Th1-Polarized, Dengue Virus-Activated Human Mast Cells Induce Endothelial Transcriptional Activation and Permeability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Lines, Virus Strains and Culture Conditions
2.2. huMC Infections and Assessments of Activation
2.3. Measurement of Endothelial Activation and Permeability
2.4. Immunofluorescence Assay
2.5. DENV Infection Kinetics in huMCs and Macrophage Cells
3. Results
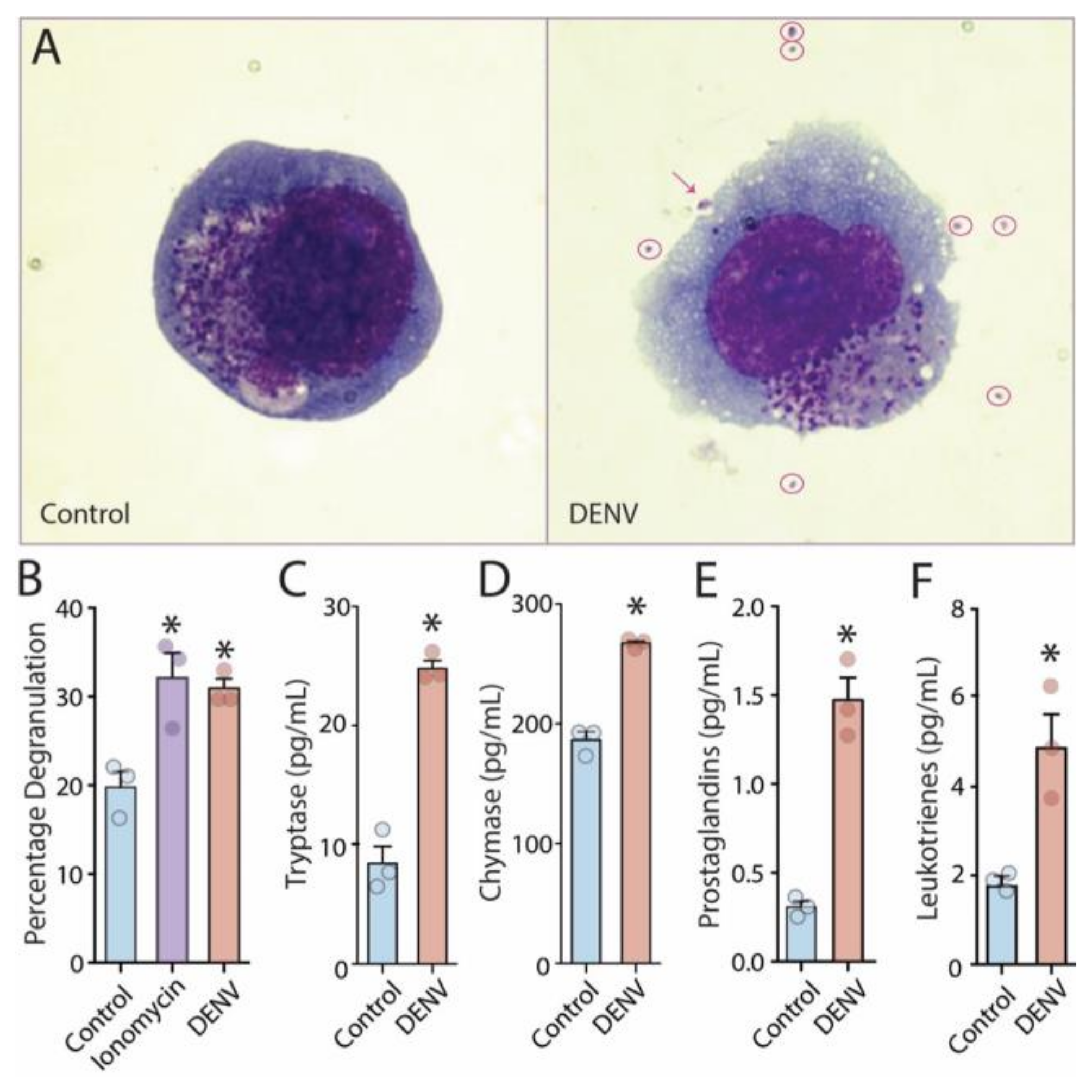
3.1. Release of Vasoactive Products by DENV-Activated Human MCs
3.2. Transcriptional Signatures in huMCs in Response to DENV
3.3. MC-Induced Endothelial Activation
3.4. DENV-Induced huMC Products Promote Endothelial Permeability
4. Discussion
Author Contributions
Funding
Conflicts of Interest
References
- St. John, A.L.; Abraham, S.N. Innate Immunity and Its Regulation by Mast Cells. J. Immunol. 2013, 190, 4458–4463. [Google Scholar] [CrossRef] [PubMed]
- Abraham, S.N.; St. John, A.L. Mast cell-orchestrated immunity to pathogens. Nat. Rev. Immunol. 2010, 10, 440–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collington, S.J.; Williams, T.J.; Weller, C.L. Mechanisms underlying the localisation of mast cells in tissues. Trends Immunol. 2011, 32, 478–485. [Google Scholar] [CrossRef]
- Kunder, C.A.; St. John, A.L.; Abraham, S.N. Mast cell modulation of the vascular and lymphatic endothelium. Blood 2011, 118, 5383–5393. [Google Scholar] [CrossRef]
- Rathore, A.P.; St. John, A.L. Protective and pathogenic roles for mast cells during viral infections. Curr. Opin. Immunol. 2020, 66, 74–81. [Google Scholar] [CrossRef]
- Lawrence, C.E.; Paterson, Y.Y.; Wright, S.H.; Knight, P.A.; Miller, H.R. Mouse mast cell protease-1 is required for the enteropathy induced by gastrointestinal helminth infection in the mouse. Gastroenterology 2004, 127, 155–165. [Google Scholar] [CrossRef]
- St. John, A.L.; Rathore, A.P.S.; Yap, H.; Ng, M.-L.; Metcalfe, D.D.; Vasudevan, S.G.; Abraham, S.N. Immune surveillance by mast cells during dengue infection promotes natural killer (NK) and NKT-cell recruitment and viral clearance. Proc. Natl. Acad. Sci. USA 2011, 108, 9190–9195. [Google Scholar] [CrossRef] [Green Version]
- Graham, A.C.; Hilmer, K.M.; Zickovich, J.M.; Obar, J.J. Inflammatory Response of Mast Cells during Influenza A Virus Infection Is Mediated by Active Infection and RIG-I Signaling. J. Immunol. 2013, 190, 4676–4684. [Google Scholar] [CrossRef]
- St. John, A.L. Influence of Mast Cells on Dengue Protective Immunity and Immune Pathology. PLOS Pathog. 2013, 9, e1003783. [Google Scholar] [CrossRef] [Green Version]
- Tassaneetrithep, B.; Burgess, T.H.; Granelli-Piperno, A.; Trumpfheller, C.; Finke, J.; Sun, W.; Eller, M.A.; Pattanapanyasat, K.; Sarasombath, S.; Birx, D.L.; et al. DC-SIGN (CD209) Mediates Dengue Virus Infection of Human Dendritic Cells. J. Exp. Med. 2003, 197, 823–829. [Google Scholar] [CrossRef] [Green Version]
- Marchette, N.J.; Halstead, S.B.; Falkler, W.A.; Stenhouse, A.; Nash, D. Studies on the Pathogenesis of Dengue Infection in Monkeys. III. Sequential Distribution of Virus in Primary and Heterologous Infections. J. Infect. Dis. 1973, 128, 23–30. [Google Scholar] [CrossRef]
- St. John, A.L.; Abraham, S.N.; Gubler, D.J. Barriers to preclinical investigations of anti-dengue immunity and dengue pathogenesis. Nat. Rev. Genet. 2013, 11, 420–426. [Google Scholar] [CrossRef]
- WHO. Dengue: Guidelines for Diagnosis, Treatment, Prevention and Control: New Edition; WHO Guidelines Approved by the Guidelines Review Committee; WHO: Geneva, Switzerland, 2009. [Google Scholar]
- St. John, A.L.; Rathore, A.P.S.; Raghavan, B.; Ng, M.-L.; Abraham, S.N. Contributions of mast cells and vasoactive products, leukotrienes and chymase, to dengue virus-induced vascular leakage. eLife 2013, 2, e00481. [Google Scholar] [CrossRef]
- Morrison, J.; Rathore, A.P.S.; Mantri, C.K.; Aman, S.A.B.; Nishida, A.; St. John, A.L. Transcriptional Profiling Confirms the Therapeutic Effects of Mast Cell Stabilization in a Dengue Disease Model. J. Virol. 2017, 91, e00617-17. [Google Scholar] [CrossRef] [Green Version]
- Syenina, A.; Jagaraj, C.J.; Ab Aman, S.; Sridharan, A.; St. John, A.L. Dengue vascular leakage is augmented by mast cell degranulation mediated by immunoglobulin Fcγ receptors. eLife 2015, 4. [Google Scholar] [CrossRef] [Green Version]
- Tissera, H.; Rathore, A.P.S.; Leong, W.Y.; Pike, B.L.; Warkentien, T.E.; Farouk, F.S.; Syenina, A.; Ooi, E.E.; Gubler, D.J.; Wilder-Smith, A.; et al. Chymase Level is a Predictive Biomarker of Dengue Hemorrhagic Fever in Pediatric and Adult Patients. J. Infect. Dis. 2017, 216, 1112–1121. [Google Scholar] [CrossRef] [Green Version]
- Rathore, A.P.; Mantri, C.K.; Aman, S.A.; Syenina, A.; Ooi, J.; Jagaraj, C.J.; Goh, C.C.; Tissera, H.; Wilder-Smith, A.; Ng, L.G.; et al. Dengue virus–elicited tryptase induces endothelial permeability and shock. J. Clin. Investig. 2019, 129, 4180–4193. [Google Scholar] [CrossRef] [Green Version]
- Nilsson, G.; Blom, T.; Kusche-Gullberg, M.; Kjellen, L.; Butterfield, J.H.; Sundstrom, C.; Hellman, L. Phenotypic Characterization of the Human Mast-Cell Line HMC-1. Scand. J. Immunol. 1994, 39, 489–498. [Google Scholar] [CrossRef]
- Passante, E. Mast Cell and Basophil Cell Lines: A Compendium. Adv. Struct. Saf. Stud. 2014, 1192, 101–113. [Google Scholar] [CrossRef]
- King, C.A.; Marshall, J.S.; Alshurafa, H.; Anderson, R. Release of Vasoactive Cytokines by Antibody-Enhanced Dengue Virus Infection of a Human Mast Cell/Basophil Line. J. Virol. 2000, 74, 7146–7150. [Google Scholar] [CrossRef] [Green Version]
- Saleh, R.; Wedeh, G.; Herrmann, H.; Bibi, S.; Cerny-Reiterer, S.; Sadovnik, I.; Blatt, K.; Hadzijusufovic, E.; Jeanningros, S.; Blanc, C.; et al. A new human mast cell line expressing a functional IgE receptor converts to tumorigenic growth by KIT D816V transfection. Blood 2014, 124, 111–120. [Google Scholar] [CrossRef] [Green Version]
- Low, J.G.H.; Ooi, E.E.; Tolfvenstam, T.; Leo, Y.-S.; Hibberd, M.L.; Ng, L.C.; Lai, Y.-L.; Yap, G.S.L.; Li, C.S.C.; Vasudevan, S.G.; et al. Early Dengue infection and outcome study (EDEN)—Study design and preliminary findings. Ann. Acad. Med. Singap. 2006, 35, 783. [Google Scholar]
- Fulton, S.A.; Johnsen, J.M.; Wolf, S.F.; Sieburth, D.S.; Boom, W.H. Interleukin-12 production by human monocytes infected with Mycobacterium tuberculosis: Role of phagocytosis. Infect. Immun. 1996, 64, 2523–2531. [Google Scholar] [CrossRef] [Green Version]
- Schulze, I.T.; Schlesinger, R. Plaque assay of dengue and other group B arthropod-borne viruses under methyl cellulose overlay media. Virology 1963, 19, 40–48. [Google Scholar] [CrossRef]
- Pejler, G.; Åbrink, M.; Ringvall, M.; Wernersson, S. Mast Cell Proteases. Adv. Immunol. 2007, 95, 167–255. [Google Scholar] [CrossRef]
- Moreno-Altamirano, M.M.; Sánchez-García, F.J.; Legorreta-Herrera, M.; Aguilar-Carmona, I. Susceptibility of Mouse Macrophage J774 to Dengue Virus Infection. Intervirology 2007, 50, 237–239. [Google Scholar] [CrossRef]
- Mombouli, J.V.; Vanhoutte, P.M. Kinins and Endothelial Control of Vascular Smooth Muscle. Annu. Rev. Pharmacol. Toxicol. 1995, 35, 679–705. [Google Scholar] [CrossRef]
- Chambers, T.J.; Hahn, C.S.; Galler, R.; Rice, C.M. Flavivirus Genome Organization, Expression, and Replication. Annu. Rev. Microbiol. 1990, 44, 649–688. [Google Scholar] [CrossRef]
- Kitamura, Y.; Hatanaka, K.; Murakami, M.; Shibata, H. Presence of mast cell precursors in peripheral blood of mice demonstrated by parabiosis. Blood 1979, 53, 1085–1088. [Google Scholar] [CrossRef] [Green Version]
- Rathore, A.P.S.; Farouk, F.S.; St. John, A.L. Risk factors and biomarkers of severe dengue. Curr. Opin. Virol. 2020, 43, 1–8. [Google Scholar] [CrossRef]
- Goncalvez, A.P.; Engle, R.E.; Claire, M.S.; Purcell, R.H.; Lai, C.-J. Monoclonal antibody-mediated enhancement of dengue virus infection in vitro and in vivo and strategies for prevention. Proc. Natl. Acad. Sci. USA 2007, 104, 9422–9427. [Google Scholar] [CrossRef] [Green Version]
- Halstead, S.B.; O’Rourke, E.J. Antibody-enhanced dengue virus infection in primate leukocytes. Nat. Cell Biol. 1977, 265, 739–741. [Google Scholar] [CrossRef]
- Yoshimoto, T.; Okamura, H.; Tagawa, Y.I.; Iwakura, Y.; Nakanishi, K. Interleukin 18 together with interleukin 12 inhibits IgE production by induction of interferon-gamma production from activated B cells. Proc. Natl. Acad. Sci. USA 1997, 94, 3948–3953. [Google Scholar] [CrossRef] [Green Version]
- Hoshino, T.; Yagita, H.; Ortaldo, J.R.; Wiltrout, R.H.; Young, H.A. In vivo administration of IL-18 can induce IgE production through Th2 cytokine induction and up-regulation of CD40 ligand (CD154) expression on CD4+ T cells. Eur. J. Immunol. 2000, 30, 1998–2006. [Google Scholar] [CrossRef]
- Lodolce, J.P.; Boone, D.L.; Chai, S.; Swain, R.E.; Dassopoulos, T.; Trettin, S.; Ma, A. IL-15 Receptor Maintains Lymphoid Homeostasis by Supporting Lymphocyte Homing and Proliferation. Immunity 1998, 9, 669–676. [Google Scholar] [CrossRef] [Green Version]
- Hoylaerts, M.; Rijken, D.; Lijnen, R.H.; Collen, D. Kinetics of the activation of plasminogen by human tissue plasminogen activator. Role of fibrin. J. Biol. Chem. 1982, 257, 2912–2919. [Google Scholar]
- Michida, T.; Kawano, S.; Masuda, E.; Kobayashi, I.; Nishimura, Y.; Tsujii, M.; Hayashi, N.; Takei, Y.; Tsuji, S.; Nagano, K.; et al. Role of endothelin 1 in hemorrhagic shock-induced gastric mucosal injury in rats. Gastroenterology 1994, 106, 988–993. [Google Scholar] [CrossRef]
- Wypij, D.M.; Nichols, J.S.; Novak, P.J.; Stacy, D.L.; Berman, J.; Wiseman, J.S. Role of mast cell chymase in the extracellular processing of big-endothelin-1 to endothelin-1 in the perfused rat lung. Biochem. Pharmacol. 1992, 43, 845–853. [Google Scholar] [CrossRef]
- Savage, B.; Saldívar, E.; Ruggeri, Z.M. Initiation of Platelet Adhesion by Arrest onto Fibrinogen or Translocation on von Willebrand Factor. Cell 1996, 84, 289–297. [Google Scholar] [CrossRef] [Green Version]





| Vasoactive Factor | Major Described Biological Functions | |
|---|---|---|
| PECAM (CD31) | Platelet endothelial cell adhesion molecule | Promotes leukocyte transmigration |
| EDN1 | Endothelin-1 | Vasoconstriction |
| ENDRA * | Endothelin receptor type A | The receptor for Endothelin-1, Vasoconstriction |
| PLAT * | Plasminogen activator | A protease that converts plasminogen to plasmin, which dissolves fibrin and reduces clotting |
| THBD | Thrombomodulin | Thrombin receptor that promotes degradation of clotting factors |
| COL19A1 | Collagen, type XIX, alpha 1 | Extracellular matrix protein |
| PGF | Placental growth factor | A member of the VEGF family with diverse roles in angiogenesis and vascular function |
| TNF | Tumor Necrosis Factor | A cytokine with broad roles in host defense. Also promotes processes such as fever, cell death, and vascular leakage |
| TYMP (ECGF1) | Thymidine phosphorylase | Promotes angiogenesis |
| OCLN | Occludin | Tight junction protein that regulates paracellular permeability |
| Vasoactive Factor | Major Described Biological Functions | |
|---|---|---|
| TIMP1 | TMP metallopeptidase inhibitor | Inhibitor of matrix metalloproteinases, which degrade extracellular matrix |
| CX3CL1 (Fractalkine) | CX3CL1 chemokine (C-X3-C motif) ligand 1 | Leukocyte migration (primarily T cells and monocytes) |
| PDGFRA | Platelet-derived growth factor receptor, alpha polypeptide | Activation promotes angiogenesis |
| TNFSF10 | Tumor necrosis factor (ligand) superfamily, member 10 | Pro-apoptotic protein in stressed but not normal cells |
| PF4 | Platelet factor 4 | A member of the CXC chemokine family that promotes platelet aggregation |
| THBS1 | Thrombospondin 1 | An adhesive glycoprotein that promotes cell–cell interactions |
| VEGFA ** | Vascular endothelial growth factor A | Promotes vascular permeability and angiogenesis |
| VWF ** | von Willebrand factor | Performs a critical function in coagulation |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Syenina, A.; Saron, W.A.A.; Jagaraj, C.J.; Bibi, S.; Arock, M.; Gubler, D.J.; Rathore, A.P.S.; Abraham, S.N.; St. John, A.L. Th1-Polarized, Dengue Virus-Activated Human Mast Cells Induce Endothelial Transcriptional Activation and Permeability. Viruses 2020, 12, 1379. https://doi.org/10.3390/v12121379
Syenina A, Saron WAA, Jagaraj CJ, Bibi S, Arock M, Gubler DJ, Rathore APS, Abraham SN, St. John AL. Th1-Polarized, Dengue Virus-Activated Human Mast Cells Induce Endothelial Transcriptional Activation and Permeability. Viruses. 2020; 12(12):1379. https://doi.org/10.3390/v12121379
Chicago/Turabian StyleSyenina, Ayesa, Wilfried A. A. Saron, Cyril J. Jagaraj, Siham Bibi, Michel Arock, Duane J. Gubler, Abhay P. S. Rathore, Soman N. Abraham, and Ashley L. St. John. 2020. "Th1-Polarized, Dengue Virus-Activated Human Mast Cells Induce Endothelial Transcriptional Activation and Permeability" Viruses 12, no. 12: 1379. https://doi.org/10.3390/v12121379
APA StyleSyenina, A., Saron, W. A. A., Jagaraj, C. J., Bibi, S., Arock, M., Gubler, D. J., Rathore, A. P. S., Abraham, S. N., & St. John, A. L. (2020). Th1-Polarized, Dengue Virus-Activated Human Mast Cells Induce Endothelial Transcriptional Activation and Permeability. Viruses, 12(12), 1379. https://doi.org/10.3390/v12121379







