Berberine Hampers Influenza A Replication through Inhibition of MAPK/ERK Pathway
Abstract
:1. Introduction
2. Materials and Methods
2.1. Compounds
2.2. Virus and Cells
2.3. Replication Inhibition Assay
2.4. Mechanism of Action Assays
- (I)
- Virus inactivation assay shows the influence of tested compounds on the viral particle. Influenza A virus stock was incubated with BBR under constant mixing for 1 h at room temperature. Samples were diluted 100 times to ensure that the BBR concentration was below the lower limit of the effective range. Samples were then titrated according to the Reed and Muench method [24].
- (II)
- Cell protection assay examines the effect of the compound on the cell surface. In this assay, cells were incubated with BBR for 2 h at 37 °C. After incubation, the media were removed, and cells were washed with PBS. Then, cells were infected with the influenza A virus (TCID50 = 400) or mock control for 2 h at 37 °C. Next, media were discarded, cells were washed thrice with PBS, and fresh infection medium was added. Cells were incubated for 48 h at 37 °C.
- (III)
- Virus attachment assay examines the effect of the compound on the virus–receptor interaction. Confluent cells were pre-cooled to 4 °C and overlaid with ice-cold influenza A virus in BBR-containing medium (TCID50 = 400) or mock control. Samples were incubated at 4 °C for 1 h to allow for virus attachment to the host cell but not for virus internalization [25]. Then, cells were rinsed thrice with cold PBS to remove the residual virus and fresh medium was applied. Cells were incubated for 48 h at 37 °C.
- (IV)
- Virus internalization assay for evaluation of virus entry into the susceptible cell. Pre-cooled, confluent cells were infected with a virus (TCID50 = 400; ice-cold solution) for 2 h at 4 °C to avoid virus internalization to cells. After incubation, the medium was discarded, and cells were washed thrice with cold PBS. Then, media supplemented with BBR were applied, and samples were incubated for 2 h at 37 °C to allow for virus internalization. Next, cells were washed with an acidic buffer (pH 3.0; 0.1 M glycine, 0.1 M sodium chloride) to inactivate uninternalized virions. Cultures were rinsed once with PBS and fresh media were applied. Cells were incubated for 48 h at 37 °C.
- (V)
- Virus replication, assembly, and egress assay evaluates virus replication and production of infectious progeny. Cell cultures were infected with influenza A virus (TCID50 = 400) for 2 h at 37 °C. Then, the residual virus was washed out thrice with PBS. Medium supplemented with BBR was applied, and cells were incubated for 48 h at 37 °C.
2.5. Cell Viability
2.6. Confocal Microscopy and Image Analysis
2.7. Quantitative Real-Time PCR
2.8. Isolation of Nuclear and Cytoplasmic Proteins
2.9. Statistics
3. Results
3.1. The Antiviral Effect of BBR Is Cell-Type Dependent
3.2. BBR Inhibits the Influenza A Virus Replication at Late Stages of the Infection
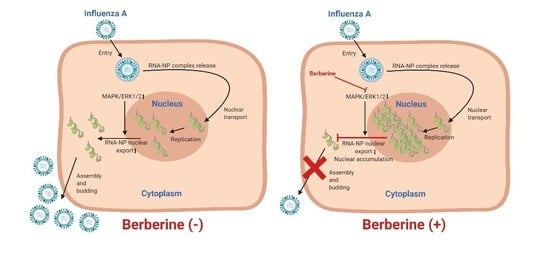
3.3. BBR Inhibits the Influenza A Virus Replication through Downregulation of the MAPK/ERK Pathway
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shaw, M.L.; Palese, P. Orthomyxoviridae: The viruses and their replication. Fields Virol. 2007, 2, 1647–1689. [Google Scholar]
- Morens, D.M.; Taubenberger, J.K.; Harvey, H.A.; Memoli, M.J. The 1918 influenza pandemic: Lessons for 2009 and the future. Crit. Care Med. 2010, 38. [Google Scholar] [CrossRef] [PubMed]
- Dawood, F.S.; Iuliano, A.D.; Reed, C.; Meltzer, M.I.; Shay, D.K.; Cheng, P.Y.; Bandaranayake, D.; Breiman, R.F.; Brooks, W.A.; Buchy, P.; et al. Estimated global mortality associated with the first 12 months of 2009 pandemic influenza A H1N1 virus circulation: A modelling study. Lancet Infect. Dis. 2012, 12, 687–695. [Google Scholar] [CrossRef] [Green Version]
- Nair, H.; Brooks, W.A.; Katz, M.; Roca, A.; Berkley, J.A.; Madhi, S.A.; Simmerman, J.M.; Gordon, A.; Sato, M.; Howie, S.; et al. Global burden of respiratory infections due to seasonal influenza in young children: A systematic review and meta-analysis. Lancet 2011, 378, 1917–1930. [Google Scholar] [CrossRef] [Green Version]
- Reperant, L.A.; Moesker, F.M.; Osterhaus, A.D.M.E. Influenza: From zoonosis to pandemic. ERJ Open Res. 2016, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thompson, M.G.; Pierse, N.; Sue Huang, Q.; Prasad, N.; Duque, J.; Claire Newbern, E.; Baker, M.G.; Turner, N.; McArthur, C. Influenza vaccine effectiveness in preventing influenza-associated intensive care admissions and attenuating severe disease among adults in New Zealand 2012–2015. Vaccine 2018, 36, 5916–5925. [Google Scholar] [CrossRef]
- Ferdinands, J.M.; Olsho, L.E.W.; Agan, A.A.; Bhat, N.; Sullivan, R.M.; Hall, M.; Mourani, P.M.; Thompson, M.; Randolph, A.G. Effectiveness of influenza vaccine against life-threatening RT-PCR-confirmed influenza illness in US children, 2010-2012. J. Infect. Dis. 2014, 210, 674–683. [Google Scholar] [CrossRef] [Green Version]
- Hussain, M.; Galvin, H.D.; Haw, T.Y.; Nutsford, A.N.; Husain, M. Drug resistance in influenza a virus: The epidemiology and management. Infect. Drug Resist. 2017, 10, 121–134. [Google Scholar] [CrossRef] [Green Version]
- Hayden, F.G.; Sugaya, N.; Hirotsu, N.; Lee, N.; de Jong, M.D.; Hurt, A.C.; Ishida, T.; Sekino, H.; Yamada, K.; Portsmouth, S.; et al. Baloxavir Marboxil for Uncomplicated Influenza in Adults and Adolescents. N. Engl. J. Med. 2018, 379, 913–923. [Google Scholar] [CrossRef]
- Gan, R. Bioactivities of Berberine: An Update. Int. J. Mod. Biol. Med. 2012, 1, 48–81. [Google Scholar]
- Neag, M.A.; Mocan, A.; Echeverría, J.; Pop, R.M.; Bocsan, C.I.; Crisan, G.; Buzoianu, A.D. Berberine: Botanical Occurrence, traditional uses, extraction methods, and relevance in cardiovascular, metabolic, hepatic, and renal disorders. Front. Pharmacol. 2018, 9, 557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imenshahidi, M.; Hosseinzadeh, H. Berberis Vulgaris and Berberine: An Update Review. Phyther. Res. 2016, 30, 1745–1764. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Ekavali; Chopra, K.; Mukherjee, M.; Pottabathini, R.; Dhull, D.K. Current knowledge and pharmacological profile of berberine: An update. Eur. J. Pharmacol. 2015, 761, 288–297. [Google Scholar] [CrossRef] [PubMed]
- Qiu, S.; Sun, H.; Zhang, A.H.; Xu, H.Y.; Yan, G.L.; Han, Y.; Wang, X.J. Natural alkaloids: Basic aspects, biological roles, and future perspectives. Chin. J. Nat. Med. 2014, 12, 401–406. [Google Scholar] [CrossRef]
- Batista, M.N.; Braga, A.C.S.; Fernandes Campos, G.R.; Michel Souza, M.; de Matos, R.P.A.; Zara Lopes, T.; Maria Candido, N.; Duarte Lima, M.L.; Cristina Machado, F.; de Andrade, S.T.Q.; et al. Natural products isolated from oriental medicinal herbs inactivate zika virus. Viruses 2019, 11, 49. [Google Scholar] [CrossRef] [Green Version]
- Robinson, C.L.; Chong, A.C.N.; Ashbrook, A.W.; Jeng, G.; Jin, J.; Chen, H.; Tang, E.I.; Martin, L.A.; Kim, R.S.; Kenyon, R.M.; et al. Male germ cells support long-term propagation of Zika virus. Nat. Commun. 2018, 9. [Google Scholar] [CrossRef]
- Marchant, D.; Singhera, G.K.; Utokaparch, S.; Hackett, T.L.; Boyd, J.H.; Luo, Z.; Si, X.; Dorscheid, D.R.; McManus, B.M.; Hegele, R.G. Toll-Like Receptor 4-Mediated Activation of p38 Mitogen-Activated Protein Kinase Is a Determinant of Respiratory Virus Entry and Tropism. J. Virol. 2010, 84, 11359–11373. [Google Scholar] [CrossRef] [Green Version]
- Zou, K.; Li, Z.; Zhang, Y.; Zhang, H.Y.; Li, B.; Zhu, W.L.; Shi, J.Y.; Jia, Q.; Li, Y.M. Advances in the study of berberine and its derivatives: A focus on anti-inflammatory and anti-tumor effects in the digestive system. Acta Pharmacol. Sin. 2017, 38, 157–167. [Google Scholar] [CrossRef] [Green Version]
- Cecil, C.E.; Davis, J.M.; Cech, N.B.; Laster, S.M. Inhibition of H1N1 influenza A virus growth and induction of inflammatory mediators by the isoquinoline alkaloid berberine and extracts of goldenseal (Hydrastis canadensis). Int. Immunopharmacol. 2011, 11, 1706–1714. [Google Scholar] [CrossRef]
- Wu, Y.; Li, J.; Kim, Y.; Wu, J.; Wang, Q.; Hao, Y. In vivo and in vitro antiviral effects of berberine on influenza virus. Chin. J. Integr. Med. 2011, 17, 444–452. [Google Scholar] [CrossRef]
- Yan, Y.Q.; Fu, Y.J.; Wu, S.; Qin, H.Q.; Zhen, X.; Song, B.M.; Weng, Y.S.; Wang, P.C.; Chen, X.Y.; Jiang, Z.Y. Anti-influenza activity of berberine improves prognosis by reducing viral replication in mice. Phyther. Res. 2018, 32, 2560–2567. [Google Scholar] [CrossRef]
- Ciejka, J.; Milewska, A.; Wytrwal, M.; Wojarski, J.; Golda, A.; Ochman, M.; Nowakowska, M.; Szczubialka, K.; Pyrc, K. Novel polyanions inhibiting replication of influenza viruses. Antimicrob. Agents Chemother. 2016, 60, 1955–1966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciejka, J.; Botwina, P.; Nowakowska, M.; Szczubiałka, K.; Pyrc, K. Synthetic sulfonated derivatives of poly (allylamine hydrochloride) as inhibitors of human metapneumovirus. PLoS ONE 2019, 14, e0214646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reed, L.J.; Muench, H. A simple method of estimating fifty per cent endpoints. Am. J. Hyg. 1938, 27, 493–497. [Google Scholar]
- Harden, E.A.; Falshaw, R.; Carnachan, S.M.; Kern, E.R.; Prichard, M.N. Virucidal activity of polysaccharide extracts from four algal species against herpes simplex virus. Antiviral Res. 2009, 83, 282–289. [Google Scholar] [CrossRef] [Green Version]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]
- Kula, A.; Guerra, J.; Knezevich, A.; Kleva, D.; Myers, M.P.; Marcello, A. Characterization of the HIV-1 RNA associated proteome identifies Matrin 3 as a nuclear cofactor of Rev function. Retrovirology 2011, 8. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.H.; Weeratunga, P.; Kim, M.S.; Nikapitiya, C.; Lee, B.H.; Uddin, M.B.; Kim, T.H.; Yoon, J.E.; Park, C.; Ma, J.Y.; et al. Inhibitory effects of an aqueous extract from Cortex Phellodendri on the growth and replication of broad-spectrum of viruses in vitro and in vivo. BMC Complement. Altern. Med. 2016, 16. [Google Scholar] [CrossRef] [Green Version]
- Qi, H.W.; Xin, L.Y.; Xu, X.; Ji, X.X.; Fan, L.H. Epithelial-to-mesenchymal transition markers to predict response of Berberine in suppressing lung cancer invasion and metastasis. J. Transl. Med. 2014, 12, 22. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Liu, F.; Jiang, S.; Liu, J.; Chen, X.; Zhang, S.; Zhao, H. Berberine hydrochloride inhibits cell proliferation and promotes apoptosis of non-small cell lung cancer via the suppression of the MMP2 and Bcl-2/bax signaling pathways. Oncol. Lett. 2018, 15, 7409–7414. [Google Scholar] [CrossRef]
- Rosenberger, C.M.; Podyminogin, R.L.; Askovich, P.S.; Navarro, G.; Kaiser, S.M.; Sanders, C.J.; McClaren, J.L.; Tam, V.C.; Dash, P.; Noonan, J.G.; et al. Characterization of innate responses to influenza virus infection in a novel lung type I epithelial cell model. J. Gen. Virol. 2014, 95, 350–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milewska, A.; Nowak, P.; Stozek, K.; Potempa, J.; Pyrc, K.; Zarebski, M. Human coronavirus NL63 utilizes heparan sulfate proteoglycans for attachment to target cells. J. Virol. 2014, 88, 13221–13230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Milewska, A.; Nowak, P.; Owczarek, K.; Szczepanski, A.; Zarebski, M.; Hoang, A.; Berniak, K.; Wojarski, J.; Zeglen, S.; Baster, Z.; et al. Entry of Human Coronavirus NL63 into the Cell. J. Virol. 2017, 92, e01933-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaur, P.; Munjhal, A.; Lal, S.K. Influenza virus and cell signaling pathways. Med. Sci. Monit. 2011, 17, RA148–RA154. [Google Scholar] [CrossRef] [Green Version]
- Varghese, F.S.; Ahola, T.; Thaa, B.; McInerney, G.M.; Amrun, S.N.; Simarmata, D.; Ng, L.F.P.; Rausalu, K.; Merits, A.; Nyman, T.A. The antiviral alkaloid berberine reduces Chikungunya virus-induced mitogen-activated protein kinase signaling. J. Virol. 2016, 90, 9743–9757. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Li, K.; Ma, L.; Wu, S.; Hu, J.; Yan, H.; Jiang, J.; Li, Y. Berberine inhibits enterovirus 71 replication by downregulating the MEK/ERK signaling pathway and autophagy. Virol. J. 2017, 14, 2. [Google Scholar] [CrossRef] [Green Version]
- Pleschka, S.; Wolff, T.; Ehrhardt, C.; Hobom, G.; Planz, O.; Rapp, U.R.; Ludwig, S. Influenza virus propagation is impaired by inhibition of the Raf/MEK/ERK signalling cascade. Nat. Cell Biol. 2001, 3, 301–305. [Google Scholar] [CrossRef]
- Ehrhardt, C.; Marjuki, H.; Wolff, T.; Nürnberg, B.; Planz, O.; Pleschka, S.; Ludwig, S. Bivalent role of the phosphatidylinositol-3-kinase (PI3K) during influenza virus infection and host cell defence. Cell. Microbiol. 2006, 8, 1336–1348. [Google Scholar] [CrossRef]
- Warmka, J.K.; Solberg, E.L.; Zeliadt, N.A.; Srinivasan, B.; Charlson, A.T.; Xing, C.; Wattenberg, E.V. Inhibition of mitogen activated protein kinases increases the sensitivity of A549 lung cancer cells to the cytotoxicity induced by a kava chalcone analog. Biochem. Biophys. Res. Commun. 2012. [Google Scholar] [CrossRef] [Green Version]
- Lu, C.L.; Lin, H.I.; Chen, B.F.; Jow, G.M. Beauvericin-induced cell apoptosis through the mitogen-activated protein kinase pathway in human nonsmall cell lung cancer A549 cells. J. Toxicol. Sci. 2016, 41, 429–437. [Google Scholar] [CrossRef] [Green Version]
- Verheijen, M.; Lienhard, M.; Schrooders, Y.; Clayton, O.; Nudischer, R.; Boerno, S.; Timmermann, B.; Selevsek, N.; Schlapbach, R.; Gmuender, H.; et al. DMSO induces drastic changes in human cellular processes and epigenetic landscape in vitro. Sci. Rep. 2019, 9, 4641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, E.R.; Hadidian, Z.; Mason, M.M. THE SINGLE–AND REPEATED–DOSE TOXICITY OF DIMETHYL SULFOXIDE. Ann. N. Y. Acad. Sci. 1967. [Google Scholar] [CrossRef] [PubMed]
- Uyeki, T.M.; Bernstein, H.H.; Bradley, J.S.; Englund, J.A.; File, T.M.; Fry, A.M.; Gravenstein, S.; Hayden, F.G.; Harper, S.A.; Hirshon, J.M.; et al. Clinical Practice Guidelines by the Infectious Diseases Society of America: 2018 Update on Diagnosis, Treatment, Chemoprophylaxis, and Institutional Outbreak Management of Seasonal Influenzaa. Clin. Infect. Dis. 2019. [Google Scholar] [CrossRef] [PubMed]
- Godugu, C.; Patel, A.R.; Doddapaneni, R.; Somagoni, J.; Singh, M. Approaches to improve the oral bioavailability and effects of novel anticancer drugs berberine and betulinic acid. PLoS ONE 2014. [Google Scholar] [CrossRef] [PubMed] [Green Version]







| IC50 | TC50 | SI | |
|---|---|---|---|
| A549 | 17 µM (0.006 µg/mL) | 107 µM (0.036 µg/mL) | 6 |
| MDCK | 52 µM (0.017 µg/mL) | 1035 µM (0.350 µg/mL) | 20 |
| LET1 | 4 µM (0.001 µg/mL) | 521 µM (0.176 µg/mL) | 123 |
| HAE | 16 µM (0.005 µg/mL) | - | - |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Botwina, P.; Owczarek, K.; Rajfur, Z.; Ochman, M.; Urlik, M.; Nowakowska, M.; Szczubiałka, K.; Pyrc, K. Berberine Hampers Influenza A Replication through Inhibition of MAPK/ERK Pathway. Viruses 2020, 12, 344. https://doi.org/10.3390/v12030344
Botwina P, Owczarek K, Rajfur Z, Ochman M, Urlik M, Nowakowska M, Szczubiałka K, Pyrc K. Berberine Hampers Influenza A Replication through Inhibition of MAPK/ERK Pathway. Viruses. 2020; 12(3):344. https://doi.org/10.3390/v12030344
Chicago/Turabian StyleBotwina, Paweł, Katarzyna Owczarek, Zenon Rajfur, Marek Ochman, Maciej Urlik, Maria Nowakowska, Krzysztof Szczubiałka, and Krzysztof Pyrc. 2020. "Berberine Hampers Influenza A Replication through Inhibition of MAPK/ERK Pathway" Viruses 12, no. 3: 344. https://doi.org/10.3390/v12030344
APA StyleBotwina, P., Owczarek, K., Rajfur, Z., Ochman, M., Urlik, M., Nowakowska, M., Szczubiałka, K., & Pyrc, K. (2020). Berberine Hampers Influenza A Replication through Inhibition of MAPK/ERK Pathway. Viruses, 12(3), 344. https://doi.org/10.3390/v12030344