Fatal Dengue Cases Reveal Brain Injury and Viral Replication in Brain-Resident Cells Associated with the Local Production of Pro-Inflammatory Mediators
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Procedures
2.2. Human Fatal Cases
2.3. Histopathological Analysis
2.4. Immunohistochemistry
2.5. In-Situ Hybridization
2.6. Detection of DENV RNA and HMGB1
2.7. Data Availability
3. Results
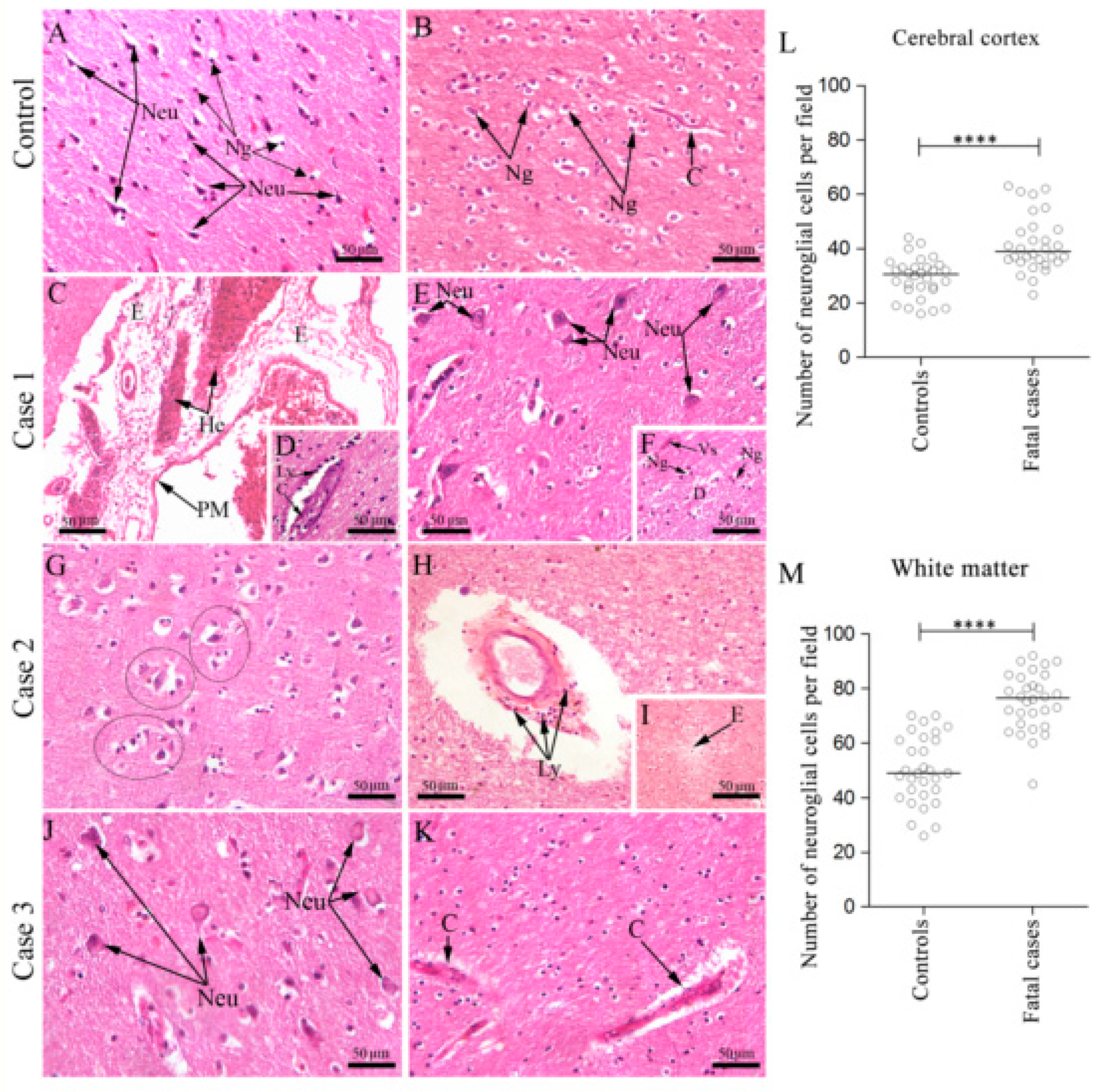
3.1. Fatal Dengue Cases Present Injury in the Cerebral Cortex and White Matter
3.1.1. Morphological Aspects
3.1.2. Quantitative Aspects
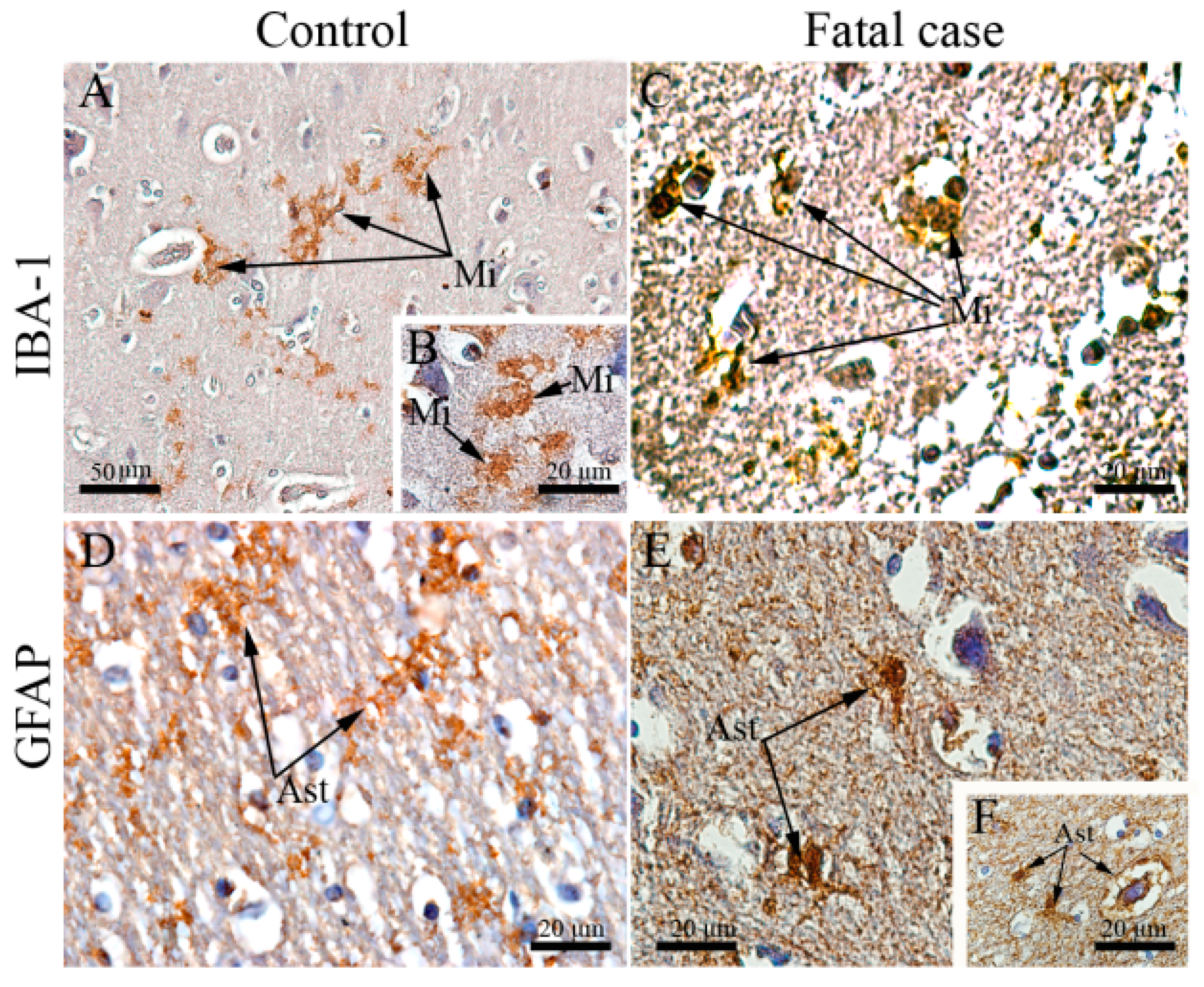
3.2. Fatal Dengue Cases Exhibit Altered Morphology of Microglial Cells and Astrocytes
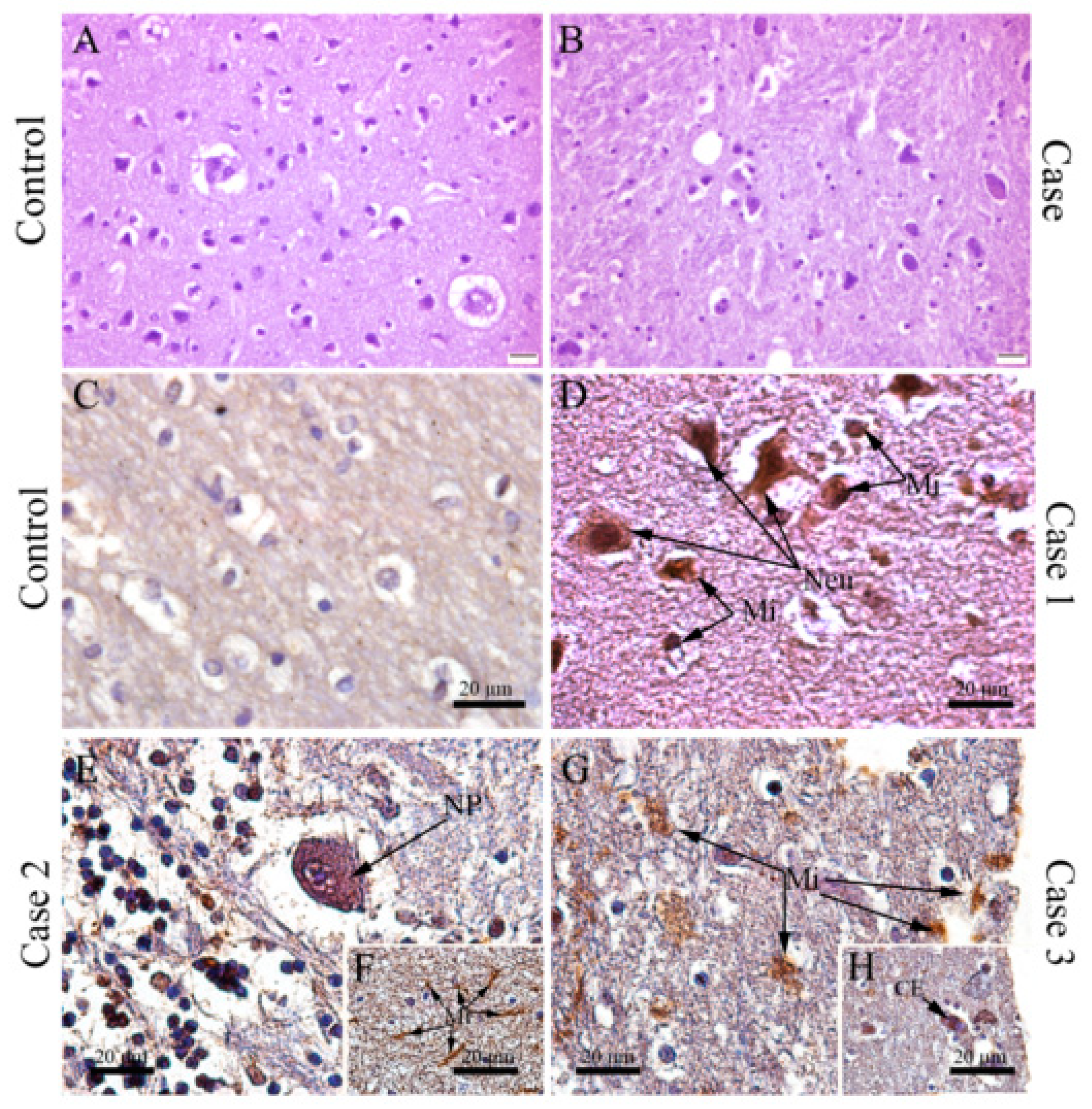
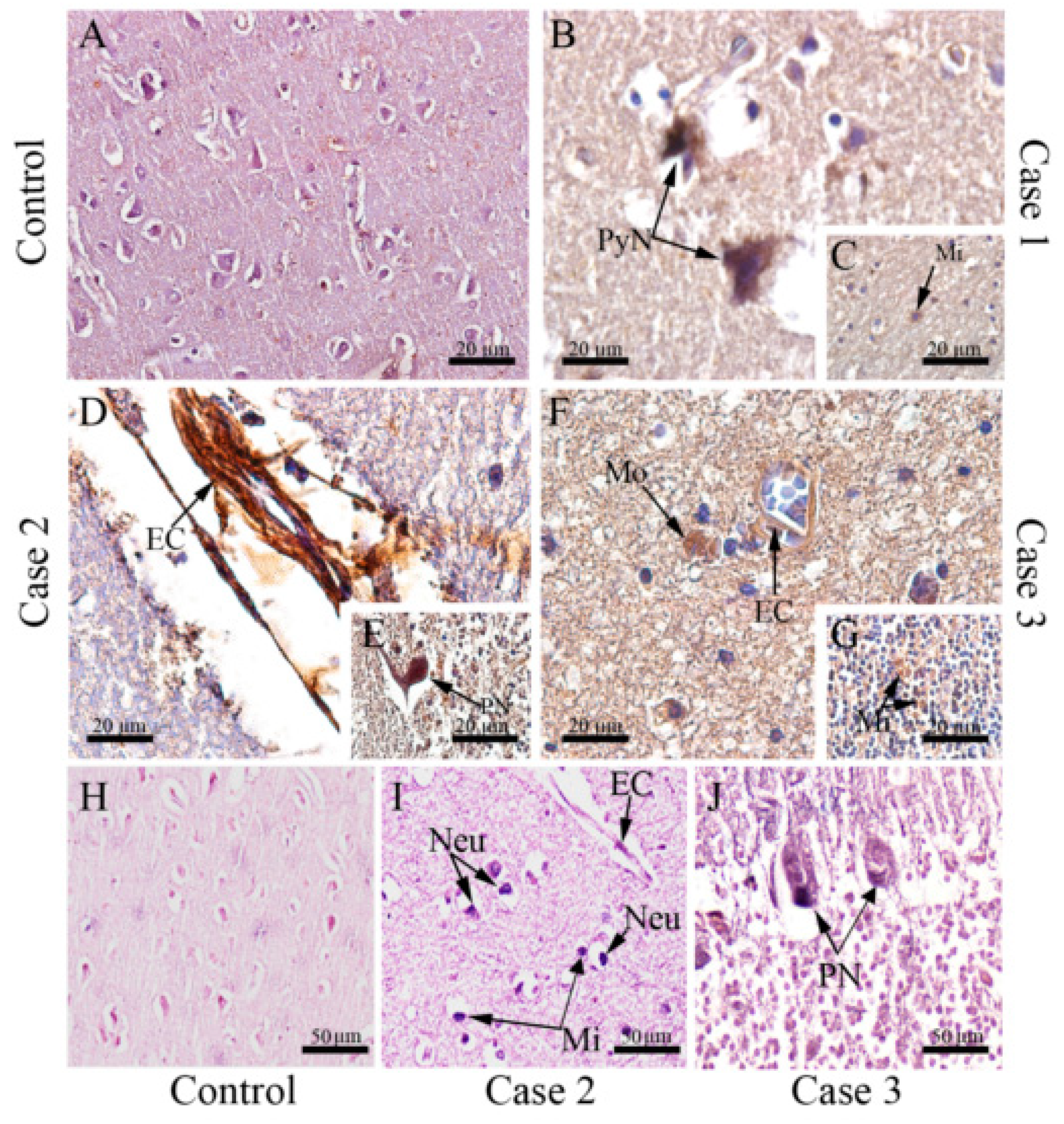
3.3. DENV Targets and Replicates in Brain-Resident Cells
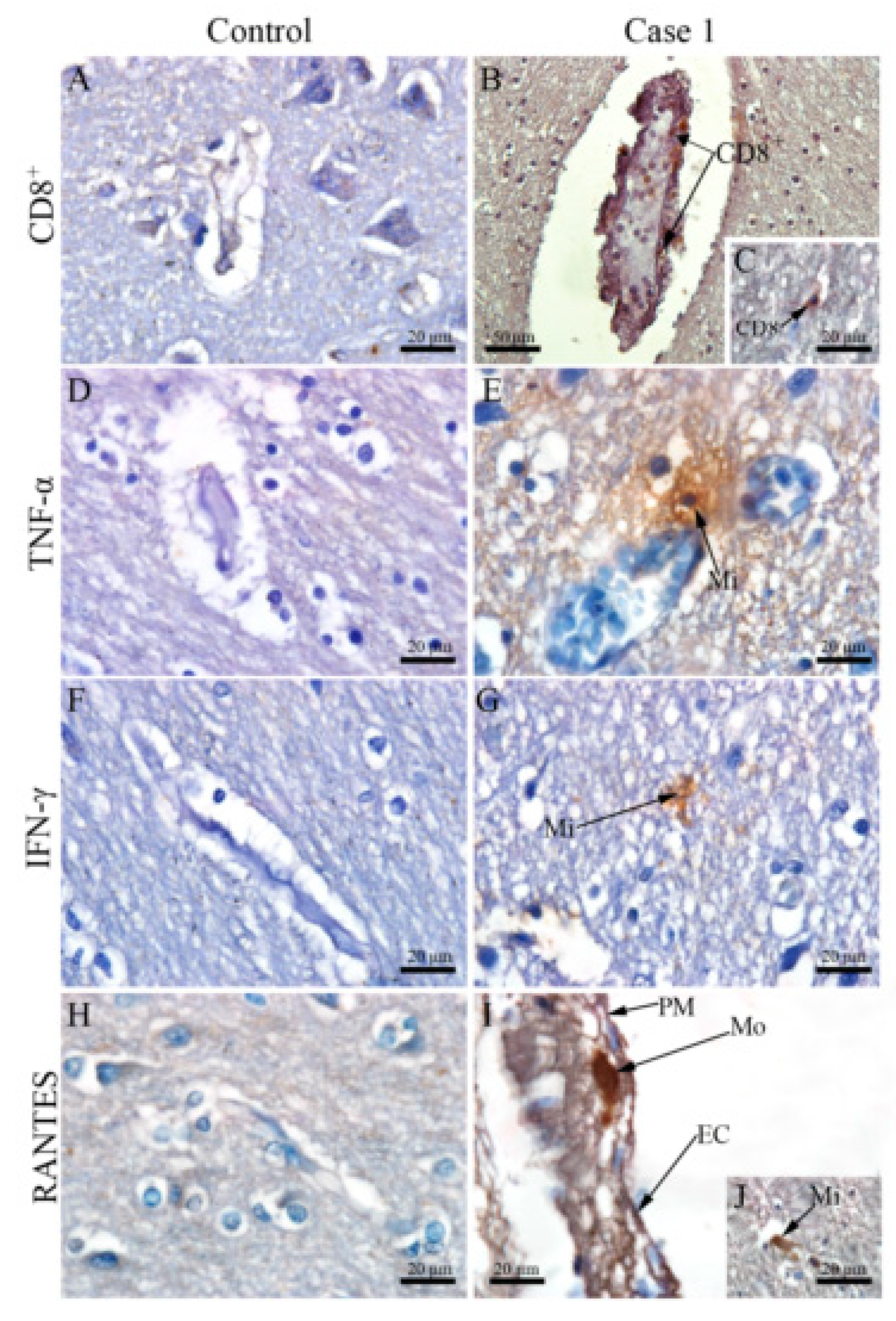
3.4. DENV Infection in the CNS Is Associated with the Local Expression of Several Pro-Inflammatory Mediators
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Myers, M.F.; et al. The global distribution and burden of dengue. 2010, 496, 504–507. [Google Scholar] [CrossRef] [PubMed]
- Halstead, S.B. Dengue. Lancet 2007, 370, 1644–1652. [Google Scholar]
- Carabali, M.; Hernandez, L.M.; Arauz, M.J.; Villar, L.A.; Ridde, V. Why are people with dengue dying? A scoping review of determinants for dengue mortality. BMC Infect. Dis. 2015, 15, 1–14. [Google Scholar] [CrossRef]
- Huy, N.T.; Van Giang, T.; Thuy, D.H.D.; Kikuchi, M.; Hien, T.T.; Zamora, J.; Hirayama, K. Factors Associated with Dengue Shock Syndrome: A Systematic Review and Meta-Analysis. PLoS Negl. Trop. Dis. 2013, 7. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, D.G. The relationship of interacting immunological components in dengue pathogenesis. Virol. J. 2009, 6, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Paes, M.V.; Lenzi, H.L.; Nogueira, A.C.M.; Nuovo, G.J.; Pinhão, Â.T.; Mota, E.M.; Basílio-De-Oliveira, C.A.; Schatzmayr, H.; Barth, O.M.; De Barcelos Alves, A.M. Hepatic damage associated with dengue-2 virus replication in liver cells of BALB/c mice. Lab. Investig. 2009, 89, 1140–1151. [Google Scholar] [CrossRef] [PubMed]
- Pancharoen, C.; Thisyakorn, U. Neurological manifestations in dengue patients. Southeast Asian J. Trop. Med. Public Health 2001, 32, 341–345. [Google Scholar]
- Pang, T.; Cardosa, M.J.; Guzman, M.G. Of cascades and perfect storms: The immunopathogenesis of dengue haemorrhagic fever-dengue shock syndrome (DHF/DSS). Immunol. Cell Biol. 2007, 85, 43–45. [Google Scholar] [CrossRef]
- Patey, O.; Ollivaud, L.; Breuil, J.; Lafaix, C. Unusual neurologic manifestations occurring during dengue fever infection. Am. J. Trop. Med. Hyg. 1993, 48, 793–802. [Google Scholar] [CrossRef]
- Puccioni-Sohler, M.; Rosadas, C.; Cabral-Castro, M.J. Neurological complications in dengue infection: A review for clinical practice. Arq. Neuropsiquiatr. 2013, 71, 667–671. [Google Scholar] [CrossRef]
- Puccioni-Sohler, M.; Soares, C.N.; Papaiz-Alvarenga, R.; Castro, M.J.C.; Faria, L.C.; Peralta, J.M. Neurologic dengue manifestations associated with intrathecal specific immune response. Neurology 2009, 73, 1413–1417. [Google Scholar] [CrossRef] [PubMed]
- Miagostovich, M.P.; Ramos, R.G.; Nicol, A.F.; Nogueira, R.M.; Cuzzi-Maya, T.; Oliveira, A.V.; Marchevsky, R.S.; Mesquita, R.P.; Schatzmayr, H.G. Retrospective study on dengue fatal cases. Clin Neuropathol. 1997, 16, 204–208. [Google Scholar]
- Angibaud, G.; Luaute, J.; Laille, M.; Gaultier, C. Brain involvement in dengue fever. J. Clin. Neurosci. 2001, 8, 63–65. [Google Scholar] [CrossRef] [PubMed]
- Araújo, F.M.C.; Brilhante, R.S.N.; Cavalcanti, L.P.G.; Rocha, M.F.G.; Cordeiro, R.A.; Perdigão, A.C.B.; Miralles, I.S.; Araújo, L.C.; Araújo, R.M.C.; Lima, E.G.; et al. Detection of the dengue non-structural 1 antigen in cerebral spinal fluid samples using a commercially available enzyme-linked immunosorbent assay. J. Virol. Methods 2011. [Google Scholar] [CrossRef] [PubMed]
- Lima, D.M.; de Paula, S.O.; França, R.F.; de Oliveira França, R.F.; Palma, P.V.B.; Morais, F.R.; Gomes-Ruiz, A.C.; de Aquino, M.T.P.; da Fonseca, B.A.L. A DNA vaccine candidate encoding the structural prM/E proteins elicits a strong immune response and protects mice against dengue-4 virus infection. Vaccine 2011, 29, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Mamdouh, K.H.; Mroog, K.M.; Hani, N.H.; Nabil, E.M. Atypical dengue meningitis in Makkah, Saudi Arabia with slow resolving, prominent migraine like headache, phobia, and arrhythmia. J. Glob. Infect. Dis. 2013. [Google Scholar] [CrossRef]
- Saini, L.; Chakrabarty, B.; Pastel, H.; Israni, A.; Kumar, A.; Gulati, S. Dengue fever triggering hemiconvulsion hemiplegia epilepsy in a child. Neurol. India 2017, 65, 636. [Google Scholar]
- Solomon, T.; Dung, N.M.; Vaughn, D.W.; Kneen, R.; Thao, L.T.T.; Raengsakulrach, B.; Loan, H.T.; Day, N.P.J.; Farrar, J.; Myint, K.S.A.; et al. Neurological manifestations of dengue infection. Lancet 2000. [Google Scholar] [CrossRef]
- Domingues, R.B.; Kuster, G.W.; Onuki-Castro, F.L.; Souza, V.A.; Levi, J.E.; Pannuti, C.S. Involvement of the central nervous system in patients with dengue virus infection. J. Neurol. Sci. 2008, 267, 36–40. [Google Scholar] [CrossRef]
- Cam, B.V.; Fonsmark, L.; Hue, N.B.; Phuong, N.T.; Poulsen, A.; Heegaard, E.D. Prospective case-control study of encephalopathy in children with dengue hemorrhagic fever. Am. J. Trop. Med. Hyg. 2001. [Google Scholar] [CrossRef]
- Thisyakorn, U.; Thisyakorn, C. Dengue infection with unusual manifestations. J. Med. Assoc. Thai. 1994, 77, 410–413. [Google Scholar]
- Thisyakorn, U.; Thisyakorn, C.; Limpitikul, W.; Nisalak, A. Dengue infection with central nervous system manifestations. Southeast Asian J. Trop. Med. Public Health 1999. [Google Scholar]
- Diagnosis, G.F.O.R. New edition 2009. Prev. Control 2009. [Google Scholar]
- Póvoa, T.F.; Alves, A.M.; Oliveira, C.A.; Nuovo, G.J.; Chagas, V.L.; Paes, M.V. The Pathology of Severe Dengue in Multiple Organs of Human Fatal Cases: Histopathology, Ultrastructure and Virus Replication. PLoS ONE 2014, 9, e83386. [Google Scholar]
- Póvoa, T.F.; Oliveira, E.R.A.; Basílio-de-Oliveira, C.A.; Nuovo, G.J.; Chagas, V.L.A.; Salomão, N.G.; Mota, E.M.; Paes, M.V. Peripheral organs of dengue fatal cases present strong pro-inflammatory response with participation of IFN-Gamma-, TNF-Alphaand RANTES-Producing cells. PLoS ONE 2016. [Google Scholar] [CrossRef]
- Correction: Peripheral organs of dengue fatal cases present strong pro-inflammatory response with participation of IFN-Gamma-, TNF-alpha- and RANTES-producing cells (PLoS ONE (2016) 11:12 (e0168973) DOI: 10.1371/journal.pone.0168973). PLoS ONE 2018. [CrossRef]
- Kuno, G.; Gomez, I.; Gubler, D.J. Detecting artificial anti-dengue IgM immune complexes using an enzyme-linked immunosorbent assay. Am. J. Trop. Med. Hyg. 1987, 36, 153–159. [Google Scholar] [CrossRef]
- Lanciotti, R.S.; Calisher, C.H.; Gubler, D.J.; Chang, G.J.; Vorndam, A.V. Rapid detection and typing of dengue viruses from clinical samples by using reverse transcriptase-polymerase chain reaction. J. Clin. Microbiol. 1992. [Google Scholar] [CrossRef]
- Costa, S.M.; Yorio, A.P.; Gonçalves, A.J.S.; Vidale, M.M.; Costa, E.C.B.; Mohana-Borges, R.; Motta, M.A.; Freire, M.S.; Alves, A.M.B. Induction of a protective response in mice by the dengue virus NS3 protein using DNA vaccines. PLoS ONE 2011. [Google Scholar] [CrossRef]
- Oliveira, E.R.A.; Póvoa, T.F.; Nuovo, G.J.; Allonso, D.; Salomaõ, N.G.; Basílio-De-Oliveira, C.A.; Geraldo, L.H.M.; Fonseca, C.G.; Lima, F.R.S.; Mohana-Borges, R.; et al. Dengue fatal cases present virus-specific HMGB1 response in peripheral organs. Sci. Rep. 2017, 7, 1–14. [Google Scholar] [CrossRef]
- Bear, M.F. A synaptic basis for memory storage in the cerebral cortex. Proc. Natl. Acad. Sci. USA 1996, 93, 13453–13459. [Google Scholar] [CrossRef]
- Kong, L.; Michalka, S.W.; Rosen, M.L.; Sheremata, S.L.; Swisher, J.D.; Shinn-Cunningham, B.G.; Somers, D.C. Auditory spatial attention representations in the human cerebral cortex. Cereb. Cortex 2014. [Google Scholar] [CrossRef]
- Butler, S.R. Organization of cerebral cortex for perception. Br. Med. J. 1971, 4. [Google Scholar] [CrossRef]
- Boly, M.; Massimini, M.; Tsuchiya, N.; Postle, B.R.; Koch, C.; Tononi, G. Are the neural correlates of consciousness in the front or in the back of the cerebral cortex? Clinical and neuroimaging evidence. J. Neurosci. 2017. [Google Scholar] [CrossRef] [PubMed]
- Fields, R.D. White matter in learning, cognition and psychiatric disorders. Trends Neurosci. 2008. [Google Scholar] [CrossRef] [PubMed]
- Quallo, M.M.; Price, C.J.; Ueno, K.; Asamizuya, T.; Cheng, K.; Lemon, R.N.; Iriki, A. Gray and white matter changes associated with tool-use learning in macaque monkeys. Proc. Natl. Acad. Sci. USA 2009. [Google Scholar] [CrossRef] [PubMed]
- Nedergaard, M.; Ransom, B.; Goldman, S.A. New roles for astrocytes: Redefining the functional architecture of the brain. Trends Neurosci. 2003. [Google Scholar] [CrossRef] [PubMed]
- Jessen, K.R.; Mirsky, R. The origin and development of glial cells in peripheral nerves. Nat. Rev. Neurosci. 2005, 6, 671–682. [Google Scholar] [CrossRef]
- Vilhardt, F.; Haslund-Vinding, J.; Jaquet, V.; McBean, G. Microglia antioxidant systems and redox signalling. Br. J. Pharmacol. 2017, 174, 1719–1732. [Google Scholar] [CrossRef]
- Ghouili, I.; Bahdoudi, S.; Morin, F.; Amri, F.; Hamdi, Y.; Coly, P.M.; Walet-Balieu, M.L.; Leprince, J.; Zekri, S.; Vaudry, H.; et al. Endogenous Expression of ODN-Related Peptides in Astrocytes Contributes to Cell Protection Against Oxidative Stress: Astrocyte-Neuron Crosstalk Relevance for Neuronal Survival. Mol. Neurobiol. 2018. [Google Scholar] [CrossRef]
- Chen, J.; Ng, M.M.L.; Chu, J.J.H. Molecular profiling of T-helper immune genes during dengue virus infection. Virol. J. 2008. [Google Scholar] [CrossRef]
- Dalrymple, N.A.; Mackow, E.R. Endothelial Cells Elicit Immune-Enhancing Responses to Dengue Virus Infection. J. Virol. 2012. [Google Scholar] [CrossRef] [PubMed]
- De Souza, K.P.R.; Silva, E.G.; De Oliveira Rocha, E.S.; Figueiredo, L.B.; De Almeida-Leite, C.M.; Arantes, R.M.E.; De Assis Silva Gomes, J.; Ferreira, G.P.; De Oliveira, J.G.; Kroon, E.G.; et al. Nitric oxide synthase expression correlates with death in an experimental mouse model of dengue with CNS involvement. Virol. J. 2013. [Google Scholar] [CrossRef]
- Ribeiro Nogueira, R.M.; Gonçalves Schatzmayr, H.; Bispo De Filippis, A.M.; Barreto Dos Santos, F.; Venâncio Da Cunha, R.; Coelho, J.O.; De Souza, L.J.; Ramos Guimarães, F.; Machado De Araújo, E.S.; Santos De Simone, T.; et al. Dengue virus type 3, Brazil, 2002. Emerg. Infect. Dis. 2005, 11, 1376–1381. [Google Scholar] [CrossRef] [PubMed]
- Bhamarapravati, N.; Tuchinda, P.; Boonyapaknavik, V. Pathology of thailand haemorrhagic fever: A study of 100 autopsy cases. Ann. Trop. Med. Parasitol. 1967. [Google Scholar] [CrossRef]
- Sam, S.S.; Omar, S.F.S.; Teoh, B.T.; Abd-Jamil, J.; AbuBakar, S. Review of Dengue Hemorrhagic Fever Fatal Cases Seen among Adults: A Retrospective Study. PLoS Negl. Trop. Dis. 2013. [Google Scholar] [CrossRef] [PubMed]
- Rathi, K.R.; Arora, M.M.; Sahai, K.; Tripathi, S.; Singh, S.P.; Raman, D.K.; Anand, K.B. Autopsy findings in fatal dengue haemorrhagic fever - 06 cases. Med. J. Armed Forces India 2013. [Google Scholar] [CrossRef]
- Chimelli, L.; Hahn, M.D.; Netto, M.B.; Ramos, R.G.; Dias, M.; Gray, F. Dengue: Neuropathological findings in 5 fatal cases from Brazil. Clin. Neuropathol. 1990. [Google Scholar]
- Tjalkens, R.B.; Popichak, K.A.; Kirkley, K.A. Inflammatory Activation of Microglia and Astrocytes in Manganese Neurotoxicity. Adv. Neurobiol. 2017, 18, 159–181. [Google Scholar]
- Pekny, M.; Nilsson, M. Astrocyte activation and reactive gliosis. Glia 2005, 50, 427–434. [Google Scholar] [CrossRef]
- Chu, J.J.H.; Ng, M.L. The mechanism of cell death during West Nile virus infection is dependent on initial infectious dose. J. Gen. Virol. 2003. [Google Scholar] [CrossRef]
- Sofroniew, M.V.; Vinters, H.V. Astrocytes: Biology and pathology. Acta Neuropathol. 2010, 119, 7–35. [Google Scholar] [CrossRef] [PubMed]
- Salomão, N.G.; Rabelo, K.; Póvoa, T.F.; Alves, A.M.B.; Da Costa, S.M.; Gonçalves, A.J.S.; Amorim, J.F.; Azevedo, A.S.; Nunes, P.C.G.; Basílio-De-Oliveira, C.A.; et al. BALB/c mice infected with DENV-2 strain 66985 by the intravenous route display injury in the central nervous system. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef]
- Ramírez, R.; Falcón, R.; Izquierdo, A.; García, A.; Alvarez, M.; Pérez, A.B.; Soto, Y.; Muné, M.; Da Silva, E.M.; Ortega, O.; et al. Recombinant dengue 2 virus NS3 protein conserves structural antigenic and immunological properties relevant for dengue vaccine design. Virus Genes 2014. [Google Scholar] [CrossRef] [PubMed]
- Lim, M.Q.; Kumaran, E.A.P.; Tan, H.C.; Lye, D.C.; Leo, Y.S.; Ooi, E.E.; MacAry, P.A.; Bertoletti, A.; Rivino, L. Cross-reactivity and anti-viral function of dengue capsid and NS3- specific memory t cells toward Zika Virus. Front. Immunol. 2018, 355, 1053–1059. [Google Scholar] [CrossRef] [PubMed]
- Kouretova, J.; Hammamy, M.Z.; Epp, A.; Hardes, K.; Kallis, S.; Zhang, L.; Hilgenfeld, R.; Bartenschlager, R.; Steinmetzer, T. Effects of NS2B-NS3 protease and furin inhibition on West Nile and Dengue virus replication. J. Enzyme Inhib. Med. Chem. 2017. [Google Scholar] [CrossRef] [PubMed]
- Ramos, C.; Sánchez, G.; Hernández Pando, R.; Baquera, J.; Hernández, D.; Mota, J.; Ramos, J.; Flores, A.; Llausás, E. Dengue virus in the brain of a fatal case of hemorrhagic dengue fever. J. Neurovirol. 1998. [Google Scholar] [CrossRef]
- Bhatt, R.S.; Kothari, S.T.; Gohil, D.J.; D’Souza, M.; Chowdhary, A.S. Novel evidence of microglial immune response in impairment of Dengue infection of CNS. Immunobiology 2015, 220, 1170–1176. [Google Scholar] [CrossRef]
- Rothman, A.L. Immunity to dengue virus: A tale of original antigenic sin and tropical cytokine storms. Nat. Rev. Immunol. 2011, 11, 532–543. [Google Scholar] [CrossRef]
- Hou, J.; Baker, L.A.; Zhou, L.; Klein, R.S. Viral interactions with the blood-brain barrier: Old dog, new tricks. Tissue Barriers 2016. [Google Scholar] [CrossRef]
- Trung, D.T.; Wills, B. Systemic Vascular Leakage Associated with Dengue Infections—The Clinical Perspective. In Dengue Virus; Springer: Berlin, Heidelberg, 2010; pp. 57–66. [Google Scholar]
- Miner, J.J.; Diamond, M.S. Mechanisms of restriction of viral neuroinvasion at the blood-brain barrier. Curr. Opin. Immunol. 2016. [Google Scholar] [CrossRef]
- Tohidpour, A.; Morgun, A.V.; Boitsova, E.B.; Malinovskaya, N.A.; Martynova, G.P.; Khilazheva, E.D.; Kopylevich, N.V.; Gertsog, G.E.; Salmina, A.B. Neuroinflammation and infection: Molecular mechanisms associated with dysfunction of neurovascular unit. Front. Cell. Infect. Microbiol. 2017, 7. [Google Scholar] [CrossRef]
- Mustafá, Y.M.; Meuren, L.M.; Coelho, S.V.A.; De Arruda, L.B. Pathways exploited by flaviviruses to counteract the blood-brain barrier and invade the central nervous system. Front. Microbiol. 2019. [Google Scholar] [CrossRef]
- Brown, G.C. Nitric oxide and neuronal death. Nitric Oxide-Biol. Chem. 2010. [Google Scholar] [CrossRef] [PubMed]
- Ballabh, P.; Braun, A.; Nedergaard, M. The blood–brain barrier: An overview. Neurobiol. Dis. 2004, 16, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Asiimwe, N.; Yeo, S.G.; Kim, M.-S.; Jung, J.; Jeong, N.Y. Nitric Oxide: Exploring the Contextual Link with Alzheimer’s Disease. Oxid. Med. Cell. Longev. 2016, 2016, 1–10. [Google Scholar] [CrossRef]
- Džoljić, E.; Grabatinić, I.; Kostić, V. Why is nitric oxide important for our brain? Funct. Neurol. 2015, 30, 159–163. [Google Scholar] [CrossRef]
- Benarroch, E.E. Nitric oxide: A pleiotropic signal in the nervous system. Neurology 2011, 77, 1568–1576. [Google Scholar] [CrossRef] [PubMed]
- Park, J.S.; Svetkauskaite, D.; He, Q.; Kim, J.Y.; Strassheim, D.; Ishizaka, A.; Abraham, E. Involvement of Toll-like Receptors 2 and 4 in Cellular Activation by High Mobility Group Box 1 Protein. J. Biol. Chem. 2004, 279, 7370–7377. [Google Scholar] [CrossRef] [PubMed]
- Harris, H.E.; Andersson, U.; Pisetsky, D.S. HMGB1: A multifunctional alarmin driving autoimmune and inflammatory disease. Nat. Rev. Rheumatol. 2012. [Google Scholar] [CrossRef] [PubMed]
- Allonso, D.; Belgrano, F.S.; Calzada, N.; Guzmán, M.G.; Vázquez, S.; Mohana-Borges, R. Elevated serum levels of high mobility group box 1 (HMGB1) protein in dengue-infected patients are associated with disease symptoms and secondary infection. J. Clin. Virol. 2012, 55, 214–219. [Google Scholar] [CrossRef]
- Fang, P.; Schachner, M.; Shen, Y.Q. HMGB1 in development and diseases of the central nervous system. Mol. Neurobiol. 2012, 45, 499–506. [Google Scholar] [CrossRef] [PubMed]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Salomão, N.; Rabelo, K.; Basílio-de-Oliveira, C.; Basílio-de-Oliveira, R.; Geraldo, L.; Lima, F.; dos Santos, F.; Nuovo, G.; Oliveira, E.R.A.; Paes, M. Fatal Dengue Cases Reveal Brain Injury and Viral Replication in Brain-Resident Cells Associated with the Local Production of Pro-Inflammatory Mediators. Viruses 2020, 12, 603. https://doi.org/10.3390/v12060603
Salomão N, Rabelo K, Basílio-de-Oliveira C, Basílio-de-Oliveira R, Geraldo L, Lima F, dos Santos F, Nuovo G, Oliveira ERA, Paes M. Fatal Dengue Cases Reveal Brain Injury and Viral Replication in Brain-Resident Cells Associated with the Local Production of Pro-Inflammatory Mediators. Viruses. 2020; 12(6):603. https://doi.org/10.3390/v12060603
Chicago/Turabian StyleSalomão, Natália, Kíssila Rabelo, Carlos Basílio-de-Oliveira, Rodrigo Basílio-de-Oliveira, Luiz Geraldo, Flávia Lima, Flávia dos Santos, Gerard Nuovo, Edson R. A. Oliveira, and Marciano Paes. 2020. "Fatal Dengue Cases Reveal Brain Injury and Viral Replication in Brain-Resident Cells Associated with the Local Production of Pro-Inflammatory Mediators" Viruses 12, no. 6: 603. https://doi.org/10.3390/v12060603
APA StyleSalomão, N., Rabelo, K., Basílio-de-Oliveira, C., Basílio-de-Oliveira, R., Geraldo, L., Lima, F., dos Santos, F., Nuovo, G., Oliveira, E. R. A., & Paes, M. (2020). Fatal Dengue Cases Reveal Brain Injury and Viral Replication in Brain-Resident Cells Associated with the Local Production of Pro-Inflammatory Mediators. Viruses, 12(6), 603. https://doi.org/10.3390/v12060603






