| Kenkeme River | Sorex caecutiens | 2006 | 24 | 4 | 4 | |
| Sorex daphaenodon | 2006 | 4 | 0 | 0 | ||
| Sorex roboratus | 2006 | 12 | 4 | 3 | ||
| Lena River | Sorex caecutiens | 2006 | 6 | 1 | 1 | |
| Sorex tundrensis | 2006 | 5 | 0 | 0 |
| Site | Species | Virus | MSB | Sex | Date | Hantavirus Sequence | ||
|---|---|---|---|---|---|---|---|---|
| S | M | L | ||||||
| Amga | Sorex caecutiens | SWSV | 148347 | male | 14 August 2006 | 956 | 731 | 751, 1304, 1046 |
| River | Sorex caecutiens | SWSV | 148436 | male | 12 August 2006 | 1094 | 2414, 476 | |
| Sorex caecutiens | SWSV | 148457 | male | 12 August 2006 | 1095 | 2414, 476 | ||
| Sorex caecutiens | SWSV | 148558 | male | 10 August 2006 | 1627 | 1088 | 4598 | |
| Sorex caecutiens | SWSV | 148559 | female | 10 August 2006 | 971 | 2414, 476 | ||
| Sorex caecutiens | ALTV | 148458 | male | 12 August 2006 | 1385 | 558 | 6535 | |
| Sorex caecutiens | ALTV | 148573 | male | 14 August 2006 | 704 | 400, 409 | ||
| Sorex caecutiens | ALTV | 148574 | male | 14 August 2006 | 804 | 409 | ||
| Sorex caecutiens | ALTV | 148575 | male | 14 August 2006 | 409 | |||
| Sorex caecutiens | ALTV | 148580 | male | 09 August 2006 | 1600 | 568 | 6535 | |
| Sorex minutissimus | ALTV | 148651 | male | 14 August 2006 | 362 | |||
| Kenkeme | Sorex caecutiens | ALTV | 148745 | male | 20 August 2006 | 703 | 241, 409 | |
| River | Sorex caecutiens | ALTV | 148793 | female | 20 August 2006 | 1600 | 890 | 6533 |
| Sorex caecutiens | ALTV | 148830 | male | 21 August 2006 | 409 | |||
| Sorex caecutiens | ALTV | 148840 | male | 20 August 2006 | 686 | 409 | ||
| Sorex roboratus | ALTV | 148679 | male | 21 August 2006 | 1664 | 907 | 6533 | |
| Sorex roboratus | ALTV | 148833 | male | 20 August 2006 | 208 | |||
| Sorex roboratus | ALTV | 148839 | female | 20 August 2006 | 409 | |||
| Sorex roboratus | KKMV | 148794 | male | 20 August 2006 | 1640 | 1002 | 4304 | |
| Lena River | Sorex caecutiens | ALTV | 146482 | male | 02 August 2006 | 1600 | 6500 | |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, H.J.; Gu, S.H.; Yashina, L.N.; Cook, J.A.; Yanagihara, R. Highly Divergent Genetic Variants of Soricid-Borne Altai Virus (Hantaviridae) in Eurasia Suggest Ancient Host-Switching Events. Viruses 2019, 11, 857. https://doi.org/10.3390/v11090857
Kang HJ, Gu SH, Yashina LN, Cook JA, Yanagihara R. Highly Divergent Genetic Variants of Soricid-Borne Altai Virus (Hantaviridae) in Eurasia Suggest Ancient Host-Switching Events. Viruses. 2019; 11(9):857. https://doi.org/10.3390/v11090857
Chicago/Turabian StyleKang, Hae Ji, Se Hun Gu, Liudmila N. Yashina, Joseph A. Cook, and Richard Yanagihara. 2019. "Highly Divergent Genetic Variants of Soricid-Borne Altai Virus (Hantaviridae) in Eurasia Suggest Ancient Host-Switching Events" Viruses 11, no. 9: 857. https://doi.org/10.3390/v11090857
APA StyleKang, H. J., Gu, S. H., Yashina, L. N., Cook, J. A., & Yanagihara, R. (2019). Highly Divergent Genetic Variants of Soricid-Borne Altai Virus (Hantaviridae) in Eurasia Suggest Ancient Host-Switching Events. Viruses, 11(9), 857. https://doi.org/10.3390/v11090857





