Survival of Human Norovirus Surrogates in Water upon Exposure to Thermal and Non-Thermal Antiviral Treatments
Abstract
1. Introduction
2. Materials and Methods
2.1. Eukaryotic Cell Lines, Bacterial Cells and Media
2.2. Virus Stocks Preparation
2.3. Physical and Chemical Treatments
2.4. Treatment with Chitosan Microparticles
2.5. RT-qPCR
2.6. TCID50 Assay
2.7. Viral Plaque Assays
2.8. Data Analysis
3. Results
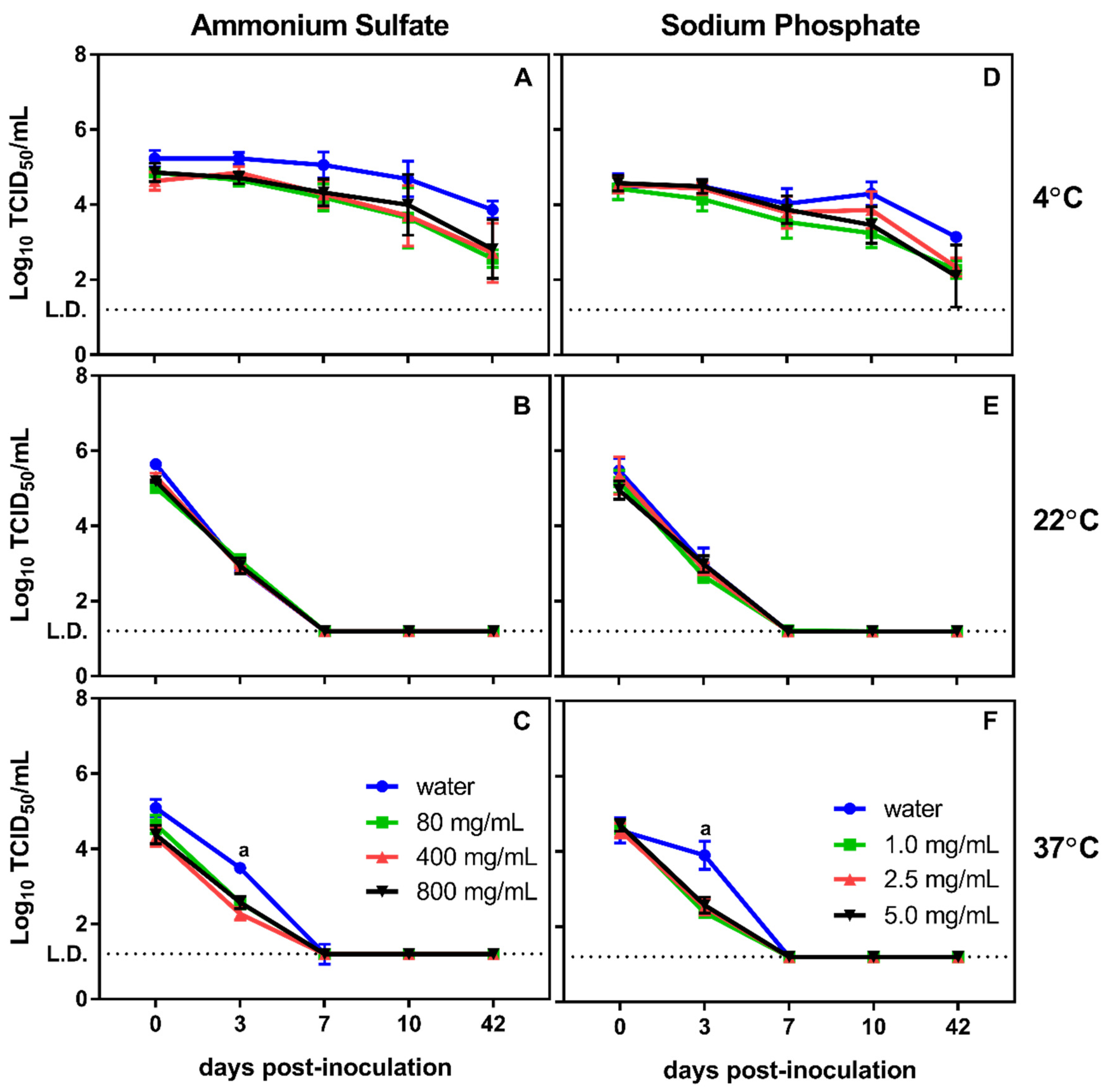
3.1. Effect of Physical and Chemical Parameters on In Vitro Survival of MNV
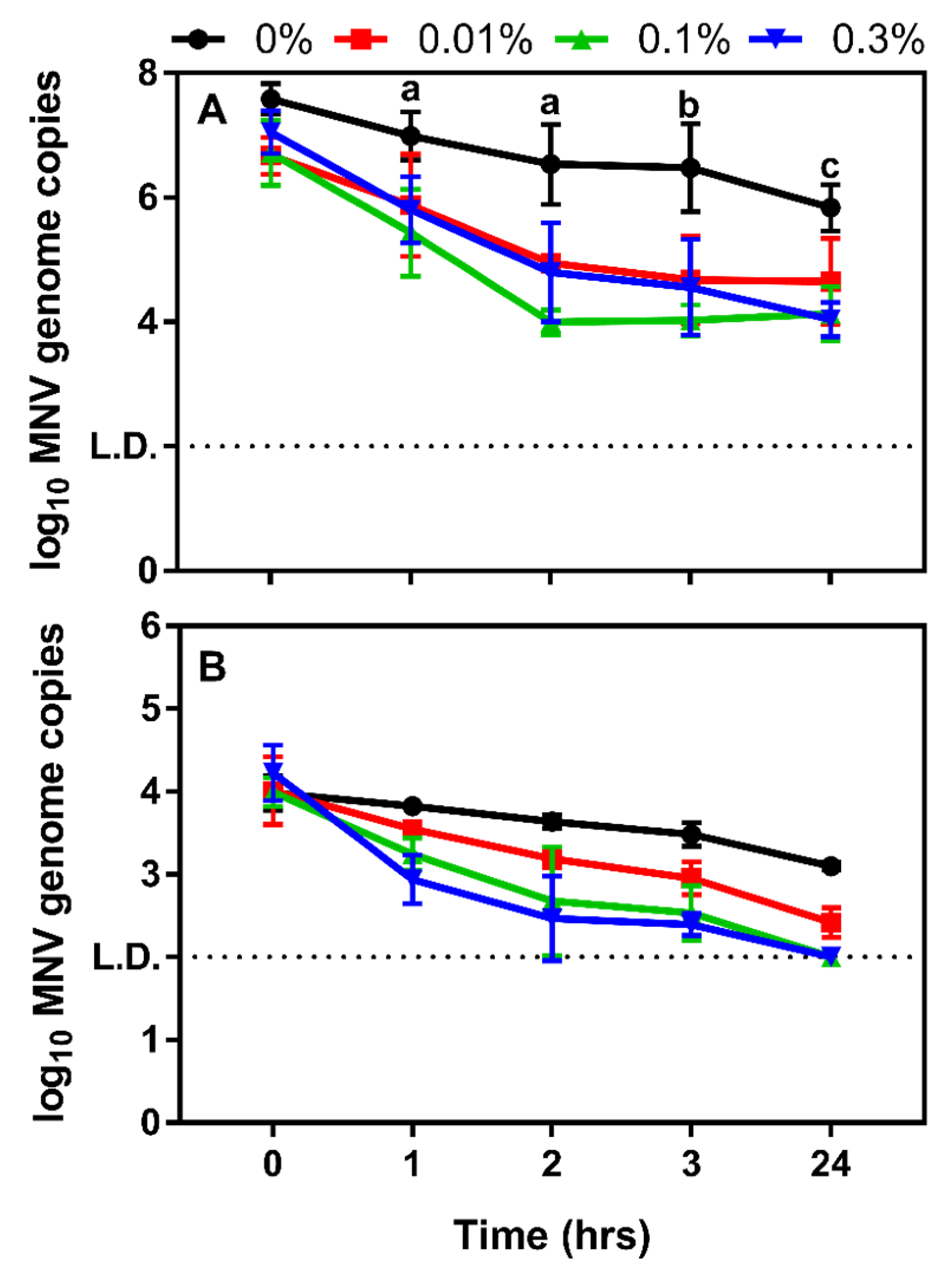
3.2. Determining the Impact Chitosan Microparticles on Human Norovirus Surrogates
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- World Health Organization. WHO Estimates of the Global Burden of Foodborne Diseases. Available online: http://www.who.int/foodsafety/areas_work/foodborne-diseases/ferg/en/ (accessed on 4 February 2018).
- Scallan, E.; Hoekstra, R.M.; Angulo, F.J.; Tauxe, R.V.; Widdowson, M.A.; Roy, S.L.; Jones, J.L.; Griffin, P.M. Foodborne illness acquired in the United States--major pathogens. Emerg. Infect. Dis. 2011, 17, 7–15. [Google Scholar] [PubMed]
- Callejón, R.M.; Rodríguez-Naranjo, M.I.; Ubeda, C.; Hornedo-Ortega, R.; Garcia-Parrilla, M.C.; Troncoso, A.M. Reported foodborne outbreaks due to fresh produce in the United States and European Union: Trends and causes. Foodborne Pathog. Dis. 2015, 12, 32–38. [Google Scholar] [PubMed]
- Kukkula, M.; Maunula, L.; Silvennoinen, E.; von Bonsdorff, C.H. Outbreak of viral gastroenteritis due to drinking water contaminated by Norwalk-like viruses. J. Infect. Dis. 1999, 180, 1771–1776. [Google Scholar] [PubMed]
- Mattison, K.; Harlow, J.; Morton, V.; Cook, A.; Pollari, F.; Bidawid, S.; Farber, J.M. Enteric viruses in ready-to-eat packaged leafy greens. Emerg Infect. Dis 2010, 16, 1815–1817, discussion 1817. [Google Scholar]
- Baert, L.; Mattison, K.; Loisy-Hamon, F.; Harlow, J.; Martyres, A.; Lebeau, B.; Stals, A.; Van Coillie, E.; Herman, L.; Uyttendaele, M. Review: Norovirus prevalence in Belgian, Canadian and French fresh produce: A threat to human health? Int. J. Food Microbiol. 2011, 151, 261–269. [Google Scholar]
- Hall, A.J.; Wikswo, M.E.; Pringle, K.; Gould, L.H.; Parashar, U.D. Vital signs: Foodborne norovirus outbreaks—United States, 2009–2012. Morb. Mortal. Weekly Report 2014, 63, 491–495. [Google Scholar]
- Matthews, J.E.; Dickey, B.W.; Miller, R.D.; Felzer, J.R.; Dawson, B.P.; Lee, A.S.; Rocks, J.J.; Kiel, J.; Montes, J.S.; Moe, C.L.; et al. The epidemiology of published norovirus outbreaks: A review of risk factors associated with attack rate and genogroup. Epidemiol. Infect. 2012, 140, 1161–1172. [Google Scholar]
- Graciaa, D.S.; Cope, J.R.; Roberts, V.A.; Cikesh, B.L.; Kahler, A.M.; Vigar, M.; Hilborn, E.D.; Wade, T.J.; Backer, L.C.; Montgomery, S.P.; et al. Outbreaks Associated with Untreated Recreational Water—United States, 2000–2014. MMWR. Morb. Mortal. Weekly Report 2018, 67, 701–706. [Google Scholar]
- Sips, G.J.; Dirven, M.J.G.; Donkervoort, J.T.; van Kolfschoten, F.M.; Schapendonk, C.M.E.; Phan, M.V.T.; Bloem, A.; van Leeuwen, A.F.; Trompenaars, M.E.; Koopmans, M.P.G.; et al. Norovirus outbreak in a natural playground: A One Health approach. Zoonoses Public Health 2020. [Google Scholar] [CrossRef]
- Steele, M.; Odumeru, J. Irrigation water as source of foodborne pathogens on fruit and vegetables. J. Food Prot. 2004, 67, 2839–2849. [Google Scholar]
- Petterson, S.R.; Ashbolt, N.J.; Sharma, A. Microbial risks from wastewater irrigation of salad crops: A screening-level risk assessment. Water Environ. Res. 2001, 73, 667–672. [Google Scholar] [CrossRef] [PubMed]
- Gelting, R.J.; Baloch, M.A.; Zarate-Bermudez, M.A.; Selman, C. Irrigation water issues potentially related to the 2006 multistate E. coli O157:H7 outbreak associated with spinach. Agric. Water Manag. 2011, 98, 1395–1402. [Google Scholar] [CrossRef]
- Montazeri, N.; Goettert, D.; Achberger, E.C.; Johnson, C.N.; Prinyawiwatkul, W.; Janes, M.E. Pathogenic enteric viruses and microbial indicators during secondary treatment of municipal wastewater. Appl. Environ. Microbiol. 2015, 81, 6436–6445. [Google Scholar] [CrossRef] [PubMed]
- Morris, J.G., Jr.; Potter, M. Foodborne Infections and Intoxications. In Food Science and Technology, 4th ed.; Elsevier Science: San Diego, CA, USA, 2013; p. 1, online resource (576 pages). [Google Scholar]
- D’Souza, D.; Sair, A.; Williams, K.; Papafragkou, E.; Jean, J.; Moore, C.; Jaykus, L. Persistence of caliciviruses on enviromnental surfaces and their transfer to food. Int. J. Food Microbiol. 2006, 108, 84–91. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Jaykus, L.A.; Wong, E.; Moe, C. Persistence of Norwalk virus, male-specific coliphage, and Escherichia coli on stainless steel coupons and in phosphate-buffered saline. J. Food Prot. 2012, 75, 2151–2157. [Google Scholar] [CrossRef]
- Teunis, P.F.; Moe, C.L.; Liu, P.; Miller, S.E.; Lindesmith, L.; Baric, R.S.; Le Pendu, J.; Calderon, R.L. Norwalk virus: How infectious is it? J. Med. Virol. 2008, 80, 1468–1476. [Google Scholar] [CrossRef]
- Seitz, S.R.; Leon, J.S.; Schwab, K.J.; Lyon, G.M.; Dowd, M.; McDaniels, M.; Abdulhafid, G.; Fernandez, M.L.; Lindesmith, L.C.; Baric, R.S.; et al. Norovirus infectivity in humans and persistence in water. Appl. Environ. Microbiol. 2011, 77, 6884–6888. [Google Scholar] [CrossRef]
- Ngazoa, E.S.; Fliss, I.; Jean, J. Quantitative study of persistence of human norovirus genome in water using TaqMan real-time RT-PCR. J. Appl. Microbiol. 2008, 104, 707–715. [Google Scholar] [CrossRef]
- Charles, K.J.; Shore, J.; Sellwood, J.; Laverick, M.; Hart, A.; Pedley, S. Assessment of the stability of human viruses and coliphage in groundwater by PCR and infectivity methods. J. Appl. Microbiol. 2009, 106, 1827–1837. [Google Scholar] [CrossRef]
- Fallahi, S.; Mattison, K. Evaluation of murine norovirus persistence in environments relevant to food production and processing. J. Food Prot. 2011, 74, 1847–1851. [Google Scholar] [CrossRef]
- Yates, M.V. Septic Tank Density and Ground-Water Contamination. Groundwater 1985, 23, 586–591. [Google Scholar] [CrossRef]
- Lymer, D.; Vrede, K. Nutrient additions resulting in phage release and formation of non-nucleoid-containing bacteria. Aquat. Microb. Ecol. 2006, 43, 107–112. [Google Scholar] [CrossRef]
- MADAN, N.J.; MARSHALL, W.A. Virus and microbial loop dynamics over an annual cycle in three contrasting Antarctic lakes. Freashwater Biol. 2005, 50, 1291–1300. [Google Scholar] [CrossRef]
- McCance, W.; Jones, O.A.H.; Edwards, M.; Surapaneni, A.; Chadalavada, S.; Currell, M. Contaminants of Emerging Concern as novel groundwater tracers for delineating wastewater impacts in urban and peri-urban areas. Water Res. 2018, 146, 118–133. [Google Scholar] [CrossRef] [PubMed]
- Badruzzaman, M.; Pinzon, J.; Oppenheimer, J.; Jacangelo, J.G. Sources of nutrients impacting surface waters in Florida: A review. J. Environ. Manag. 2012, 109, 80–92. [Google Scholar] [CrossRef]
- Burri, N.M.; Weatherl, R.; Moeck, C.; Schirmer, M. A review of threats to groundwater quality in the anthropocene. Sci. Total Environ. 2019, 684, 136–154. [Google Scholar] [CrossRef]
- Masiol, M.; Gianni, B.; Prete, M. Herbicides in river water across the northeastern Italy: Occurrence and spatial patterns of glyphosate, aminomethylphosphonic acid, and glufosinate ammonium. Environ. Sci. Pollut. Res. Int. 2018, 25, 24368–24378. [Google Scholar] [CrossRef]
- Kurilic, S.M.; Ulnikovic, V.P.; Maric, N.; Vasiljevic, M. Assessment of typical natural processes and human activities’ impact on the quality of drinking water. Environ. Monit. Assess. 2015, 187, 659. [Google Scholar] [CrossRef]
- Stuart, M.E.; Lapworth, D.J. Macronutrient status of UK groundwater: Nitrogen, phosphorus and organic carbon. Sci. Total Environ. 2016, 572, 1543–1560. [Google Scholar] [CrossRef]
- Heindel, J.J.; Chapin, R.E.; Gulati, D.K.; George, J.D.; Price, C.J.; Marr, M.C.; Myers, C.B.; Barnes, L.H.; Fail, P.A.; Grizzle, T.B.; et al. Assessment of the reproductive and developmental toxicity of pesticide/fertilizer mixtures based on confirmed pesticide contamination in California and Iowa groundwater. Fundam Appl. Toxicol. 1994, 22, 605–621. [Google Scholar] [CrossRef]
- Cook, N.; Knight, A.; Richards, G.P. Persistence and Elimination of Human Norovirus in Food and on Food Contact Surfaces: A Critical Review. J. Food Prot. 2016, 79, 1273–1294. [Google Scholar] [CrossRef] [PubMed]
- Kamarasu, P.; Hsu, H.-Y.; Moore, M.D. Research Progress in Viral Inactivation Utilizing Human Norovirus Surrogates. Front. Sustain. Food Syst. 2018, 2, 89. [Google Scholar] [CrossRef]
- Cromeans, T.; Park, G.W.; Costantini, V.; Lee, D.; Wang, Q.; Farkas, T.; Lee, A.; Vinje, J. Comprehensive comparison of cultivable norovirus surrogates in response to different inactivation and disinfection treatments. Appl. Environ. Microbiol. 2014, 80, 5743–5751. [Google Scholar] [CrossRef] [PubMed]
- Raja, M.A.; Katas, H.; Jing Wen, T. Stability, intracellular delivery, and release of siRNA from chitosan nanoparticles using different cross-linkers. PLoS ONE 2015, 10, e0128963. [Google Scholar]
- Lizardi-Mendoza, J.; Argüelles Monal, W.M.; Goycoolea Valencia, F.M. Chemical Characteristics and Functional Properties of Chitosan. In Chitosan in the Preservation of Agricultural Commodities; Bautista-Baños, S., Romanazzi, G., Jiménez Aparicio, A., Eds.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 3–31. [Google Scholar]
- Muxika, A.; Etxabide, A.; Uranga, J.; Guerrero, P.; de la Caba, K. Chitosan as a bioactive polymer: Processing, properties and applications. Int. J. Biol. Macromol. 2017, 105, 1358–1368. [Google Scholar] [CrossRef]
- Vunain, E.; Mishra, A.K.; Mamba, B.B. Fundamentals of Chitosan for Biomedical Applications. In Chitosan Based Biomaterial, Volume 1—Fundamentals; Jennings, J.A., Bumgardner, J.D., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 3–30. [Google Scholar]
- Davis, R.; Zivanovic, S.; D’Souza, D.H.; Davidson, P.M. Effectiveness of chitosan on the inactivation of enteric viral surrogates. Food Microbiol. 2012, 32, 57–62. [Google Scholar] [CrossRef]
- Davis, R.; Zivanovic, S.; Davidson, P.M.; D’Souza, D.H. Enteric Viral Surrogate Reduction by Chitosan. Food Environ. Virol. 2015, 7, 359–365. [Google Scholar] [CrossRef]
- Jeon, S.J.; Oh, M.; Yeo, W.S.; Galvao, K.N.; Jeong, K.C. Underlying mechanism of antimicrobial activity of chitosan microparticles and implications for the treatment of infectious diseases. PLoS ONE 2014, 9, e92723. [Google Scholar] [CrossRef]
- Fan, Y.; Ginn, A.; Ma, Z.; Kang, M.; Jeong, K.C.; Wright, A.C. Application of chitosan microparticles for mitigation of Salmonella in agricultural water. J. Appl. Microbiol. 2017, 123, 1346–1358. [Google Scholar] [CrossRef]
- de Moraes, M.H.; Chapin, T.K.; Ginn, A.; Wright, A.C.; Parker, K.; Hoffman, C.; Pascual, D.W.; Danyluk, M.D.; Teplitski, M. Development of an Avirulent Salmonella Surrogate for Modeling Pathogen Behavior in Pre- and Postharvest Environments. Appl. Environ. Microbiol. 2016, 82, 4100–4111. [Google Scholar] [CrossRef]
- Zhu, S.; Regev, D.; Watanabe, M.; Hickman, D.; Moussatche, N.; Jesus, D.M.; Kahan, S.M.; Napthine, S.; Brierley, I.; Hunter, R.N., 3rd; et al. Identification of immune and viral correlates of norovirus protective immunity through comparative study of intra-cluster norovirus strains. PLoS Pathog. 2013, 9, e1003592. [Google Scholar] [CrossRef] [PubMed]
- Thackray, L.B.; Wobus, C.E.; Chachu, K.A.; Liu, B.; Alegre, E.R.; Henderson, K.S.; Kelley, S.T.; Virgin, H.W.t. Murine noroviruses comprising a single genogroup exhibit biological diversity despite limited sequence divergence. J. Virol. 2007, 81, 10460–10473. [Google Scholar] [CrossRef] [PubMed]
- Tung-Thompson, G.; Libera, D.A.; Koch, K.L.; de Los Reyes, F.L., 3rd; Jaykus, L.A. Aerosolization of a Human Norovirus Surrogate, Bacteriophage MS2, during Simulated Vomiting. PLoS ONE 2015, 10, e0134277. [Google Scholar] [CrossRef] [PubMed]
- Rolfe, K.J.; Parmar, S.; Mururi, D.; Wreghitt, T.G.; Jalal, H.; Zhang, H.; Curran, M.D. An internally controlled, one-step, real-time RT-PCR assay for norovirus detection and genogrouping. J. Clin. Virol. 2007, 39, 318–321. [Google Scholar] [CrossRef]
- Cannon, J.L.; Papafragkou, E.; Park, G.W.; Osborne, J.; Jaykus, L.A.; Vinje, J. Surrogates for the study of norovirus stability and inactivation in the environment: AA comparison of murine norovirus and feline calicivirus. J. Food Prot. 2006, 69, 2761–2765. [Google Scholar] [CrossRef]
- Bae, J.; Schwab, K.J. Evaluation of murine norovirus, feline calicivirus, poliovirus, and MS2 as surrogates for human norovirus in a model of viral persistence in surface water and groundwater. Appl. Environ. Microbiol. 2008, 74, 477–484. [Google Scholar] [CrossRef]
- Ma, Z.; Kim, D.; Adesogan, A.T.; Ko, S.; Galvao, K.; Jeong, K.C. Chitosan Microparticles Exert Broad-Spectrum Antimicrobial Activity against Antibiotic-Resistant Micro-organisms without Increasing Resistance. ACS Appl. Mater. Interfaces 2016, 8, 10700–10709. [Google Scholar] [CrossRef]
- Jones, M.K.; Watanabe, M.; Zhu, S.; Graves, C.L.; Keyes, L.R.; Grau, K.R.; Gonzalez-Hernandez, M.B.; Iovine, N.M.; Wobus, C.E.; Vinje, J.; et al. Enteric bacteria promote human and mouse norovirus infection of B cells. Science 2014, 346, 755–759. [Google Scholar] [CrossRef]
- Reed, L.J.; Muench, H. A simple method of estimating fifty per cent endpoints12. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Wobus, C.E.; Karst, S.M.; Thackray, L.B.; Chang, K.O.; Sosnovtsev, S.V.; Belliot, G.; Krug, A.; Mackenzie, J.M.; Green, K.Y.; Virgin, H.W. Replication of Norovirus in cell culture reveals a tropism for dendritic cells and macrophages. PLoS Biol. 2004, 2, e432. [Google Scholar] [CrossRef]
- Hirneisen, K.A.; Kniel, K.E. Comparing human norovirus surrogates: Murine norovirus and Tulane virus. J. Food Prot. 2013, 76, 139–143. [Google Scholar] [CrossRef]
- Seo, K.; Lee, J.E.; Lim, M.Y.; Ko, G. Effect of temperature, pH, and NaCl on the inactivation kinetics of murine norovirus. J. Food Prot. 2012, 75, 533–540. [Google Scholar] [CrossRef] [PubMed]
- Su, X.; Zivanovic, S.; D’Souza, D.H. Effect of chitosan on the infectivity of murine norovirus, feline calicivirus, and bacteriophage MS2. J. Food Prot. 2009, 72, 2623–2628. [Google Scholar] [CrossRef] [PubMed]
- Mathijs, E.; Stals, A.; Baert, L.; Botteldoorn, N.; Denayer, S.; Mauroy, A.; Scipioni, A.; Daube, G.; Dierick, K.; Herman, L.; et al. A review of known and hypothetical transmission routes for noroviruses. Food Environ. Virol. 2012, 4, 131–152. [Google Scholar] [CrossRef]
- Brie, A.; Razafimahefa, R.; Loutreul, J.; Robert, A.; Gantzer, C.; Boudaud, N.; Bertrand, I. The Effect of Heat and Free Chlorine Treatments on the Surface Properties of Murine Norovirus. Food Environ. Virol. 2017, 9, 149–158. [Google Scholar] [CrossRef]
- Isquith, A.J.; Abbott, E.A.; Walters, P.A. Surface-bonded antimicrobial activity of an organosilicon quaternary ammonium chloride. Appl. Microbiol. 1972, 24, 859–863. [Google Scholar] [CrossRef] [PubMed]
- Thomson, J.E.; Bailey, J.S.; Cox, N.A. Phosphate and heat treatments to control Salmonella and reduce spoilage and rancidity on broiler carcasses. Poult. Sci. 1979, 58, 139–143. [Google Scholar] [CrossRef]
- Yang, E.J.; Kim, J.G.; Kim, J.Y.; Kim, S.C.; Lee, N.H.; Hyun, C.G. Anti-inflammatory effect of chitosan oligosaccharides in RAW 264.7 cells. Cent. Eur. J. Biol. 2010, 5, 95–102. [Google Scholar] [CrossRef]
- Kochkina, Z.M.; Surgucheva, N.A.; Chirkov, S.N. Inactivation of coliphages by chitosan derivatives. Microbiology 2000, 69, 212–216. [Google Scholar] [CrossRef]






| Category | Chemical Used | Concentration Range Tested |
|---|---|---|
| Temperature | N/A | 4–65 °C |
| UV radiation | N/A | 10,000–250,000 µJ/cm2 |
| Phosphate | NaH2PO4 | 0–5 mg/L |
| Ammonium | NH4Cl | 50–800 mg/L |
| Polysaccharide | Chitosan microparticles | 0–0.3% (w/v) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, S.; Barnes, C.; Bhar, S.; Hoyeck, P.; Galbraith, A.N.; Devabhaktuni, D.; Karst, S.M.; Montazeri, N.; Jones, M.K. Survival of Human Norovirus Surrogates in Water upon Exposure to Thermal and Non-Thermal Antiviral Treatments. Viruses 2020, 12, 461. https://doi.org/10.3390/v12040461
Zhu S, Barnes C, Bhar S, Hoyeck P, Galbraith AN, Devabhaktuni D, Karst SM, Montazeri N, Jones MK. Survival of Human Norovirus Surrogates in Water upon Exposure to Thermal and Non-Thermal Antiviral Treatments. Viruses. 2020; 12(4):461. https://doi.org/10.3390/v12040461
Chicago/Turabian StyleZhu, Shu, Candace Barnes, Sutonuka Bhar, Papa Hoyeck, Annalise N. Galbraith, Divya Devabhaktuni, Stephanie M. Karst, Naim Montazeri, and Melissa K. Jones. 2020. "Survival of Human Norovirus Surrogates in Water upon Exposure to Thermal and Non-Thermal Antiviral Treatments" Viruses 12, no. 4: 461. https://doi.org/10.3390/v12040461
APA StyleZhu, S., Barnes, C., Bhar, S., Hoyeck, P., Galbraith, A. N., Devabhaktuni, D., Karst, S. M., Montazeri, N., & Jones, M. K. (2020). Survival of Human Norovirus Surrogates in Water upon Exposure to Thermal and Non-Thermal Antiviral Treatments. Viruses, 12(4), 461. https://doi.org/10.3390/v12040461





