Detection of Ebola Virus Antibodies in Fecal Samples of Great Apes in Gabon
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Sample Collection and RNA Isolation
2.3. EBOV, SUDV, BDBV, and MARV RNA Detection
2.4. Multiplexed Analysis of Anti-EBOV Antibodies in Great Ape Fecal Samples
2.4.1. Antigens
2.4.2. Recombinant Antigen Coupling to Microsphere
2.4.3. Multiplexed Immunoassay
2.5. Statistical Analysis
3. Results
3.1. No Viral RNA Detection
3.2. Feasibility of Antibody Detection in a Small Fecal Sample Volumes
3.3. Prevalence of IgG and IgM Antibodies Against EBOV in Fecal Samples of Gorillas and Chimpanzees
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Goldstein, T.; Anthony, S.J.; Gbakima, A.; Bird, B.H.; Bangura, J.; Tremeau-Bravard, A.; Belaganahalli, M.N.; Wells, H.L.; Dhanota, J.K.; Liang, E.; et al. The discovery of Bombali virus adds further support for bats as hosts of ebolaviruses. Nat. Microbiol. 2018, 3, 1084–1089. [Google Scholar] [CrossRef]
- WHO. Ebola haemorrhagic fever in Zaire, 1976. Bull. World Health Organ. 1978, 56, 271–293. [Google Scholar]
- Malvy, D.; McElroy, A.K.; de Clerck, H.; Gunther, S.; van Griensven, J. Ebola virus disease. Lancet 2019, 393, 936–948. [Google Scholar] [CrossRef] [Green Version]
- Formenty, P.; Boesch, C.; Wyers, M.; Steiner, C.; Donati, F.; Dind, F.; Walker, F.; Le Guenno, B. Ebola virus outbreak among wild chimpanzees living in a rain forest of Cote d’Ivoire. J. Infect. Dis. 1999, 179 (Suppl. S1), S120–S126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Le Guenno, B.; Formenty, P.; Wyers, M.; Gounon, P.; Walker, F.; Boesch, C. Isolation and partial characterisation of a new strain of Ebola virus. Lancet 1995, 345, 1271–1274. [Google Scholar] [CrossRef]
- Hayes, C.G.; Burans, J.P.; Ksiazek, T.G.; Del Rosario, R.A.; Miranda, M.E.; Manaloto, C.R.; Barrientos, A.B.; Robles, C.G.; Dayrit, M.M.; Peters, C.J. Outbreak of fatal illness among captive macaques in the Philippines caused by an Ebola-related filovirus. Am. J. Trop. Med. Hyg. 1992, 46, 664–671. [Google Scholar] [CrossRef] [PubMed]
- Miranda, M.E.; White, M.E.; Dayrit, M.M.; Hayes, C.G.; Ksiazek, T.G.; Burans, J.P. Seroepidemiological study of filovirus related to Ebola in the Philippines. Lancet 1991, 337, 425–426. [Google Scholar] [CrossRef] [Green Version]
- Martell, H.J.; Masterson, S.G.; McGreig, J.E.; Michaelis, M.; Wass, M.N. Is the Bombali virus pathogenic in humans? Bioinformatics (Oxf. Engl.) 2019, 35, 3553–3558. [Google Scholar] [CrossRef]
- Rouquet, P.; Froment, J.M.; Bermejo, M.; Kilbourn, A.; Karesh, W.; Reed, P.; Kumulungui, B.; Yaba, P.; Delicat, A.; Rollin, P.E.; et al. Wild animal mortality monitoring and human Ebola outbreaks, Gabon and Republic of Congo, 2001–2003. Emerg. Infect. Dis. 2005, 11, 283–290. [Google Scholar] [CrossRef] [PubMed]
- Bermejo, M.; Rodriguez-Teijeiro, J.D.; Illera, G.; Barroso, A.; Vila, C.; Walsh, P.D. Ebola outbreak killed 5000 gorillas. Science 2006, 314, 1564. [Google Scholar] [CrossRef] [Green Version]
- Walsh, P.D.; Abernethy, K.A.; Bermejo, M.; Beyers, R.; De Wachter, P.; Akou, M.E.; Huijbregts, B.; Mambounga, D.I.; Toham, A.K.; Kilbourn, A.M.; et al. Catastrophic ape decline in western equatorial Africa. Nature 2003, 422, 611–614. [Google Scholar] [CrossRef] [PubMed]
- Lahm, S.A.; Kombila, M.; Swanepoel, R.; Barnes, R.F. Morbidity and mortality of wild animals in relation to outbreaks of Ebola haemorrhagic fever in Gabon, 1994–2003. Trans. R. Soc. Trop. Med. Hyg. 2007, 101, 64–78. [Google Scholar] [CrossRef] [PubMed]
- Georges, A.J.; Leroy, E.M.; Renaut, A.A.; Benissan, C.T.; Nabias, R.J.; Ngoc, M.T.; Obiang, P.I.; Lepage, J.P.; Bertherat, E.J.; Benoni, D.D.; et al. Ebola hemorrhagic fever outbreaks in Gabon, 1994–1997: Epidemiologic and health control issues. J. Infect. Dis. 1999, 179 (Suppl. S1), S65–S75. [Google Scholar] [CrossRef]
- Leroy, E.M.; Rouquet, P.; Formenty, P.; Souquiere, S.; Kilbourne, A.; Froment, J.M.; Bermejo, M.; Smit, S.; Karesh, W.; Swanepoel, R.; et al. Multiple Ebola virus transmission events and rapid decline of central African wildlife. Science 2004, 303, 387–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maganga, G.D.; Kapetshi, J.; Berthet, N.; Kebela Ilunga, B.; Kabange, F.; Mbala Kingebeni, P.; Mondonge, V.; Muyembe, J.J.; Bertherat, E.; Briand, S.; et al. Ebola virus disease in the Democratic Republic of Congo. N. Engl. J. Med. 2014, 371, 2083–2091. [Google Scholar] [CrossRef]
- Leroy, E.M.; Kumulungui, B.; Pourrut, X.; Rouquet, P.; Hassanin, A.; Yaba, P.; Delicat, A.; Paweska, J.T.; Gonzalez, J.P.; Swanepoel, R. Fruit bats as reservoirs of Ebola virus. Nature 2005, 438, 575–576. [Google Scholar] [CrossRef]
- Pourrut, X.; Delicat, A.; Rollin, P.E.; Ksiazek, T.G.; Gonzalez, J.P.; Leroy, E.M. Spatial and temporal patterns of Zaire ebolavirus antibody prevalence in the possible reservoir bat species. J. Infect. Dis. 2007, 196 (Suppl. S2), S176–S183. [Google Scholar] [CrossRef] [Green Version]
- Ayouba, A.; Ahuka-Mundeke, S.; Butel, C.; Mbala Kingebeni, P.; Loul, S.; Tagg, N.; Villabona-Arenas, C.J.; Lacroix, A.; Ndimbo-Kumugo, S.P.; Keita, A.K.; et al. Extensive Serological Survey of Multiple African Nonhuman Primate Species Reveals Low Prevalence of Immunoglobulin G Antibodies to 4 Ebola Virus Species. J. Infect. Dis. 2019, 220, 1599–1608. [Google Scholar] [CrossRef]
- Leroy, E.M.; Telfer, P.; Kumulungui, B.; Yaba, P.; Rouquet, P.; Roques, P.; Gonzalez, J.P.; Ksiazek, T.G.; Rollin, P.E.; Nerrienet, E. A serological survey of Ebola virus infection in central African nonhuman primates. J. Infect. Dis. 2004, 190, 1895–1899. [Google Scholar] [CrossRef] [Green Version]
- Ogunro, B.N.; Olugasa, B.O.; Verschoor, E.J.; Olarinmoye, A.O.; Theyse, I.; Niphuis, H. Serological Detection of Ebola Virus Exposures in Native Non-human Primates of Southern Nigeria. J. Epidemiol. Glob. Health 2018, 8, 162–170. [Google Scholar] [CrossRef]
- Reed, P.E.; Mulangu, S.; Cameron, K.N.; Ondzie, A.U.; Joly, D.; Bermejo, M.; Rouquet, P.; Fabozzi, G.; Bailey, M.; Shen, Z.; et al. A new approach for monitoring ebolavirus in wild great apes. PLoS Negl. Trop. Dis. 2014, 8, e3143. [Google Scholar] [CrossRef]
- Leroy, E.M.; Baize, S.; Volchkov, V.E.; Fisher-Hoch, S.P.; Georges-Courbot, M.C.; Lansoud-Soukate, J.; Capron, M.; Debre, P.; McCormick, J.B.; Georges, A.J. Human asymptomatic Ebola infection and strong inflammatory response. Lancet 2000, 355, 2210–2215. [Google Scholar] [CrossRef]
- Boundenga, L.; Ollomo, B.; Rougeron, V.; Mouele, L.Y.; Mve-Ondo, B.; Delicat-Loembet, L.M.; Moukodoum, N.D.; Okouga, A.P.; Arnathau, C.; Elguero, E.; et al. Diversity of malaria parasites in great apes in Gabon. Malar. J. 2015, 14, 111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mombo, I.M.; Lukashev, A.N.; Bleicker, T.; Brunink, S.; Berthet, N.; Maganga, G.D.; Durand, P.; Arnathau, C.; Boundenga, L.; Ngoubangoye, B.; et al. African Non-Human Primates Host Diverse Enteroviruses. PLoS ONE 2017, 12, e0169067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Towner, J.S.; Sealy, T.K.; Khristova, M.L.; Albarino, C.G.; Conlan, S.; Reeder, S.A.; Quan, P.L.; Lipkin, W.I.; Downing, R.; Tappero, J.W.; et al. Newly discovered ebola virus associated with hemorrhagic fever outbreak in Uganda. PLoS Pathog. 2008, 4, e1000212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Towner, J.S.; Khristova, M.L.; Sealy, T.K.; Vincent, M.J.; Erickson, B.R.; Bawiec, D.A.; Hartman, A.L.; Comer, J.A.; Zaki, S.R.; Stroher, U.; et al. Marburgvirus genomics and association with a large hemorrhagic fever outbreak in Angola. J. Virol. 2006, 80, 6497–6516. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayouba, A.; Toure, A.; Butel, C.; Keita, A.K.; Binetruy, F.; Sow, M.S.; Foulongne, V.; Delaporte, E.; Peeters, M. Development of a Sensitive and Specific Serological Assay Based on Luminex Technology for Detection of Antibodies to Zaire Ebola Virus. J. Clin. Microbiol. 2017, 55, 165–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- R Development Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Keele, B.F.; Van Heuverswyn, F.; Li, Y.; Bailes, E.; Takehisa, J.; Santiago, M.L.; Bibollet-Ruche, F.; Chen, Y.; Wain, L.V.; Liegeois, F.; et al. Chimpanzee reservoirs of pandemic and nonpandemic HIV-1. Science 2006, 313, 523–526. [Google Scholar] [CrossRef] [Green Version]
- Leroy, E.M.; Souquiere, S.; Rouquet, P.; Drevet, D. Re-emergence of ebola haemorrhagic fever in Gabon. Lancet 2002, 359, 712. [Google Scholar] [CrossRef]
- Boue, V.; Locatelli, S.; Boucher, F.; Ayouba, A.; Butel, C.; Esteban, A.; Okouga, A.P.; Ndoungouet, A.; Motsch, P.; Le Flohic, G.; et al. High Rate of Simian Immunodeficiency Virus (SIV) Infections in Wild Chimpanzees in Northeastern Gabon. Viruses 2015, 7, 4997–5015. [Google Scholar] [CrossRef]
- Huijbregts, B.; De Wachter, P.; Obiang, L.S.N.; Akou, M.E. Ebola and the decline of gorilla Gorilla gorilla and chimpanzee Pan troglodytes populations in Minkebe Forest, north-eastern Gabon. Oryx 2003, 37, 437–443. [Google Scholar] [CrossRef] [Green Version]
- Alexander, K.A.; Sanderson, C.E.; Marathe, M.; Lewis, B.L.; Rivers, C.M.; Shaman, J.; Drake, J.M.; Lofgren, E.; Dato, V.M.; Eisenberg, M.C.; et al. What factors might have led to the emergence of Ebola in West Africa? PLoS Negl. Trop. Dis. 2015, 9, e0003652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pourrut, X.; Souris, M.; Towner, J.S.; Rollin, P.E.; Nichol, S.T.; Gonzalez, J.P.; Leroy, E. Large serological survey showing cocirculation of Ebola and Marburg viruses in Gabonese bat populations, and a high seroprevalence of both viruses in Rousettus aegyptiacus. BMC Infect. Dis. 2009, 9, 159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pigott, D.M.; Golding, N.; Mylne, A.; Huang, Z.; Henry, A.J.; Weiss, D.J.; Brady, O.J.; Kraemer, M.U.; Smith, D.L.; Moyes, C.L.; et al. Mapping the zoonotic niche of Ebola virus disease in Africa. eLife 2014, 3, e04395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
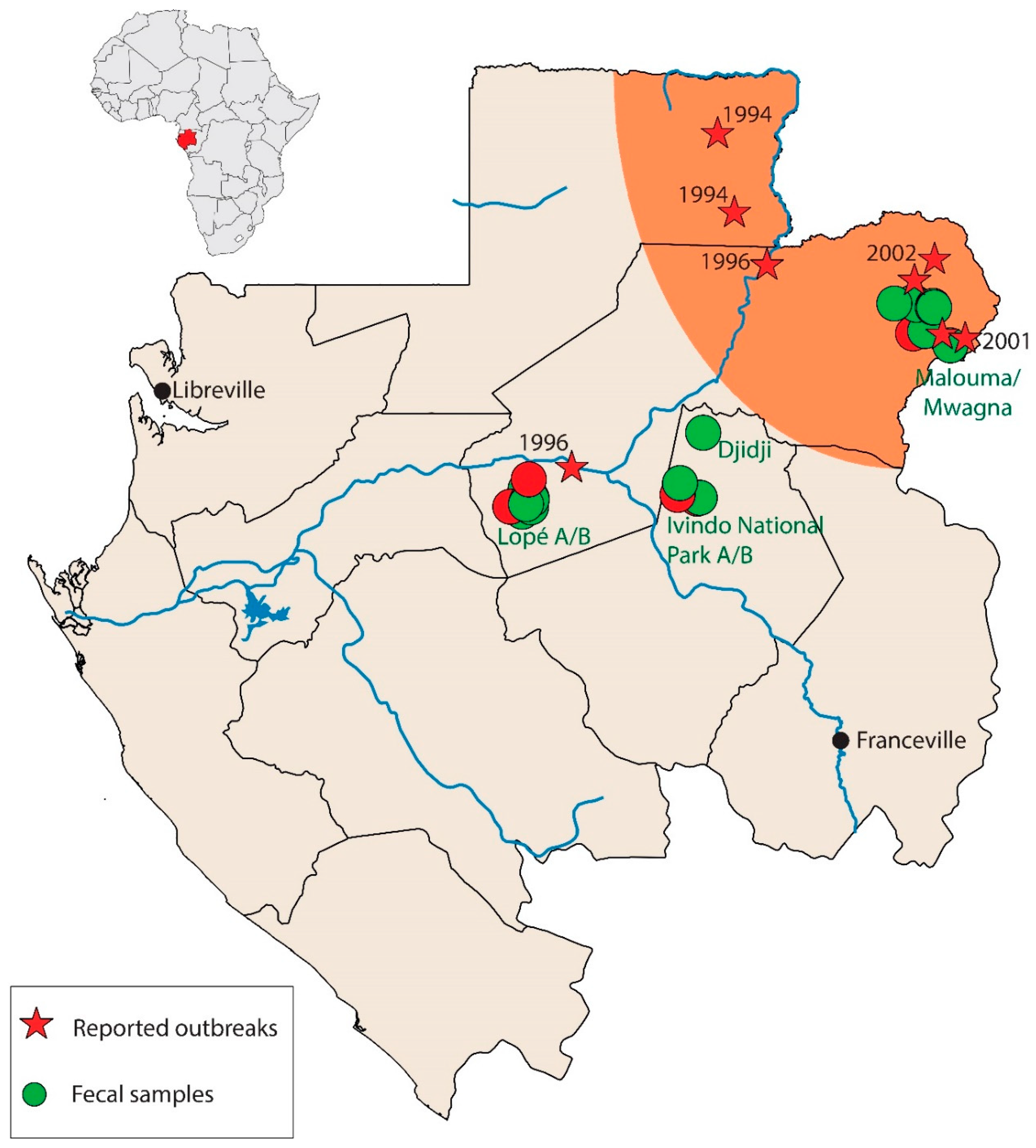
| Zone | Date Collected | Species | No. Tested | IgG Pos/Total Tested | IgM Pos/Total Tested |
|---|---|---|---|---|---|
| Lopé A | October 2009–February 2010 | Gorilla gorilla gorilla | 34 | 0/34 (0.0; 0.0–10.3) | 0/34 (0.0; 0.0–10.3) |
| Pan troglodytes troglodytes | 43 | 3/43 (7; 1.4–19.0) | 3/43 (7; 1.4–19.0) | ||
| Lopé B | April–November 2012 | Gorilla gorilla gorilla | 22 | 0/22 (0.0; 0.0–15.4) | 0/22 (0.0; 0.0–15.4) |
| Pan troglodytes troglodytes | 79 | 1/79 (1.3; 0.0–6.8) | 5/79 (6.4; 2.0–14.1) | ||
| Mwagna | July 2011 | Gorilla gorilla gorilla | 0 | 0/0 | 0/0 |
| Pan troglodytes troglodytes | 5 | 0/5 | 0/5 | ||
| Malouma | January–September 2012 | Gorilla gorilla gorilla | 10 | 0/10 (0.0; 0.0–30.8) | 0/10 (0.0; 30.8) |
| Pan troglodytes troglodytes | 90 | 5/90 (5.5; 1.8–12.5) | 0/90 (0.0; 0.0–4.0) | ||
| INP B | April 2012 | Gorilla gorilla gorilla | 32 | 10/32 (31.2; 16.1–50.0) | 3/32 (9.4; 2.0–25.0) |
| Pan troglodytes troglodytes | 1 | 0/1 | 0/1 | ||
| Djidi | June 2011 | Gorilla gorilla gorilla | 1 | 0/1 | 0/1 |
| Pan troglodytes troglodytes | 4 | 0/4 | 0/4 | ||
| INP A | July–September 2010 | Gorilla gorilla gorilla | 26 | 1/26 (3.8; 0.0–19.6) | 0/26 (0.0; 0.0–13.2) |
| Pan troglodytes troglodytes | 8 | 0/8 | 0/8 |
| Virus | Primers and Probes | Sequences 5′ to 3′ | References |
|---|---|---|---|
| EBOV | EBOZNPCONF1862 | AGCTACGGCGAATACCAGAGTT | |
| EBOZNPCONR1943 | CGTCCTCGTCTAGATCGAATAGG | ||
| EBOZNPCONP1885 | 6 FAM-CTCGGAAAACGGCATGAATGCACC-BHQ1 | ||
| SUDV | Soudan-F | GCCATGGITTCAGGTTTGAG | [25] |
| Soudan-R | GGTIACATTGGGCAACAATTCA | ||
| Soudan-P | 6-FAM-ACGGTGCACATTCTCCTTTTCTCGGA-BHQ1 | ||
| BDBV | Uganda-F | GAGAAAAGGCCTGTCTGGAGAA | |
| Uganda-R | TCGGGTATTGAATCAGACCTTGTT | ||
| Uganda-P | 6 FAM-TTCAACGACAAATCCAAGTGCACGCA-BHQ1 | ||
| MARV | MBGCONTAQMF1 | GGACCACTGCTGGCCATATC | [26] |
| MBGCONTAQMR3-1 | GAGAACATITCGGCAGGAAG | ||
| MBG 5313 | 6 FAM-CCTAAACAGGCTTGTCTTCTCTGGGACTT-BHQ1 | ||
| MBG 5313- Prb RAV | 6 FAM-ATCCTGAATAAGCTCGTCTTCTCTGGGACTT-BHQ1 | ||
| Panfilo | Filo A | TATMGRAATTTTTCYTTYTCATT | [9] |
| Filo B | ATGTGGTGGGYTATAAWARTCACTRACAT | ||
| Filo C | GCWAAAGCMTTYCCWAGYAAYATGATGG | ||
| Filo D | ATAAWARTCACTRACATGCATATAACA |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mombo, I.M.; Fritz, M.; Becquart, P.; Liegeois, F.; Elguero, E.; Boundenga, L.; Mebaley, T.N.; Prugnolle, F.; Maganga, G.D.; Leroy, E.M. Detection of Ebola Virus Antibodies in Fecal Samples of Great Apes in Gabon. Viruses 2020, 12, 1347. https://doi.org/10.3390/v12121347
Mombo IM, Fritz M, Becquart P, Liegeois F, Elguero E, Boundenga L, Mebaley TN, Prugnolle F, Maganga GD, Leroy EM. Detection of Ebola Virus Antibodies in Fecal Samples of Great Apes in Gabon. Viruses. 2020; 12(12):1347. https://doi.org/10.3390/v12121347
Chicago/Turabian StyleMombo, Illich M., Matthieu Fritz, Pierre Becquart, Florian Liegeois, Eric Elguero, Larson Boundenga, Telstar N. Mebaley, Franck Prugnolle, Gael D. Maganga, and Eric M. Leroy. 2020. "Detection of Ebola Virus Antibodies in Fecal Samples of Great Apes in Gabon" Viruses 12, no. 12: 1347. https://doi.org/10.3390/v12121347







