Bacteriophages for Chronic Wound Treatment: From Traditional to Novel Delivery Systems
Abstract
:1. Introduction
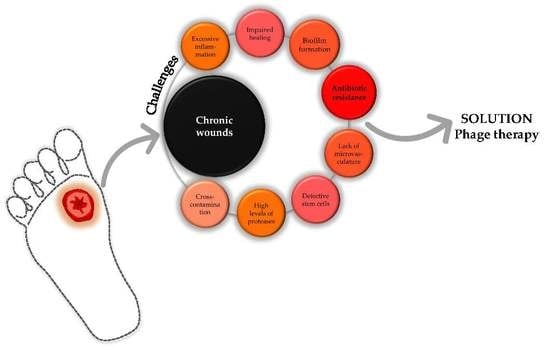
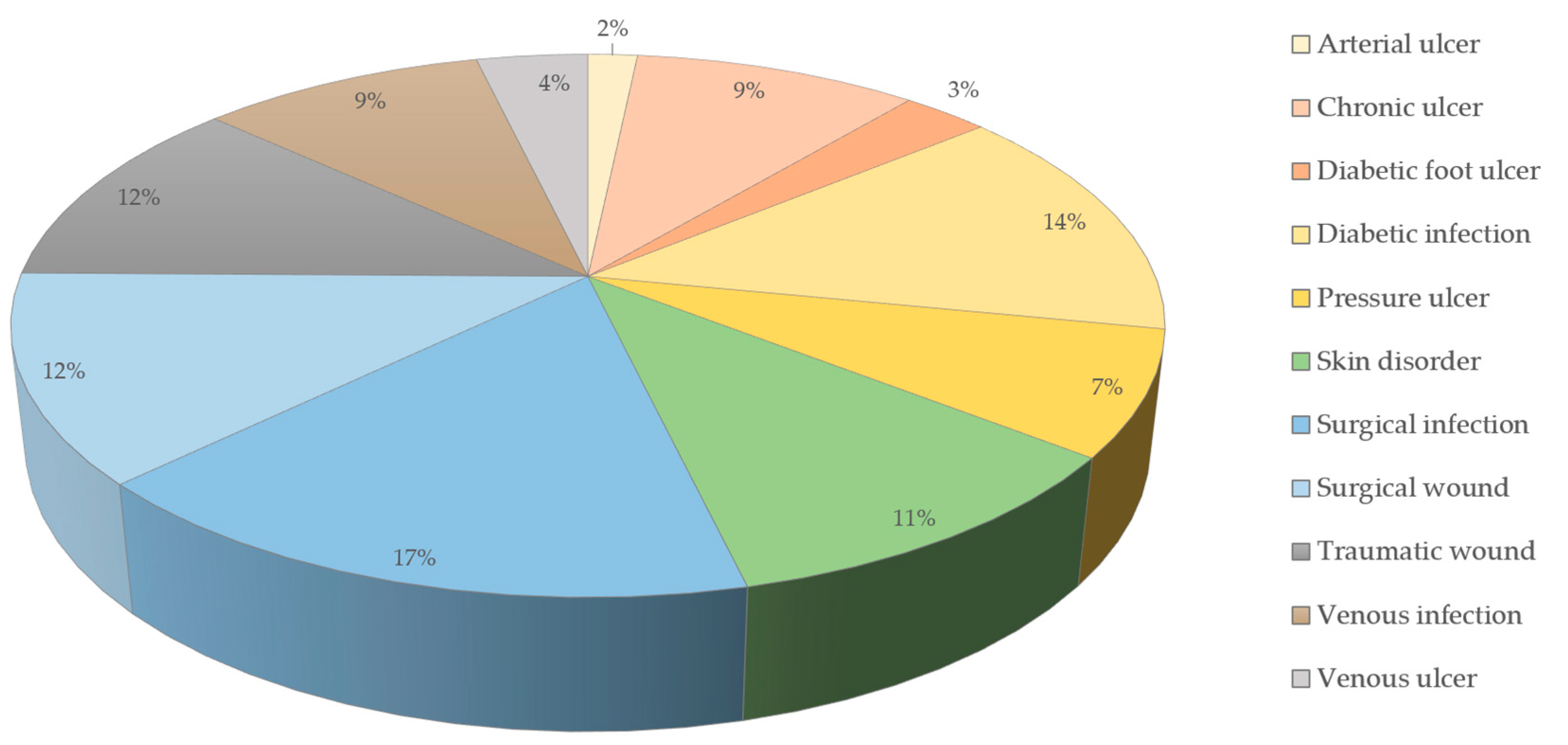
2. Impact of Chronic Wounds
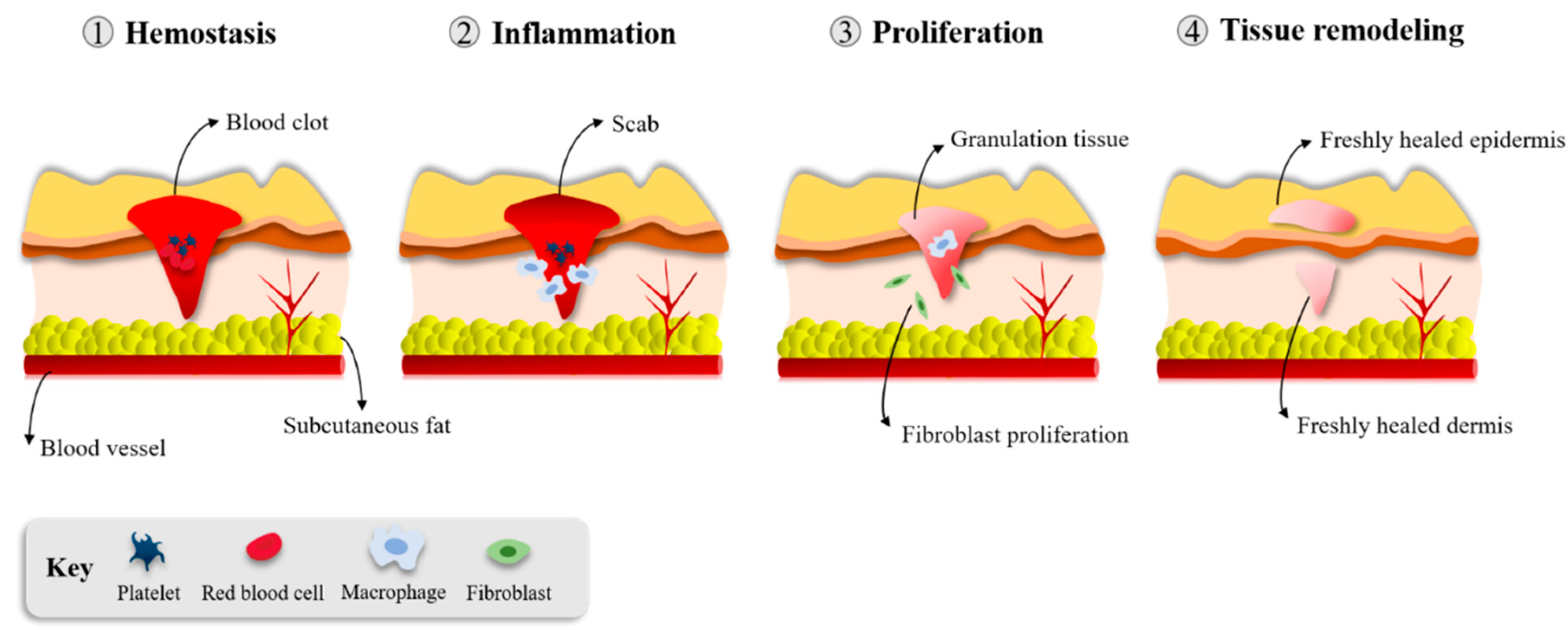
3. Wound Healing
3.1. Hemostasis/Inflammation
3.2. Proliferation and Repair
3.3. Tissue Remodeling
4. Microorganisms Present in Chronic Wounds
5. Biofilms
6. Non-Phage-Based Wound Treatments
7. Phage Therapy
7.1. Phage Therapy Reference Institutions
7.2. Phage Therapy for Chronic Wound Healing—Ex Vivo and Animal In Vivo Models
7.3. Clinical Phage Therapy Trials on Wounds
8. Phage Delivery
8.1. Individual Phages versus Cocktails
8.2. Routes of Administration
8.3. Phage Delivery Systems
9. Phage Therapy Regulation
9.1. Regulation Hurdles
9.2. Recent Regulatory Decisions Regarding the Use of Phages
9.3. Prospective Future Issues for Use
10. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Lazarus, G.S.; Cooper, D.M.; Knighton, D.R.; Margolis, D.J.; Percoraro, R.E.; Rodeheaver, G.; Robson, M.C. Definitions and guidelines for assessment of wounds and evaluation of healing. Wound Repair Regen. 1994, 2, 165–170. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, A.R.; Bernstein, J.M. Chronic wound infection: Facts and controversies. Clin. Dermatol. 2010, 28, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.S.; Borrelli, M.R.; Lorenz, H.P.; Longaker, M.T.; Wan, D.C. Mesenchymal stromal cells and cutaneous wound healing: A comprehensive review of the background, role, and therapeutic potential. Stem Cells Int. 2018, 2018, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aljghami, M.E.; Saboor, S.; Amini-Nik, S. Emerging innovative wound dressings. Ann. Biomed. Eng. 2019, 47, 659–675. [Google Scholar] [CrossRef]
- Mustoe, T.A.; O’Shaughnessy, K.; Kloeters, O. Chronic wound pathogenesis and current treatment strategies: A unifying hypothesis. Plast. Reconstr. Surg. 2006, 117, 35S–41S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.S.; Sun, X.; Lee, J.-H.; Kim, H.-W.; Fu, X.; Leong, K.W. Advanced drug delivery systems and artificial skin grafts for skin wound healing. Adv. Drug Deliv. Rev. 2018, 146, 209–239. [Google Scholar] [CrossRef]
- Chang, F.; Yang, C.W.; Lu, W. Chronic wound: Pathogenesis and current treatments. Acad. J. Second Mil. Med. Univ. 2007, 28, 1259–1261. [Google Scholar]
- Macdonald, J. Global Initiative for Wound and Lymphoedema Care (GIWLC). J. Lymphoedema 2009, 4, 92–95. [Google Scholar]
- Reilly, E.; Karakousis, G.; Schrag, S.; Stawicki, S. Pressure ulcers in the intensive care unit: The ‘forgotten’enemy. Opus 2007, 12, 17–30. [Google Scholar]
- World Health Organization. Antimicrobial Resistance. Available online: https://www.who.int/news-room/fact-sheets/detail/antimicrobial-resistance (accessed on 8 January 2020).
- Manohar, P.; Nachimuthu, R.; Lopes, B.S. The therapeutic potential of bacteriophages targeting gram-negative bacteria using Galleria mellonella infection model. BMC Microbiol. 2018, 18, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Pence, B.D.; Woods, J.A. Exercise, obesity, and cutaneous wound healing: Evidence from rodent and human studies. Adv. Wound Care 2014, 3, 71–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Werdin, F.; Tennenhaus, M.; Schaller, H. Evidence-based management strategies for treatment of chronic wounds. ePlasty Open Access J. Plast. Surg. 2009, 9, 169–179. [Google Scholar]
- Cazander, G.; Pritchard, D.I.; Nigam, Y.; Jung, W.; Nibbering, P.H. Multiple actions of Lucilia sericata larvae in hard-to-heal wounds: Larval secretions contain molecules that accelerate wound healing, reduce chronic inflammation and inhibit bacterial infection. BioEssays 2013, 35, 1083–1092. [Google Scholar] [CrossRef] [PubMed]
- Graves, N.; Zheng, H. The prevalence and incidence of chronic wounds: A literature review. Wound Pract. Res. J. Aust. Wound Manag. Assoc. 2014, 22, 4. [Google Scholar]
- Martinengo, L.; Olsson, M.; Bajpai, R.; Soljak, M.; Upton, Z.; Schmidtchen, A.; Car, J.; Järbrink, K. Prevalence of chronic wounds in the general population: Systematic review and meta-analysis of observational studies. Ann. Epidemiol. 2018, 29, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Ellis, S.; Lin, E.J.; Tartar, D. Immunology of wound healing. Curr. Dermatol. Rep. 2018, 7, 350–358. [Google Scholar] [CrossRef] [Green Version]
- Kalan, L.R.; Brennan, M.B. The role of the microbiome in nonhealing diabetic wounds. Ann. N. Y. Acad. Sci. 2019, 1435, 79–92. [Google Scholar] [CrossRef] [Green Version]
- Krasner, D.; Rodeheaver, G.; Sibbald, R. Chronic Wound Care: A Clinical Source Book for Healthcare Professionals, 4th ed.; HMP Communications: Malvern, PA, USA, 2007. [Google Scholar]
- Vos, T.; Allen, C.; Arora, M.; Barber, R.M.; Brown, A.; Carter, A.; Casey, D.C.; Charlson, F.J.; Chen, A.Z.; Coggeshall, M.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 310 diseases and injuries, 1990–2015: A systematic analysis for the Global Burden of Disease Study 2015. Lancet 2016, 388, 1545–1602. [Google Scholar] [CrossRef] [Green Version]
- James, S.L.; Abate, D.; Abate, K.H.; Abay, S.M.; Abbafati, C.; Abbasi, N.; Abbastabar, H.; Abd-Allah, F.; Abdela, J.; Abdelalim, A.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar] [CrossRef] [Green Version]
- Lim, H.W.; Collins, S.A.B.; Resneck, J.S.; Bolognia, J.L.; Hodge, J.A.; Rohrer, T.A.; Van Beek, M.J.; Margolis, D.J.; Sober, A.J.; Weinstock, M.A.; et al. The burden of skin disease in the United States. J. Am. Acad. Dermatol. 2017, 76, 958–972.e2. [Google Scholar] [CrossRef] [Green Version]
- Sen, C.K.; Gordillo, G.M.; Roy, S.; Kirsner, R.; Lambert, L.; Hunt, T.K.; Gottrup, F.; Gurtner, G.C.; Longaker, M.T. Human skin wounds: A major and snowballing threat to public health and the economy: Perspective article. Wound Repair Regen. 2009, 17, 763–771. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dealey, C.; Posnett, J.; Walker, A. The cost of pressure ulcers in the United Kingdom. J. Wound Care 2012, 21, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Nussbaum, S.R.; Carter, M.J.; Fife, C.E.; DaVanzo, J.; Haught, R.; Nusgart, M.; Cartwright, D. An economic evaluation of the impact, cost, and Medicare policy implications of chronic nonhealing wounds. Value Health 2018, 21, 27–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, P.-H.; Huang, B.-S.; Horng, H.-C.; Yeh, C.-C.; Chen, Y.-J. Wound healing. J. Chin. Med. Assoc. 2018, 81, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Roşca, A.-M.; Ţuţuianu, R.; Domnica Titorencu, I. Mesenchymal stromal cells derived exosomes as tools for chronic wound healing therapy. Rom. J. Morphol. Embryol. 2018, 59, 655–662. [Google Scholar] [PubMed]
- Ibrahim, N.‘I.; Wong, S.K.; Mohamed, I.N.; Mohamed, N.; Chin, K.-Y.; Ima-Nirwana, S.; Shuid, A.N. Wound healing properties of selected natural products. Int. J. Environ. Res. Public Health 2018, 15, 2360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Powers, J.G.; Higham, C.; Broussard, K.; Phillips, T.J. Wound healing and treating wounds: Chronic wound care and management. J. Am. Acad. Dermatol. 2016, 74, 607–625. [Google Scholar] [CrossRef]
- Swanson, T.; Angel, D.; Sussman, G.; Cooper, R.; Haesler, E.; Ousey, K.; Carville, K.; Fletcher, J.; Kalan, L.; Keast, D.; et al. International Wound Infection Institute (IWII) Wound infection in clinical practice: Principles of best practice. Wounds Int. 2016, 32, 21. [Google Scholar]
- Bowler, P.G.; Duerden, B.I.; Armstrong, D.G. Wound microbiology and associated approaches to wound management. Clin. Microbiol. Rev. 2001, 14, 244–269. [Google Scholar] [CrossRef] [Green Version]
- Méric, G.; Mageiros, L.; Pensar, J.; Laabei, M.; Yahara, K.; Pascoe, B.; Kittiwan, N.; Tadee, P.; Post, V.; Lamble, S.; et al. Disease-associated genotypes of the commensal skin bacterium Staphylococcus Epidermidis. Nat. Commun. 2018, 9, 5034. [Google Scholar] [CrossRef] [Green Version]
- Evangelista, S.; Guimaraes, N.; Garcia, N.; Santos, S.; Oliveira, A. Effectiveness of manual versus automated cleaning on Staphylococcus epidermidis biofilm removal from the surface of surgical instruments. Am. J. Infect. Control 2019. [Google Scholar] [CrossRef] [PubMed]
- Mody, L.; Washer, L.L.; Kaye, K.S.; Gibson, K.; Saint, S.; Reyes, K.; Cassone, M.; Mantey, J.; Cao, J.; Altamimi, S.; et al. Multidrug-resistant organisms in hospitals: What is on patient hands and in their rooms? Clin. Infect. Dis. 2019, 69, 1837–1844. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carikas, B.K.; Matthews, S. Hospital privacy curtains – What’s hanging around? Dissector 2019, 47, 20–22. [Google Scholar]
- Moremi, N.; Claus, H.; Silago, V.; Kabage, P.; Abednego, R.; Matee, M.; Vogel, U.; Mshana, S.E. Hospital surface contamination with antimicrobial-resistant Gram-negative organisms in Tanzanian regional and tertiary hospitals: The need to improve environmental cleaning. J. Hosp. Infect. 2019, 102, 98–100. [Google Scholar] [CrossRef]
- Haque, M.; Sartelli, M.; McKimm, J.; Bakar, M.A. Health care-associated infections—An overview. Infect. Drug Resist. 2018, 11, 2321. [Google Scholar] [CrossRef] [Green Version]
- Patel, S. Understanding wound infection and colonisation. Wound Essentials 2007, 2, 132–142. [Google Scholar]
- Gjødsbøl, K.; Christensen, J.J.; Karlsmark, T.; Jørgensen, B.; Klein, B.M.; Krogfelt, K.A. Multiple bacterial species reside in chronic wounds: A longitudinal study. Int. Wound J. 2006, 3, 225–231. [Google Scholar] [CrossRef]
- Oliveira, A.; Ribeiro, H.G.; Silva, A.C.; Silva, M.D.; Sousa, J.C.; Rodrigues, C.F.; Melo, L.D.R.; Henriques, A.F.; Sillankorva, S. Synergistic antimicrobial interaction between honey and phage against Escherichia coli biofilms. Front. Microbiol. 2017, 8, 1–18. [Google Scholar] [CrossRef]
- Jneid, J.; Cassir, N.; Schuldiner, S.; Jourdan, N.; Sotto, A.; Lavigne, J.P.; Scola, B. La Exploring the microbiota of diabetic foot infections with culturomics. Front. Cell. Infect. Microbiol. 2018, 8, 282. [Google Scholar] [CrossRef] [Green Version]
- Otta, S.; Debata, N.K.; Swain, B. Bacteriological profile of diabetic foot ulcers. J. Health Res. 2019, 6, 7–11. [Google Scholar]
- Ogba, O.M.; Nsan, E.; Eyam, E.S. Aerobic bacteria associated with diabetic foot ulcers and their susceptibility pattern. Biomed. Dermatol. 2019, 3, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Lachiewicz, A.M.; Hauck, C.G.; Weber, D.J.; Cairns, B.A.; Van Duin, D. Bacterial Infections after Burn Injuries: Impact of Multidrug Resistance. Clin. Infect. Dis. 2017, 65, 2130–2136. [Google Scholar] [CrossRef] [PubMed]
- Oruko, R.O.; Odiyo, J.O.; Edokpayi, J.N. The role of leather microbes in human health. IntechOpen 2019. [Google Scholar] [CrossRef] [Green Version]
- Findley, K.; Oh, J.; Yang, J.; Conlan, S.; Deming, C.; Meyer, J.A.; Schoenfeld, D.; Nomicos, E.; Park, M.; Becker, J.; et al. Topographic diversity of fungal and bacterial communities in human skin. Nature 2013, 498, 367–370. [Google Scholar] [CrossRef]
- Cui, L.; Morris, A.; Ghedin, E. The human mycobiome in health and disease. Genome Med. 2013, 5, 63. [Google Scholar] [CrossRef] [Green Version]
- Hansson, C.; Hoborn, J.; Möller, A.; Swanbeck, G. The microbial flora in venous leg ulcers without clinical signs of infection. Repeated culture using a validated standardised microbiological technique. Acta Derm. Venereol. 1995, 75, 24–30. [Google Scholar]
- Krüger, W.; Vielreicher, S.; Kapitan, M.; Jacobsen, I.D.; Niemiec, M.J. Fungal-bacterial interactions: In health and disease. Candida albicans Cell. Mol. Biol. Second Ed. 2019, 8, 70. [Google Scholar] [CrossRef] [Green Version]
- Kalan, L.; Loesche, M.; Hodkinson, B.P.; Heilmann, K.; Ruthel, G.; Gardner, S.E.; Grice, E.A. Redefining the chronic-wound microbiome: Fungal communities are prevalent, dynamic, and associated with delayed healing. MBio 2016, 7, e01058-16. [Google Scholar] [CrossRef] [Green Version]
- Chellan, G.; Shivaprakash, S.; Ramaiyar, S.K.; Varma, A.K.; Varma, N.; Sukumaran, M.T.; Vasukutty, J.R.; Bal, A.; Kumar, H. Spectrum and prevalence of fungi infecting deep tissues of lower-limb wounds in patients with type 2 diabetes. J. Clin. Microbiol. 2010, 48, 2097–2102. [Google Scholar] [CrossRef] [Green Version]
- Gupta, N.; Haque, A.; Mukhopadhyay, G.; Narayan, R.P.; Prasad, R. Interactions between bacteria and Candida in the burn wound. Burns 2005, 31, 375–378. [Google Scholar] [CrossRef]
- Kalan, L.; Grice, E.A. Fungi in the wound microbiome. Adv. Wound Care 2018, 7, 247–255. [Google Scholar] [CrossRef] [PubMed]
- Dowd, S.E.; Delton Hanson, J.; Rees, E.; Wolcott, R.D.; Zischau, A.M.; Sun, Y.; White, J.; Smith, D.M.; Kennedy, J.; Jones, C.E. Survey of fungi and yeast in polymicrobial infections in chronic wounds. J. Wound Care 2011, 20, 40–47. [Google Scholar] [CrossRef] [PubMed]
- Que, A.T.; Nguyen, N.M.T.; Do, N.A.; Nguyen, N.L.; Tran, N.D.; Le, T.A. Infection of burn wound by Aspergillus fumigatus with gross appearance of fungal colonies. Med. Mycol. Case Rep. 2019, 24, 30–32. [Google Scholar] [CrossRef] [PubMed]
- Costerton, J.W.; Geesey, G.G.; Cheng, K.J. How bacteria stick in. Sci. Am. Inc 1978, 238, 86–95. [Google Scholar] [CrossRef]
- Percival, S.L.; Hill, K.E.; Williams, D.W.; Hooper, S.J.; Thomas, D.W.; Costerton, J.W. A review of the scientific evidence for biofilms in wounds. Wound Repair Regen. 2012, 20, 647–657. [Google Scholar] [CrossRef]
- Clinton, A.; Carter, T. Chronic wound biofilms: Pathogenesis and potential therapies. Lab. Med. 2015, 46, 277–284. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Fu, K.; Wu, C.; Qin, K.; Li, F.; Zhou, L. “In-group” communication in marine Vibrio: A review of N-acyl homoserine lactones-driven quorum sensing. Front. Cell. Infect. Microbiol. 2018, 8, 1–17. [Google Scholar] [CrossRef]
- Maunders, E.; Welch, M. Matrix exopolysaccharides; the sticky side of biofilm formation. FEMS Microbiol. Lett. 2017, 364, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Costerton, J.W.; Lewandowski, Z.; Caldwell, D.E.; Korber, D.R.; Lappin-Scott, H.M. Microbial biofilms. Annu. Rev. Microbiol. 1995, 49, 711–745. [Google Scholar] [CrossRef]
- Klausen, M.; Heydorn, A.; Ragas, P.; Lambertsen, L.; Aaes-Jørgensen, A.; Molin, S.; Tolker-Nielsen, T. Biofilm formation by Pseudomonas aeruginosa wild type, flagella and type IV pili mutants. Mol. Microbiol. 2003, 48, 1511–1524. [Google Scholar] [CrossRef]
- Stewart, P.S. Diffusion in biofilms. J. Bacteriol. 2003, 185, 1485–1491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Donlan, R.M. Biofilms: Microbial life on surfaces. Emerg. Infect. Dis. 2002, 8, 881–890. [Google Scholar] [CrossRef] [PubMed]
- Pires, D.P.; Melo, L.D.R.; Vilas Boas, D.; Sillankorva, S.; Azeredo, J. Phage therapy as an alternative or complementary strategy to prevent and control biofilm-related infections. Curr. Opin. Microbiol. 2017, 39, 48–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shah, D.; Zhang, Z.; Khodursky, A.; Kaldalu, N.; Kurg, K.; Lewis, K. Persisters: A distinct physiological state of E. coli. BMC Microbiol. 2006, 6, 53. [Google Scholar] [CrossRef] [Green Version]
- Kwan, B.W.; Valenta, J.A.; Benedik, M.J.; Wood, T.K. Arrested protein synthesis increases persister-like cell formation. Antimicrob. Agents Chemother. 2013, 57, 1468–1473. [Google Scholar] [CrossRef] [Green Version]
- Wood, T.K.; Knabel, S.J.; Kwan, B.W. Bacterial persister cell formation and dormancy. Appl. Environ. Microbiol. 2013, 79, 7116–7121. [Google Scholar] [CrossRef] [Green Version]
- Conlon, B.P.; Rowe, S.E.; Gandt, A.B.; Nuxoll, A.S.; Donegan, N.P.; Zalis, E.A.; Clair, G.; Adkins, J.N.; Cheung, A.L.; Lewis, K. Persister formation in Staphylococcus aureus is associated with ATP depletion. Nat. Microbiol. 2016, 1, 16051. [Google Scholar] [CrossRef] [Green Version]
- Lewis, K. Persister Cells. Annu. Rev. Microbiol. 2010, 64, 357–372. [Google Scholar] [CrossRef]
- Ng, W.-L.; Bassler, B.L. Bacterial quorum-sensing network architectures. Annu. Rev. Genet. 2009, 43, 197–222. [Google Scholar] [CrossRef] [Green Version]
- Eickhoff, M.J.; Bassler, B.L. SnapShot: Bacterial Quorum Sensing. Cell 2018, 174, 1328–1328.e1. [Google Scholar] [CrossRef]
- Mendoza, R.A.; Hsieh, J.C.; Galiano, R.D. The impact of biofilm formation on wound healing. IntechOpen 2019. [Google Scholar] [CrossRef] [Green Version]
- Leid, J.G. Bacterial biofilms resist key host defenses. Microbe 2009, 4, 66–70. [Google Scholar]
- Tacconelli, E.; Margrini, N. Global Priority List of Antibiotic-Resistant Bacteria to Guide Research, Discovery, and Development of New Antibiotics. Available online: https://www.who.int/medicines/publications/WHO-PPL-Short_Summary_25Feb-ET_NM_WHO.pdf?ua=1 (accessed on 10 January 2020).
- Gaddy, J.A.; Actis, L.A. Regulation of Acinetobacter baumannii biofilm formation. Future Microbiol. 2009, 4, 273–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rasamiravaka, T.; Labtani, Q.; Duez, P.; El Jaziri, M. The formation of biofilms by Pseudomonas aeruginosa: A review of the natural and synthetic compounds interfering with control mechanisms. Biomed Res. Int. 2015, 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vuotto, C.; Longo, F.; Balice, M.P.; Donelli, G.; Varaldo, P.E. Antibiotic resistance related to biofilm formation in Klebsiella pneumoniae. Pathogens 2014, 3, 743–758. [Google Scholar] [CrossRef] [Green Version]
- Beloin, C.; Roux, A.; Ghigo, J.M. Escherichia coli biofilms. In Current Topics in Microbiology and Immunology-Bacterial Biofilms; Springer: Berlin/Heidelberg, Germany, 2008; pp. 249–289. ISBN 9783540754176. [Google Scholar]
- Bianchi, T.; Wolcott, R.D.; Peghetti, A.; Leaper, D.; Cutting, K.; Polignano, R.; Rosa Rita, Z.; Moscatelli, A.; Greco, A.; Romanelli, M.; et al. Recommendations for the management of biofilm: A consensus document. J. Wound Care 2016, 25, 305–317. [Google Scholar] [CrossRef]
- Parsek, M.R.; Singh, P.K. Bacterial biofilms: An emerging link to disease pathogenesis. Annu. Rev. Microbiol. 2003, 57, 677–701. [Google Scholar] [CrossRef]
- Vachhrajani, V.; Khakhkhar, P. Other types of dressings. In Science of Wound Healing and Dressing Materials; Springer: Singapore, 2020; pp. 73–84. [Google Scholar]
- Molan, P.C. The antibacterial activity of honey: 1. The nature of the antibacterial activity. Bee World 1992, 73, 5–28. [Google Scholar] [CrossRef]
- Brudzynski, K. Effect of hydrogen peroxide on antibacterial activities of Canadian honeys. Can. J. Microbiol. 2006, 52, 1228–1237. [Google Scholar] [CrossRef]
- Gethin, G.T.; Cowman, S.; Conroy, R.M. The impact of Manuka honey dressings on the surface pH of chronic wounds. Int. Wound J. 2008, 5, 185–194. [Google Scholar] [CrossRef]
- Majtan, J.; Bohova, J.; Prochazka, E.; Klaudiny, J. Methylglyoxal may affect hydrogen peroxide accumulation in manuka honey through the inhibition of glucose oxidase. J. Med. Food 2014, 17, 290–293. [Google Scholar] [CrossRef] [PubMed]
- Dhivya, S.; Padma, V.V.; Santhini, E. Wound dressings-A review. BioMedicine 2015, 5, 22. [Google Scholar] [CrossRef] [PubMed]
- Negut, I.; Grumezescu, V.; Grumezescu, A.M. Treatment strategies for infected wounds. Molecules 2018, 23, 2392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mostafalu, P.; Kiaee, G.; Giatsidis, G.; Khalilpour, A.; Nabavinia, M.; Dokmeci, M.R.; Sonkusale, S.; Orgill, D.P.; Tamayol, A.; Khademhosseini, A. A textile dressing for temporal and dosage controlled drug delivery. Adv. Funct. Mater. 2017, 27, 1–10. [Google Scholar] [CrossRef]
- Schultz, G.S.; Sibbald, R.G.; Falanga, V.; Ayello, E.A.; Dowsett, C.; Harding, K.; Romanelli, M.; Stacey, M.C. Wound bed preparation: A systematic approach to wound management. Wound Repair Regen. 2003, 11, S1–S28. [Google Scholar] [CrossRef] [PubMed]
- Jull, A.B.; Arroll, B.; Parag, V.; Waters, J. Pentoxifylline for treating venous leg ulcers. Cochrane Database Syst. Rev. 2012. [Google Scholar] [CrossRef] [PubMed]
- Cruciani, M.; Lipsky, B.A.; Mengoli, C.; de Lalla, F. Granulocyte-colony stimulating factors as adjunctive therapy for diabetic foot infections. Cochrane Database Syst. Rev. 2013. [Google Scholar] [CrossRef] [Green Version]
- Kranke, P.; Bennett, M.H.; Martyn-St James, M.; Schnabel, A.; Debus, S.E.; Weibel, S. Hyperbaric oxygen therapy for chronic wounds. Cochrane Database Syst. Rev. 2015. [Google Scholar] [CrossRef]
- Helen, T.; Liz, C.; Laura, C.; Illary, S.; Martin, B.; Hannah, B.; Ian, C.; Jo, D.; Chris, F.; Rachael, F.; et al. Aspirin versus placebo for the treatment of venous leg ulcers-A phase II, pilot, randomised trial (AVURT). Trials 2019, 20, 459. [Google Scholar] [CrossRef] [Green Version]
- Polera, N.; Badolato, M.; Perri, F.; Carullo, G.; Aiello, F. Quercetin and its natural sources in wound healing management. Curr. Med. Chem. 2018, 25, 1–22. [Google Scholar] [CrossRef]
- Wu, B.; Lu, J.; Yang, M.; Xu, T. Sulodexide for treating venous leg ulcers. Cochrane Database Syst. Rev. 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeschke, M.G.; Kamolz, L.P.; Sjöberg, F.; Wolf, S.E. Handbook of Burns: Acute Burn Care, Volume 1, 2nd ed.; Jeschke, M.G., Kamolz, L.P., Sjöberg, F., Wolf, S.E., Eds.; Springer-Verlag: Wien, Austria, 2012; ISBN 9783709103487. [Google Scholar]
- Murray, C.K. Field wound care: Prophylactic antibiotics. Wilderness Environ. Med. 2017, 28, S90–S102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bigliardi, P.L.; Alsagoff, S.A.L.; El-Kafrawi, H.Y.; Pyon, J.K.; Wa, C.T.C.; Villa, M.A. Povidone iodine in wound healing: A review of current concepts and practices. Int. J. Surg. 2017, 44, 260–268. [Google Scholar] [CrossRef] [PubMed]
- Kramer, A.; Dissemond, J.; Kim, S.; Willy, C.; Mayer, D.; Papke, R.; Tuchmann, F.; Assadian, O. Consensus on wound antisepsis: Update 2018. Skin Pharmacol. Physiol. 2018, 31, 28–58. [Google Scholar] [CrossRef] [PubMed]
- Schedler, K.; Assadian, O.; Brautferger, U.; Müller, G.; Koburger, T.; Classen, S.; Kramer, A. Proposed phase 2/ step 2 in-vitro test on basis of EN 14561 for standardised testing of the wound antiseptics PVP-iodine, chlorhexidine digluconate, polihexanide and octenidine dihydrochloride. BMC Infect. Dis. 2017, 17, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leaper, D.J.; Schultz, G.; Carville, K.; Fletcher, J.; Swanson, T.; Drake, R. Extending the TIME concept: What have we learned in the past 10 years? Int. Wound J. 2012, 9, 1–19. [Google Scholar] [CrossRef] [PubMed]
- König, B.; Reimer, K.; Fleischer, W.; König, W. Effects of Betaisodona® on parameters of host defense. Dermatology 1997, 195, 42–48. [Google Scholar] [CrossRef]
- Costa, S.S.; Viveiros, M.; Amaral, L.; Couto, I. Multidrug efflux pumps in Staphylococcus aureus: An update. Open Microbiol. J. 2013, 59–71. [Google Scholar] [CrossRef] [Green Version]
- Poole, K. Efflux pumps as antimicrobial resistance mechanisms. Ann. Med. 2007, 39, 162–176. [Google Scholar] [CrossRef]
- Park, S.R.; Kim, J.W.; Jun, H.S.; Roh, J.Y.; Lee, H.Y.; Hong, I.S. Stem cell secretome and its effect on cellular mechanisms relevant to wound healing. Mol. Ther. 2018, 26, 606–617. [Google Scholar] [CrossRef] [Green Version]
- Kutter, E.; Kuhl, S.; Abedon, S.; Alavidze, Z.; Gvasalia, G.; De Vos, D.; Gogokhia, L. Phage therapy in clinical practice: Treatment of human infections. Curr. Pharm. Biotechnol. 2010, 11, 69–86. [Google Scholar] [CrossRef] [PubMed]
- Maciejewska, B.; Olszak, T.; Drulis-Kawa, Z. Applications of bacteriophages versus phage enzymes to combat and cure bacterial infections: An ambitious and also a realistic application? Appl. Microbiol. Biotechnol. 2018, 102, 2563–2581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dy, R.L.; Rigano, L.A.; Fineran, P.C. Phage-based biocontrol strategies and their application in agriculture and aquaculture. Biochem. Soc. Trans. 2018, 46, 1605–1613. [Google Scholar] [CrossRef] [PubMed]
- Chanishvili, N. Phage therapy-History from Twort and d’Herelle through Soviet experience to current approaches. In Advances in Virus Research; Academic Press: New York, NY, USA, 2012. [Google Scholar]
- D’Herelle, F. Bacteriophage as a treatment in acute medical and surgical infections. Bull. N. Y. Acad. Med. 1931, 7, 329–348. [Google Scholar]
- Abedon, S.T.; Kuhl, S.J.; Blasdel, B.G.; Kutter, E.M. Phage treatment of human infections. Bacteriophage 2011, 1, 66–85. [Google Scholar] [CrossRef] [Green Version]
- Sulakvelidze, A.; Alavidze, Z.; Morris, G.J. Bacteriophage therapy. Antimicrob Agents Chemother 2001, 45, 649–659. [Google Scholar] [CrossRef] [Green Version]
- Summers, W.C. Felix d’Hérelle and the Origins of Molecular Biology; Yale University Press: London, UK, 1999. [Google Scholar]
- Bruynoghe, R.; Maisin, J. Essais de the rapeutique au moyen du bacteriophage. C. R. Soc. Biol. 1921, 85, 1120–1121. [Google Scholar]
- Myelnikov, D. An alternative cure: The adoption and survival of bacteriophage therapy in the USSR, 1922-1955. J. Hist. Med. Allied Sci. 2018, 73, 385–411. [Google Scholar] [CrossRef] [Green Version]
- Malik, D.J.; Sokolov, I.J.; Vinner, G.K.; Mancuso, F.; Cinquerrui, S.; Vladisavljevic, G.T.; Clokie, M.R.J.; Garton, N.J.; Stapley, A.G.F.; Kirpichnikova, A. Formulation, stabilisation and encapsulation of bacteriophage for phage therapy. Adv. Colloid Interface Sci. 2017, 249, 100–133. [Google Scholar] [CrossRef] [Green Version]
- Sarker, S.A.; Sultana, S.; Reuteler, G.; Moine, D.; Descombes, P.; Charton, F.; Bourdin, G.; McCallin, S.; Ngom-Bru, C.; Neville, T.; et al. Oral phage therapy of acute bacterial diarrhea with two coliphage preparations: A randomized trial in children from Bangladesh. EBioMedicine 2016, 4, 124–137. [Google Scholar] [CrossRef] [Green Version]
- Qadir, M.I.; Mobeen, T.; Masood, A. Phage therapy: Progress in pharmacokinetics. Brazilian J. Pharm. Sci. 2018, 54, 1–9. [Google Scholar] [CrossRef]
- McCallin, S.; Sarker, S.A.; Sultana, S.; Oechslin, F.; Brüssow, H. Metagenome analysis of Russian and Georgian Pyophage cocktails and a placebo-controlled safety trial of single phage versus phage cocktail in healthy Staphylococcus aureus carriers. Environ. Microbiol. 2018, 20, 3278–3293. [Google Scholar] [CrossRef] [PubMed]
- Milho, C.; Andrade, M.; Vilas Boas, D.; Alves, D.; Sillankorva, S. Antimicrobial assessment of phage therapy using a porcine model of biofilm infection. Int. J. Pharm. 2019, 557, 112–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, A.; Sousa, J.C.; Silva, A.C.; Melo, L.D.R.; Sillankorva, S. Chestnut honey and bacteriophage application to control Pseudomonas aeruginosa and Escherichia coli biofilms: Evaluation in an ex vivo wound model. Front. Microbiol. 2018, 9, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alves, D.R.; Booth, S.P.; Scavone, P.; Schellenberger, P.; Salvage, J.; Dedi, C.; Thet, N.-T.; Jenkins, A.T.A.; Waters, R.; Ng, K.W.; et al. Development of a high-throughput ex-vivo burn wound model using porcine skin, and its application to evaluate new approaches to control wound infection. Front. Cell. Infect. Microbiol. 2018, 8, 196. [Google Scholar] [CrossRef] [PubMed]
- Gomes-Fernandes, M.; Laabei, M.; Pagan, N.; Hidalgo, J.; Molinos, S.; Villar Hernandez, R.; Domínguez-Villanueva, D.; Jenkins, A.T.A.; Lacoma, A.; Prat, C. Accessory gene regulator (Agr) functionality in Staphylococcus aureus derived from lower respiratory tract infections. PLoS ONE 2017, 12, e0175552. [Google Scholar] [CrossRef]
- Mendes, J.J.; Leandro, C.; Corte-Real, S.; Barbosa, R.; Cavaco-Silva, P.; Melo-Cristino, J.; Gõrski, A.; Garcia, M. Wound healing potential of topical bacteriophage therapy on diabetic cutaneous wounds. Wound Repair Regen. 2013, 21, 595–603. [Google Scholar] [CrossRef]
- Seth, A.K.; Geringer, M.R.; Nguyen, K.T.; Agnew, S.P.; Dumanian, Z.; Galiano, R.D.; Leung, K.P.; Mustoe, T.A.; Hong, S.J. Bacteriophage therapy for Staphylococcus aureus biofilm-infected wounds: A new approach to chronic wound care. Plast. Reconstr. Surg. 2013, 131, 225–234. [Google Scholar] [CrossRef]
- Djebara, S.; Maussen, C.; De Vos, D.; Merabishvili, M.; Damanet, B.; Pang, K.W.; De Leenheer, P.; Strachinaru, I.; Soentjens, P.; Pirnay, J.P. Processing phage therapy requests in a Brussels military hospital: Lessons identified. Viruses 2019, 11, 265. [Google Scholar] [CrossRef] [Green Version]
- Jault, P.; Leclerc, T.; Jennes, S.; Pirnay, J.P.; Que, Y.A.; Resch, G.; Rousseau, A.F.; Ravat, F.; Carsin, H.; Le Floch, R.; et al. Efficacy and tolerability of a cocktail of bacteriophages to treat burn wounds infected by Pseudomonas aeruginosa (PhagoBurn): A randomised, controlled, double-blind phase 1/2 trial. Lancet Infect. Dis. 2019, 19, 35–45. [Google Scholar] [CrossRef]
- Rhoads, D.D.; Wolcott, R.D.; Kuskowski, M.A.; Wolcott, B.M.; Ward, L.S.; Sulakvelidze, A. Bacteriophage therapy of venous leg ulcers in humans: Results of a phase I safety trial. J. Wound Care 2009, 18, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Fish, R.; Kutter, E.; Wheat, G.; Blasdel, B.; Kutateladze, M.; Kuhl, S. Bacteriophage treatment of intransigent diabetic toe ulcers: A case series. J. Wound Care 2016, 25, S27–S33. [Google Scholar] [CrossRef]
- Fish, R.; Kutter, E.; Wheat, G.; Blasdel, B.; Kutateladze, M.; Kuhl, S. Compassionate use of bacteriophage therapy for foot ulcer treatment as an effective step for moving toward clinical trials. In Methods in Molecular Biology; Humana Press: New York, NY, USA, 2018; pp. 159–170. [Google Scholar]
- Morozova, V.V.; Kozlova, Y.N.; Ganichev, D.A.; Tikunova, N.V. Bacteriophage treatment of infected diabetic foot ulcers. In Methods in Molecular Biology; Humana Press: New York, NY, USA, 2018; pp. 151–158. [Google Scholar]
- Abo-elmaaty, S.; El Dougdoug, N.K.; Hazaa, M.M. Improved antibacterial efficacy of bacteriophage-cosmetic formulation for treatment of Staphylococcus aureus in vitro. Ann. Agric. Sci. 2016, 61, 201–206. [Google Scholar] [CrossRef] [Green Version]
- Gupta, P.; Singh, H.S.; Shukla, V.K.; Nath, G.; Bhartiya, S.K. Bacteriophage therapy of chronic nonhealing wound: Clinical study. Int. J. Low. Extrem. Wounds 2019, 18, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Ooi, M.L.; Drilling, A.J.; Morales, S.; Fong, S.; Moraitis, S.; MacIas-Valle, L.; Vreugde, S.; Psaltis, A.J.; Wormald, P.J. Safety and tolerability of bacteriophage therapy for chronic rhinosinusitis due to Staphylococcus aureus. JAMA Otolaryngol.-Head Neck Surg. 2019, 145, 723–729. [Google Scholar] [CrossRef] [PubMed]
- Tagliaferri, T.L.; Jansen, M.; Horz, H.P. Fighting pathogenic bacteria on two fronts: Phages and antibiotics as combined strategy. Front. Cell. Infect. Microbiol. 2019, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Furfaro, L.L.; Payne, M.S.; Chang, B.J. Bacteriophage therapy: Clinical trials and regulatory hurdles. Front. Cell. Infect. Microbiol. 2018, 8, 376. [Google Scholar] [CrossRef] [Green Version]
- Kortright, K.E.; Chan, B.K.; Koff, J.L.; Turner, P.E. Phage therapy: A renewed approach to combat antibiotic-resistant bacteria. Cell Host Microbe 2019, 25, 219–232. [Google Scholar] [CrossRef] [Green Version]
- Górski, A.; Miedzybrodzki, R.; Weber-Dabrowska, B.; Fortuna, W.; Letkiewicz, S.; Rogóz, P.; Jończyk-Matysiak, E.; Dabrowska, K.; Majewska, J.; Borysowski, J. Phage therapy: Combating infections with potential for evolving from merely a treatment for complications to targeting diseases. Front. Microbiol. 2016, 7, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Huh, H.; Wong, S.; St. Jean, J.; Slavcev, R. Bacteriophage interactions with mammalian tissue: Therapeutic applications. Adv. Drug Deliv. Rev. 2019, 145, 4–17. [Google Scholar] [CrossRef]
- Fauconnier, A. Phage therapy regulation: From night to dawn. Viruses 2019, 11, 352. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weld, R.J.; Butts, C.; Heinemann, J.A. Models of phage growth and their applicability to phage therapy. J. Theor. Biol. 2004, 227, 1–11. [Google Scholar] [CrossRef]
- Weiss, M.; Denou, E.; Bruttin, A.; Serra-Moreno, R.; Dillmann, M.L.; Brüssow, H. In vivo replication of T4 and T7 bacteriophages in germ-free mice colonized with Escherichia coli. Virology 2009, 393, 16–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harada, L.K.; Silva, E.C.; Campos, W.F.; Del Fiol, F.S.; Vila, M.; Dąbrowska, K.; Krylov, V.N.; Balcão, V.M. Biotechnological applications of bacteriophages: State of the art. Microbiol. Res. 2018, 212, 38–58. [Google Scholar] [CrossRef]
- Chan, B.K.; Abedon, S.T.; Loc-Carrillo, C. Phage cocktails and the future of phage therapy. Future Microbiol. 2013, 8, 769–783. [Google Scholar] [CrossRef]
- Jasim, H.N.; Hafidh, R.R.; Abdulamir, A.S. Formation of therapeutic phage cocktail and endolysin to highly studymulti-drug resistant Acinetobacter baumannii: In vitro and in vivo. Iran. J. Basic Med. Sci. 2018, 21, 1100–1108. [Google Scholar]
- Roach, D.R.; Debarbieux, L. Phage therapy: Awakening a sleeping giant. Emerg. Top. Life Sci. 2017, 1, 93–103. [Google Scholar]
- Weber-Dabrowska, B.; Jończyk-Matysiak, E.; Zaczek, M.; Łobocka, M.; Łusiak-Szelachowska, M.; Górski, A. Bacteriophage procurement for therapeutic purposes. Front. Microbiol. 2016, 7, 1177. [Google Scholar] [CrossRef]
- Schmerer, M.; Molineux, I.J.; Bull, J.J. Synergy as a rationale for phage therapy using phage cocktails. PeerJ 2014, 2, e590. [Google Scholar] [CrossRef] [Green Version]
- Merabishvili, M.; Pirnay, J.P.; De Vos, D. Guidelines to compose an ideal bacteriophage cocktail. In Methods in Molecular Biology; Humana Press: New York, NY, USA, 2018. [Google Scholar]
- Allen, R.C.; Pfrunder-Cardozo, K.R.; Meinel, D.; Egli, A.; Hall, A.R. Associations among antibiotic and phage resistance phenotypes in natural and clinical Escherichia coli isolates. MBio 2017, 8, e01341-17. [Google Scholar] [CrossRef] [Green Version]
- Ma, Y.; Pacan, J.C.; Wang, Q.; Xu, Y.; Huang, X.; Korenevsky, A.; Sabour, P.M. Microencapsulation of bacteriophage felix o1 into chitosan-alginate microspheres for oral delivery. Appl. Environ. Microbiol. 2008, 74, 4799–4805. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colom, J.; Otero, J.; Cortés, P.; Llagostera, M.; Cano-Sarabia, M.; Maspoch, D. Liposome-encapsulated bacteriophages for enhanced oral phage therapy against Salmonella spp. Appl. Environ. Microbiol. 2015, 81, 4841–4849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McVay, C.S.; Velásquez, M.; Fralick, J.A. Phage therapy of Pseudomonas aeruginosa infection in a mouse burn wound model. Antimicrob. Agents Chemother. 2007, 51, 1934–1938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, J.; Liu, Y.; Xiao, C.; He, S.; Yao, H.; Bao, G. Efficacy of phage therapy in controlling rabbit colibacillosis and Changes in cecal microbiota. Front. Microbiol. 2017, 8, 957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, H.; Sillankorva, S.; Merabishvili, M.; Kluskens, L.D.; Azeredo, J. Unexploited opportunities for phage therapy. Front. Pharmacol. 2015, 6, 1–4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brown, T.L.; Petrovski, S.; Chan, H.T.; Angove, M.J.; Tucci, J. Semi-solid and solid dosage forms for the delivery of phage therapy to epithelia. Pharmaceuticals 2018, 11, 26. [Google Scholar] [CrossRef] [Green Version]
- El-Shibiny, A.; El-Sahhar, S. Bacteriophages: The possible solution to treat infections caused by pathogenic bacteria. Can. J. Microbiol. 2017, 63, 865–879. [Google Scholar] [CrossRef] [Green Version]
- Subils, T.; Aquili, V.; Ebner, G.; Balagué, C. Effect of preservatives on Shiga toxigenic phages and Shiga toxin of Escherichia coli O157:H7. J. Food Prot. 2012, 75, 959–965. [Google Scholar] [CrossRef]
- Merabishvili, M.; Monserez, R.; Van Belleghem, J.; Rose, T.; Jennes, S.; De Vos, D.; Verbeken, G.; Vaneechoutte, M.; Pirnay, J.P. Stability of bacteriophages in burn wound care products. PLoS ONE 2017, 12, e0182121. [Google Scholar] [CrossRef] [Green Version]
- Rose, T.; Verbeken, G.; De Vos, D.; Merabishvili, M.; Vaneechoutte, M.; Lavigne, R.; Jennes, S.; Zizi, M.; Pirnay, J.-P. Experimental phage therapy of burn wound infection: Difficult first steps. Int. J. Burns Trauma 2014, 4, 66–73. [Google Scholar]
- Zhvania, P.; Hoyle, N.S.; Nadareishvili, L.; Nizharadze, D.; Kutateladze, M. Phage therapy in a 16-year-old boy with netherton syndrome. Front. Med. 2017, 4, 94. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melo, L.D.R.; Ferreira, R.; Costa, A.R.; Oliveira, H.; Azeredo, J. Efficacy and safety assessment of two enterococci phages in an in vitro biofilm wound model. Sci. Rep. 2019, 9, 6643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jennes, S.; Merabishvili, M.; Soentjens, P.; Pang, K.W.; Rose, T.; Keersebilck, E.; Soete, O.; François, P.M.; Teodorescu, S.; Verween, G.; et al. Use of bacteriophages in the treatment of colistin-only-sensitive Pseudomonas aeruginosa septicaemia in a patient with acute kidney injury-a case report. Crit. Care 2017, 21, 129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chadha, P.; Katare, O.P.; Chhibber, S. Liposome loaded phage cocktail: Enhanced therapeutic potential in resolving Klebsiella pneumoniae mediated burn wound infections. Burns 2017, 43, 1532–1543. [Google Scholar] [CrossRef] [PubMed]
- Chhibber, S.; Kaur, J.; Kaur, S. Liposome entrapment of bacteriophages improves wound healing in a diabetic mouse MRSA infection. Front. Microbiol. 2018, 9, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Hathaway, H.; Alves, D.R.; Bean, J.; Esteban, P.P.; Ouadi, K.; Mark Sutton, J.; Jenkins, A.T.A. Poly(N-isopropylacrylamide-co-allylamine) (PNIPAM-co-ALA) nanospheres for the thermally triggered release of bacteriophage K. Eur. J. Pharm. Biopharm. 2015, 96, 437–441. [Google Scholar] [CrossRef]
- Esteban, P.P.; Alves, D.R.; Enright, M.C.; Bean, J.E.; Gaudion, A.; Jenkins, A.T.A.; Young, A.E.R.; Arnot, T.C. Enhancement of the antimicrobial properties of bacteriophage-K via stabilization using oil-in-water nano-emulsions. Biotechnol. Prog. 2014, 30, 932–944. [Google Scholar] [CrossRef] [Green Version]
- Rubalskii, E.; Ruemke, S.; Salmoukas, C.; Aleshkin, A.; Bochkareva, S.; Modin, E.; Mashaqi, B.; Boyle, E.C.; Boethig, D.; Rubalsky, M.; et al. Fibrin glue as a local drug-delivery system for bacteriophage PA5. Sci. Rep. 2019, 9, 2091. [Google Scholar] [CrossRef] [Green Version]
- Nogueira, F.; Karumidze, N.; Kusradze, I.; Goderdzishvili, M.; Teixeira, P.; Gouveia, I.C. Immobilization of bacteriophage in wound-dressing nanostructure. Nanomed. Nanotechnol. Biol. Med. 2017, 13, 2475–2484. [Google Scholar] [CrossRef] [Green Version]
- Cheng, W.; Zhang, Z.; Xu, R.; Cai, P.; Kristensen, P.; Chen, M.; Huang, Y. Incorporation of bacteriophages in polycaprolactone/collagen fibers for antibacterial hemostatic dual-function. J. Biomed. Mater. Res.-Part B Appl. Biomater. 2018, 106, 2588–2595. [Google Scholar] [CrossRef]
- Bean, J.E.; Alves, D.R.; Laabei, M.; Esteban, P.P.; Thet, N.T.; Enright, M.C.; Jenkins, A.T.A. Triggered release of bacteriophage K from agarose/hyaluronan hydrogel matrixes by Staphylococcus aureus virulence factors. Chem. Mater. 2014, 26, 7201–7208. [Google Scholar] [CrossRef] [Green Version]
- Kaur, P.; Gondil, V.S.; Chhibber, S. A novel wound dressing consisting of PVA-SA hybrid hydrogel membrane for topical delivery of bacteriophages and antibiotics. Int. J. Pharm. 2019, 118779. [Google Scholar] [CrossRef] [PubMed]
- Choińska-Pulit, A.; Mituła, P.; Śliwka, P.; Łaba, W.; Skaradzińska, A. Bacteriophage encapsulation: Trends and potential applications. Trends Food Sci. Technol. 2015, 45, 212–221. [Google Scholar] [CrossRef]
- Cui, H.; Yuan, L.; Lin, L. Novel chitosan film embedded with liposome-encapsulated phage for biocontrol of Escherichia coli O157:H7 in beef. Carbohydr. Polym. 2017, 177, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Ju, Z.; Sun, W. Drug delivery vectors based on filamentous bacteriophages and phage-mimetic nanoparticles. Drug Deliv. 2017, 24, 1898–1908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rägo, L.; Santoso, B. Drug regulation: History, present and future. In Drug Benefits and Risks: International Textbook of Clinical Pharmacology; IOS Press: Washington, DC, USA, 2008; pp. 65–77. ISBN 9781586038809. [Google Scholar]
- Withington, R. Regulatory issues for phage-based clinical products. J. Chem. Technol. Biotechnol. 2001, 76, 673–676. [Google Scholar] [CrossRef]
- Verbeken, G.; De Vos, D.; Vaneechoutte, M.; Merabishvils, M.; Zizi, M.; Pirnay, J.P. European regulatory conundrum of phage therapy. Future Microbiol. 2007, 2, 485–491. [Google Scholar] [CrossRef]
- Cooper, C.J.; Mirzaei, M.K.; Nilsson, A.S. Adapting drug approval pathways for bacteriophage-based therapeutics. Front. Microbiol. 2016, 7, 1209. [Google Scholar] [CrossRef]
- McCallin, S.; Sacher, J.C.; Zheng, J.; Chan, B.K. Current state of compassionate phage therapy. Viruses 2019, 11, 343. [Google Scholar] [CrossRef] [Green Version]
- Kutateladze, M.; Adamia, R. Phage therapy experience at the Eliava Institute. Med. Mal. Infect. 2008, 38, 426–430. [Google Scholar] [CrossRef]
- World medical association declaration of helsinki: Ethical principles for medical research involving human subjects. JAMA-J. Am. Med. Assoc. 2013, 310, 2191–2194. [CrossRef] [PubMed] [Green Version]
- Food and Drug Administration. Expanded Access Program Report. Available online: https://www.fda.gov/media/119971/download (accessed on 13 January 2020).
- Paris, V.; Slawomirski, L.; Colbert, A.; Delaunay, N.; Oderkirk, J. Innovation, access and value in pharmaceuticals. In New Health Technologies—Managing Access, Value and Sustainability; OECD Publishing: Paris, France, 2017; pp. 81–116. [Google Scholar]
- Department of Health-Therapeutics Goods Administration. Accessing Unapproved Products. Available online: https://www.tga.gov.au/accessing-unapproved-products (accessed on 13 January 2020).
- Department of Health-Therapeutics Goods Administration. Personal Import Scheme. Available online: https://www.tga.gov.au/publication/personal-import-scheme (accessed on 13 January 2020).
- Pirnay, J.P.; Verbeken, G.; Ceyssens, P.J.; Huys, I.; de Vos, D.; Ameloot, C.; Fauconnier, A. The magistral phage. Viruses 2018, 10, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fauconnier, A. Guidelines for bacteriophage product certification. Methods Mol. Biol. 2018, 1693, 253–268. [Google Scholar] [PubMed]
- Górski, A.; Międzybrodzki, R.; Łobocka, M.; Głowacka-Rutkowska, A.; Bednarek, A.; Borysowski, J.; Jończyk-Matysiak, E.; Łusiak-Szelachowska, M.; Weber-Dabrowska, B.; Bagińska, N.; et al. Phage therapy: What have we learned? Viruses 2018, 10, 288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Venturini, C.; Fabjian, A.P.; Cy Lin, R. Bacteriophage therapy for severe infections. Microbiol. Aust. 2019, 40, 20–23. [Google Scholar] [CrossRef]
- Abedon, S.T. Information phage therapy research should report. Pharmaceuticals 2017, 10, 43. [Google Scholar] [CrossRef]


| Authors | Year | Individual/Cocktail Phages | Phage Name | Host Organism | Study Model | Dosage | Main Conclusions | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| S. aureus | E. coli | P. aeruginosa | A. baumannii | P. mirabilis | K. pneumoniae | E. faecium | E. faecalis | Streptococcus sp. | Proteus sp. | In vitro | In vivo | Ex vivo | Human | ||||||
| Non-encapsulated phage strategies | |||||||||||||||||||
| [125] | 2013 | Cocktail of 5 species-specific phages | F44/10 | × | × | 108 to 109 PFU/mL | Decrease of the bacterial counts. Wound healing improvement. | ||||||||||||
| F125/10 | × | ||||||||||||||||||
| F510/08 | × | ||||||||||||||||||
| F770/05 | × | ||||||||||||||||||
| F1245/05 | × | ||||||||||||||||||
| [126] | 2013 | Species-specific phage | 1ND | × | × | 106 PFU/mL | The combination of phage treatment with debridement improved healing and reduced bacterial counts. | ||||||||||||
| [161] | 2014 | Cocktail of 3 species-specific phages | 14/1 | × | × | 109 PFU/mL | The topical application of this cocktail did not show any adverse effects; however, its efficacy was not adequately studied. | ||||||||||||
| PNM | × | ||||||||||||||||||
| ISP | × | ||||||||||||||||||
| [162] | 2017 | Individual phage and cocktails | Sb-1 | × | × | 107 PFU/mL | No allergic reactions observed and after seven days, bacterial loads decreased and wounds improved. | ||||||||||||
| Pyophage | × | × | × | × | × | ||||||||||||||
| Fersis | × | × | |||||||||||||||||
| [122] | 2018 | Cocktail of 2 species-specific phages | vB_EcoS_CEB_EC3a | × | × | × | 109 PFU/mL | Phage-honey acted synergistically and reduced CFU counts. | |||||||||||
| vB_PaeP_PAO1-D | × | ||||||||||||||||||
| [123] | 2018 | Individual phage and cocktails | DRA88 | × | × | 4 h treatment: 5 μL containing 106 PFU/mL 24 h treatment: 107 PFU/mL | Reduction of viable cells and biofilm formation. | ||||||||||||
| SAB4328-A | × | ||||||||||||||||||
| [134] | 2019 | Cocktail of 3 species-specific phages | ND | × | × | × | × | 0.1 mL/cm2 and 109 PFU/mL | 3 to 5 doses of topical phage resulted in no signs of infection. Seven patients achieved complete healing on day 21. | ||||||||||
| [121] | 2019 | Individual phage and cocktails | EC7a | × | × | × | 100 µL of phage or phage cocktail at different multiplicities of infection (MOI) | Decrease of viable cells in biofilms formed on porcine skins for phages applied alone or in a cocktail. | |||||||||||
| EC7b | × | ||||||||||||||||||
| EC3a | × | ||||||||||||||||||
| P2 | × | ||||||||||||||||||
| P1 | × | ||||||||||||||||||
| AB7a | × | ||||||||||||||||||
| PA1 | × | ||||||||||||||||||
| PA4 | × | ||||||||||||||||||
| Pm5460 | × | ||||||||||||||||||
| Pm5461 | × | ||||||||||||||||||
| [163] | 2019 | Individual phage and cocktails | vB_EfaS-Zip | × | × | 108 PFU/mL | Three hours of treatment with the phage cocktail led to a 2.5 log CFU/mL reduction. | ||||||||||||
| vB_EfaP-Max | × | ||||||||||||||||||
| Injection of phages into the soft tissue | |||||||||||||||||||
| [164] | 2017 | Cocktail of 2 species-specific phages | BFC1 | × | × | ND | Blood cultures were negative for the presence of bacteria. However, the wounds remained colonized. The patient succumbed to blood sepsis derived from Klebsiella pneumoniae colonization. | ||||||||||||
| [131] | 2018 | Species-specific phage | Sb-1 | × | × | PFU/mL not referred. Injections of 0.7 cc of phage, once a week for seven weeks (total of 4.9 cc) | Ulcer healed. Re-ossification of the distal phalanx occurred and the patient discharged after three months. | ||||||||||||
| Phage encapsulation in liposomes | |||||||||||||||||||
| [165] | 2017 | Cocktail | KØ1 | × | × | 108 PFU/mL | Phage cocktail entrapped within liposomes reduced more cells and led to a faster resolution of the infection. | ||||||||||||
| KØ2 | × | ||||||||||||||||||
| KØ3 | × | ||||||||||||||||||
| KØ4 | × | ||||||||||||||||||
| KØ5 | × | ||||||||||||||||||
| [166] | 2018 | Cocktail | MR-5 | × | × | × | 109 PFU/50 µL | Cocktail of two phages reduced more bacteria and led to faster healings compared to individual phages. Liposomal phage cocktail entrapment persisted longer at the wound site. | |||||||||||
| MR-10 | × | ||||||||||||||||||
| Phage encapsulation in nanospheres | |||||||||||||||||||
| [167] | 2015 | Species-specific phage | K | × | × | 109 PFU/mL | The formulation of the phage with poly(N-isopropylacrylamid) nanospheres copolymerized with allylamine, anchored onto a simulated dressing via plasma deposition, demonstrated to lyse bacterial isolates under body temperature of 37 °C. | ||||||||||||
| Incorporation of bacteriophage into emulsions | |||||||||||||||||||
| [168] | 2014 | Species-specific phage | K | × | × | 105 PFU/mL | Higher antibacterial activity found in phage-loaded emulsions compared to free phages. The three strains studied here were rapidly and entirely killed by nanoemulsions. | ||||||||||||
| Incorporation of phages within adhesives | |||||||||||||||||||
| [169] | 2019 | Species-specific phage | PA5 | × | × | 1011 PFU | Phage immobilization within fibrin glue resulted in the release of high titers of viable phages during 11 days. | ||||||||||||
| Incorporation of phages within fibers | |||||||||||||||||||
| [170] | 2017 | Species-specific phage | vB_Pae_Kakheti25 | × | × | ND | The use of polycaprolactone to immobilize the phage eradicated the bacterium. | ||||||||||||
| [171] | 2018 | Species-specific phage | T4 | × | × | × | 1013 PFU/mL | Polycaprolactone/collagen I B in vivo fully degraded in 8 weeks without adverse reactions to muscle and subcutaneous tissues. | |||||||||||
| Incorporation of phages within hydrogels | |||||||||||||||||||
| [172] | 2014 | Species-specific phage | ΦK | × | × | 108 PFU/mL | Phage release facilitated by hyaluronidase, which degraded the hyaluronic acid methacrylate present in the upper layer of the hydrogel, promoting the subsequent killing of bacteria. | ||||||||||||
| [173] | 2019 | Species-specific phage | MR10 | × | × | × | MOI 10 | PVA-Sodium alginate hydrogel-based dressings with minocycline and phages were effective against infected burn wounds, reducing bacterial colonization and inflammation significantly. | |||||||||||
| Kpn5 | × | ||||||||||||||||||
| PA5 | × | ||||||||||||||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pinto, A.M.; Cerqueira, M.A.; Bañobre-Lópes, M.; Pastrana, L.M.; Sillankorva, S. Bacteriophages for Chronic Wound Treatment: From Traditional to Novel Delivery Systems. Viruses 2020, 12, 235. https://doi.org/10.3390/v12020235
Pinto AM, Cerqueira MA, Bañobre-Lópes M, Pastrana LM, Sillankorva S. Bacteriophages for Chronic Wound Treatment: From Traditional to Novel Delivery Systems. Viruses. 2020; 12(2):235. https://doi.org/10.3390/v12020235
Chicago/Turabian StylePinto, Ana M., Miguel A. Cerqueira, Manuel Bañobre-Lópes, Lorenzo M. Pastrana, and Sanna Sillankorva. 2020. "Bacteriophages for Chronic Wound Treatment: From Traditional to Novel Delivery Systems" Viruses 12, no. 2: 235. https://doi.org/10.3390/v12020235
APA StylePinto, A. M., Cerqueira, M. A., Bañobre-Lópes, M., Pastrana, L. M., & Sillankorva, S. (2020). Bacteriophages for Chronic Wound Treatment: From Traditional to Novel Delivery Systems. Viruses, 12(2), 235. https://doi.org/10.3390/v12020235