Rotaviruses Associate with Distinct Types of Extracellular Vesicles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and Viral Propagation
2.2. Viral Infectivity Assays
2.3. Kinetics of Viral Release
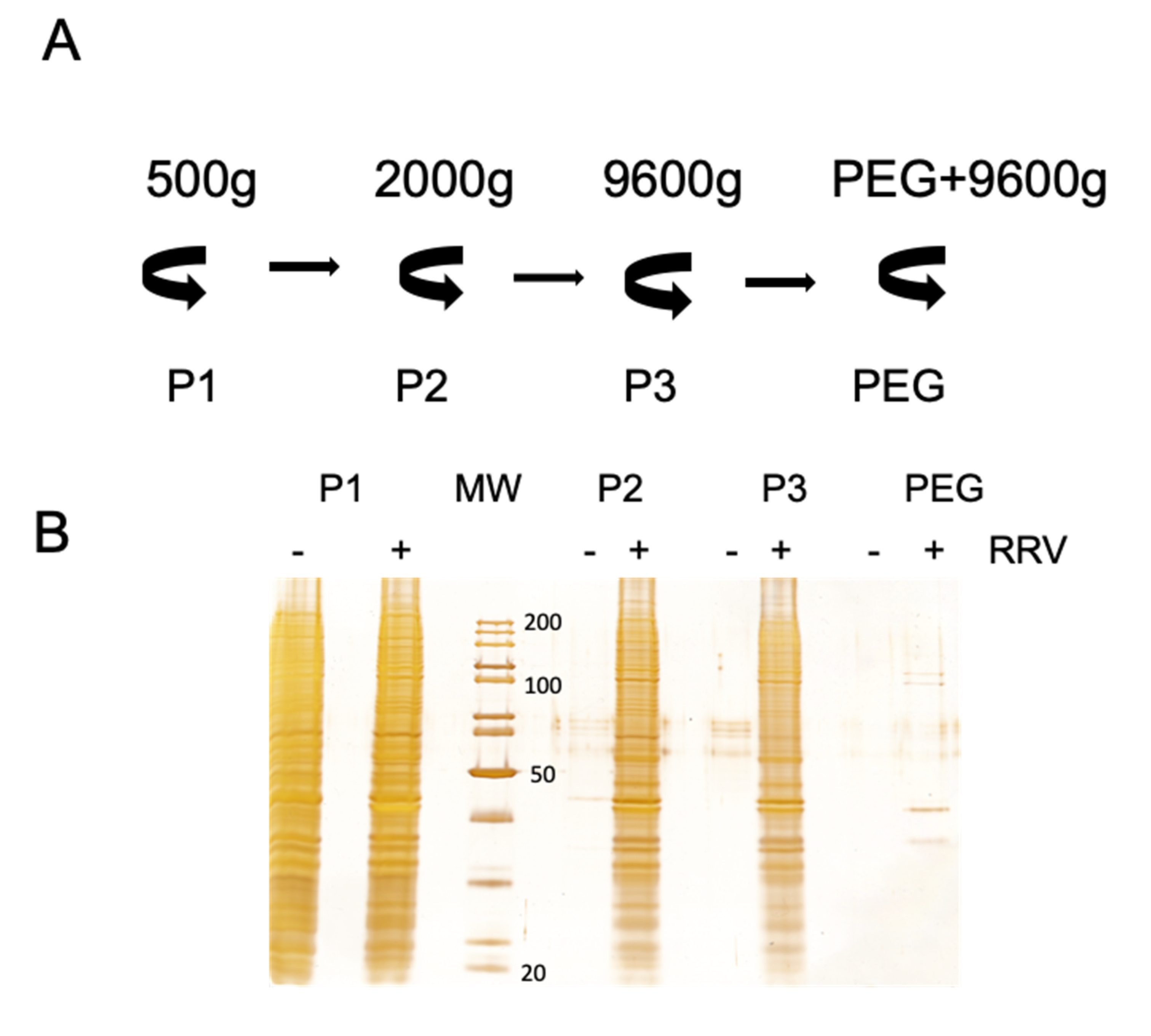
2.4. Vesicle Purification
2.5. Infectivity Associated to the EV
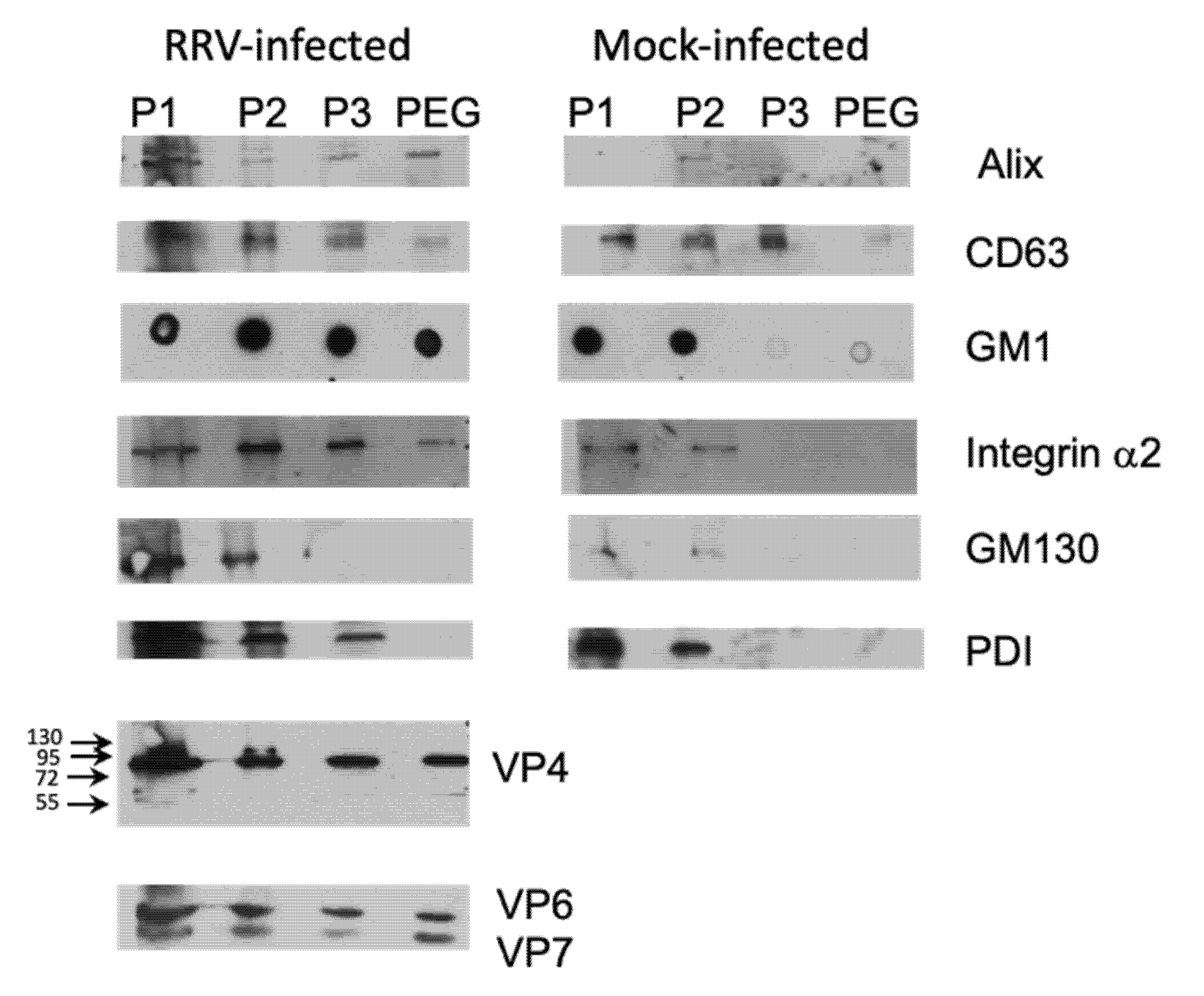
2.6. Immunodetection of Cellular or Viral Proteins
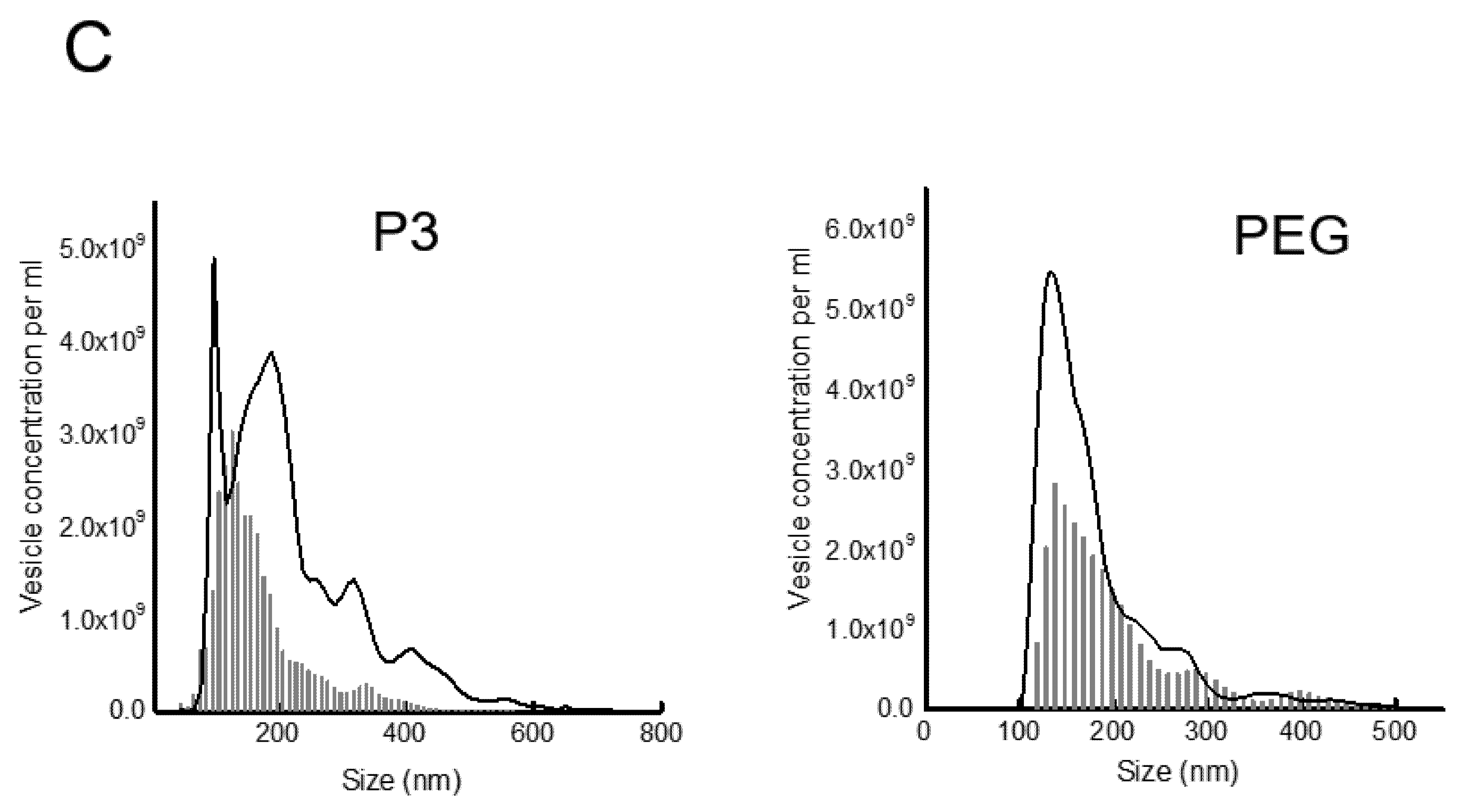
2.7. Nanoparticle Tracking Analysis
2.8. Transmission Electron Microscopy
2.9. Statistical Analysis
3. Results
3.1. Kinetics of Rotavirus Release from MA104 and Caco2 Cells
3.2. Rotavirus Infection Increases Vesicle Secretion from Caco-2 Cells
3.3. Presence of Infectious Virus and Vesicular Markers in Purified Fractions
3.4. Particles Resembling Rotaviruses are Observed Associated with Extracellular Vesicles from the Outside and Inside
3.5. Vesicle Associated Rotavirus Infectivity
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Estes, M.K.; Greenberg, H.B. Rotaviruses. In Fields Viroñlogy; Knipe, D.M., Howley, P.M., Eds.; Wiliams&Wilkins: Philadelphia, PA, USA, 2013; Volume 1, pp. 1347–1401. [Google Scholar]
- Crawford, S.E.; Ramani, S.; Tate, J.E.; Parashar, U.D.; Svensson, L.; Hagbom, M.; Franco, M.A.; Greenberg, H.B.; O’Ryan, M.; Kang, G.; et al. Rotavirus infection. Nat. Rev. Dis. Primers 2017, 3, 17083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cevallos Porta, D.; Lopez, S.; Arias, C.F.; Isa, P. Polarized rotavirus entry and release from differentiated small intestinal cells. Virology 2016, 499, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Isa, P.; Sanchez-Aleman, M.A.; Lopez, S.; Arias, C.F. Dissecting the role of integrin subunits alpha 2 and beta 3 in rotavirus cell entry by rna silencing. Virus Res. 2009, 145, 251–259. [Google Scholar] [CrossRef] [PubMed]
- Patton, J.T.; Silvestri, L.S.; Tortorici, M.A.; Vasquez-Del Carpio, R.; Taraporewala, Z.F. Rotavirus genome replication and morphogenesis: Role of the viroplasm. Curr. Top. Microbiol. Immunol. 2006, 309, 169–187. [Google Scholar] [CrossRef]
- Saxena, K.; Blutt, S.E.; Ettayebi, K.; Zeng, X.L.; Broughman, J.R.; Crawford, S.E.; Karandikar, U.C.; Sastri, N.P.; Conner, M.E.; Opekun, A.R.; et al. Human intestinal enteroids: A new model to study human rotavirus infection, host restriction, and pathophysiology. J. Virol. 2016, 90, 43–56. [Google Scholar] [CrossRef] [Green Version]
- Lopez, T.; Camacho, M.; Zayas, M.; Najera, R.; Sanchez, R.; Arias, C.F.; Lopez, S. Silencing the morphogenesis of rotavirus. J. Virol. 2005, 79, 184–192. [Google Scholar] [CrossRef] [Green Version]
- Taylor, J.A.; O’Brien, J.A.; Yeager, M. The cytoplasmic tail of nsp4, the endoplasmic reticulum-localized non-structural glycoprotein of rotavirus, contains distinct virus binding and coiled coil domains. EMBO J. 1996, 15, 4469–4476. [Google Scholar] [CrossRef]
- Martinez, J.L.; Arnoldi, F.; Schraner, E.M.; Eichwald, C.; Silva-Ayala, D.; Lee, E.; Sztul, E.; Burrone, O.R.; Lopez, S.; Arias, C.F. The guanine nucleotide exchange factor gbf1 participates in rotavirus replication. J. Virol. 2019, 93. [Google Scholar] [CrossRef] [Green Version]
- Musalem, C.; Espejo, R.T. Release of progeny virus from cells infected with simian rotavirus sa11. J. Gen. Virol. 1985, 66, 2715–2724. [Google Scholar] [CrossRef]
- Jourdan, N.; Maurice, M.; Delautier, D.; Quero, A.M.; Servin, A.L.; Trugnan, G. Rotavirus is released from the apical surface of cultured human intestinal cells through nonconventional vesicular transport that bypasses the golgi apparatus. J. Virol. 1997, 71, 8268–8278. [Google Scholar] [CrossRef] [Green Version]
- Cuadras, M.A.; Greenberg, H.B. Rotavirus infectious particles use lipid rafts during replication for transport to the cell surface in vitro and in vivo. Virology 2003, 313, 308–321. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Zhang, X.; Yu, Q.; He, J.J. Exosome-associated hepatitis c virus in cell cultures and patient plasma. Biochem. Biophys. Res. Commun. 2014, 455, 218–222. [Google Scholar] [CrossRef] [PubMed]
- Morris-Love, J.; Gee, G.V.; O’Hara, B.A.; Assetta, B.; Atkinson, A.L.; Dugan, A.S.; Haley, S.A.; Atwood, W.J. Jc polyomavirus uses extracellular vesicles to infect target cells. MBio 2019, 10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagashima, S.; Jirintai, S.; Takahashi, M.; Kobayashi, T.; Tanggis; Nishizawa, T.; Kouki, T.; Yashiro, T.; Okamoto, H. Hepatitis E virus egress depends on the exosomal pathway, with secretory exosomes derived from multivesicular bodies. J. Gen. Virol. 2014, 95, 2166–2175. [Google Scholar] [CrossRef] [PubMed]
- Silvas, J.A.; Popov, V.L.; Paulucci-Holthauzen, A.; Aguilar, P.V. Extracellular vesicles mediate receptor-independent transmission of novel tick-borne bunyavirus. J. Virol. 2016, 90, 873–886. [Google Scholar] [CrossRef] [Green Version]
- Meckes, D.G., Jr.; Raab-Traub, N. Microvesicles and viral infection. J. Virol. 2011, 85, 12844–12854. [Google Scholar] [CrossRef] [Green Version]
- Sims, B.; Gu, L.; Krendelchtchikov, A.; Matthews, Q.L. Neural stem cell-derived exosomes mediate viral entry. Int. J. Nanomed. 2014, 9, 4893–4897. [Google Scholar] [CrossRef] [Green Version]
- Van Dongen, H.M.; Masoumi, N.; Witwer, K.W.; Pegtel, D.M. Extracellular vesicles exploit viral entry routes for cargo delivery. Microbiol. Mol. Biol. Rev. 2016, 80, 369–386. [Google Scholar] [CrossRef] [Green Version]
- Deschamps, T.; Kalamvoki, M. Extracellular vesicles released by herpes simplex virus 1-infected cells block virus replication in recipient cells in a sting-dependent manner. J. Virol. 2018, 92. [Google Scholar] [CrossRef] [Green Version]
- Lai, F.W.; Lichty, B.D.; Bowdish, D.M. Microvesicles: Ubiquitous contributors to infection and immunity. J. Leukoc. Biol. 2015, 97, 237–245. [Google Scholar] [CrossRef]
- Barreto, A.; Rodriguez, L.S.; Rojas, O.L.; Wolf, M.; Greenberg, H.B.; Franco, M.A.; Angel, J. Membrane vesicles released by intestinal epithelial cells infected with rotavirus inhibit t-cell function. Viral. Immunol. 2010, 23, 595–608. [Google Scholar] [CrossRef] [Green Version]
- Santiana, M.; Ghosh, S.; Ho, B.A.; Rajasekaran, V.; Du, W.L.; Mutsafi, Y.; De Jesus-Diaz, D.A.; Sosnovtsev, S.V.; Levenson, E.A.; Parra, G.I.; et al. Vesicle-cloaked virus clusters are optimal units for inter-organismal viral transmission. Cell Host Microbe 2018, 24, 208–220.e8. [Google Scholar] [CrossRef] [Green Version]
- Guerrero, C.A.; Zarate, S.; Corkidi, G.; Lopez, S.; Arias, C.F. Biochemical characterization of rotavirus receptors in ma104 cells. J. Virol. 2000, 74, 9362–9371. [Google Scholar] [CrossRef] [Green Version]
- Rider, M.A.; Hurwitz, S.N.; Meckes, D.G., Jr. Extrapeg: A polyethylene glycol-based method for enrichment of extracellular vesicles. Sci. Rep. 2016, 6, 23978. [Google Scholar] [CrossRef] [PubMed]
- Offit, P.A.; Shaw, R.D.; Greenberg, H.B. Passive protection against rotavirus-induced diarrhea by monoclonal antibodies to surface proteins vp3 and vp7. J. Virol. 1986, 58, 700–703. [Google Scholar] [CrossRef] [Green Version]
- Wessel, D.; Flugge, U.I. A method for the quantitative recovery of protein in dilute solution in the presence of detergents and lipids. Anal. Biochem. 1984, 138, 141–143. [Google Scholar] [CrossRef]
- Padilla-Noriega, L.; Werner-Eckert, R.; Mackow, E.R.; Gorziglia, M.; Larralde, G.; Taniguchi, K.; Greenberg, H.B. Serologic analysis of human rotavirus serotypes p1a and p2 by using monoclonal antibodies. J. Clin. Microbiol. 1993, 31, 622–628. [Google Scholar] [CrossRef] [Green Version]
- Hassellöw, M.; Kaegi, R. Analysis and characterization of manufactured nanoparticles in aquatic environments. In Environmental and Human Health Impacts of Nanotechnology; Lead, J.R., Smith, E., Eds.; Wiley: Chichester, UK, 2009; pp. 211–266. [Google Scholar] [CrossRef]
- Quevedo, I.R.; Olsson, A.L.J.; Clark, R.J.; Veinot, J.G.C.; Tufenkji, N. Interpreting deposition behavior of polydisperse surface-modified nanoparticles using QCM-D and sand-packed columns. Environ. Eng. Sci. 2014, 31, 326–337. [Google Scholar] [CrossRef] [Green Version]
- Trejo-Cerro, O.; Eichwald, C.; Schraner, E.M.; Silva-Ayala, D.; Lopez, S.; Arias, C.F. Actin-dependent nonlytic rotavirus exit and infectious virus morphogenetic pathway in nonpolarized cells. J. Virol. 2018, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pérez-Delgado, A.; Instituto de Biotecnología. Caracterización de la Asociación de Rotavirus con Vesículas Extracelulares en Células MA104. Master’s Thesis, Universidad Nacional Autónoma de México, Cuernavaca, Mexico, 2019. Available online: http://132.248.9.195/ptd2019/junio/0790697/Index.html (accessed on 1 July 2020).
- Bello-Morales, R.; Praena, B.; De la Nuez, C.; Rejas, M.T.; Guerra, M.; Galan-Ganga, M.; Izquierdo, M.; Calvo, V.; Krummenacher, C.; Lopez-Guerrero, J.A. Role of microvesicles in the spread of herpes simplex virus 1 in oligodendrocytic cells. J. Virol. 2018, 92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, W.; Woodson, M.; Neupane, B.; Bai, F.; Sherman, M.B.; Choi, K.H.; Neelakanta, G.; Sultana, H. Exosomes serve as novel modes of tick-borne flavivirus transmission from arthropod to human cells and facilitates dissemination of viral rna and proteins to the vertebrate neuronal cells. PLoS Pathog. 2018, 14, e1006764. [Google Scholar] [CrossRef] [Green Version]
- Arenaccio, C.; Chiozzini, C.; Columba-Cabezas, S.; Manfredi, F.; Affabris, E.; Baur, A.; Federico, M. Exosomes from human immunodeficiency virus type 1 (hiv-1)-infected cells license quiescent cd4+ t lymphocytes to replicate hiv-1 through a nef- and adam17-dependent mechanism. J. Virol. 2014, 88, 11529–11539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kulkarni, R.; Prasad, A. Exosomes derived from hiv-1 infected DCs mediate viral trans-infection via fibronectin and galectin-3. Sci. Rep. 2017, 7, 14787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kadiu, I.; Narayanasamy, P.; Dash, P.K.; Zhang, W.; Gendelman, H.E. Biochemical and biologic characterization of exosomes and microvesicles as facilitators of hiv-1 infection in macrophages. J. Immunol. 2012, 189, 744–754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erickson, A.K.; Jesudhasan, P.R.; Mayer, M.J.; Narbad, A.; Winter, S.E.; Pfeiffer, J.K. Bacteria facilitate enteric virus co-infection of mammalian cells and promote genetic recombination. Cell Host Microbe 2018, 23, 77–88.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuss, S.K.; Best, G.T.; Etheredge, C.A.; Pruijssers, A.J.; Frierson, J.M.; Hooper, L.V.; Dermody, T.S.; Pfeiffer, J.K. Intestinal microbiota promote enteric virus replication and systemic pathogenesis. Science 2011, 334, 249–252. [Google Scholar] [CrossRef] [Green Version]
- Robinson, C.M.; Jesudhasan, P.R.; Pfeiffer, J.K. Bacterial lipopolysaccharide binding enhances virion stability and promotes environmental fitness of an enteric virus. Cell Host Microbe 2014, 15, 36–46. [Google Scholar] [CrossRef] [Green Version]
- Ramakrishnaiah, V.; Thumann, C.; Fofana, I.; Habersetzer, F.; Pan, Q.; De Ruiter, P.E.; Willemsen, R.; Demmers, J.A.; Stalin Raj, V.; Jenster, G.; et al. Exosome-mediated transmission of hepatitis C virus between human hepatoma huh7.5 cells. Proc. Natl. Acad. Sci. USA 2013, 110, 13109–13113. [Google Scholar] [CrossRef] [Green Version]
- Longatti, A.; Boyd, B.; Chisari, F.V. Virion-independent transfer of replication-competent hepatitis C virus rna between permissive cells. J. Virol. 2015, 89, 2956–2961. [Google Scholar] [CrossRef] [Green Version]
- Blutt, S.E.; Conner, M.E. Rotavirus: To the gut and beyond! Curr. Opin. Gastroenterol. 2007, 23, 39–43. [Google Scholar] [CrossRef]
- Blutt, S.E.; Fenaux, M.; Warfield, K.L.; Greenberg, H.B.; Conner, M.E. Active viremia in rotavirus-infected mice. J. Virol. 2006, 80, 6702–6705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blutt, S.E.; Matson, D.O.; Crawford, S.E.; Staat, M.A.; Azimi, P.; Bennett, B.L.; Piedra, P.A.; Conner, M.E. Rotavirus antigenemia in children is associated with viremia. PLoS Med. 2007, 4, e121. [Google Scholar] [CrossRef] [Green Version]
- Crawford, S.E.; Patel, D.G.; Cheng, E.; Berkova, Z.; Hyser, J.M.; Ciarlet, M.; Finegold, M.J.; Conner, M.E.; Estes, M.K. Rotavirus viremia and extraintestinal viral infection in the neonatal rat model. J. Virol. 2006, 80, 4820–4832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lynch, M.; Shieh, W.J.; Tatti, K.; Gentsch, J.R.; Ferebee-Harris, T.; Jiang, B.; Guarner, J.; Bresee, J.S.; Greenwald, M.; Cullen, S.; et al. The pathology of rotavirus-associated deaths, using new molecular diagnostics. Clin. Infect. Dis. 2003, 37, 1327–1333. [Google Scholar] [CrossRef] [PubMed]
- Goldwater, P.N.; Rowland, K.; Thesinger, M.; Abbott, K.; Grieve, A.; Palombo, E.A.; Masendycz, P.J.; Wilkinson, I.; Bear, J. Rotavirus encephalopathy: Pathogenesis reviewed. J. Paediatr. Child Health 2001, 37, 206–209. [Google Scholar] [CrossRef]
- Nishimura, S.; Ushijima, H.; Nishimura, S.; Shiraishi, H.; Kanazawa, C.; Abe, T.; Kaneko, K.; Fukuyama, Y. Detection of rotavirus in cerebrospinal fluid and blood of patients with convulsions and gastroenteritis by means of the reverse transcription polymerase chain reaction. Brain Dev. 1993, 15, 457–459. [Google Scholar] [CrossRef]
- Cuadras, M.A.; Bordier, B.B.; Zambrano, J.L.; Ludert, J.E.; Greenberg, H.B. Dissecting rotavirus particle-raft interaction with small interfering rnas: Insights into rotavirus transit through the secretory pathway. J. Virol. 2006, 80, 3935–3946. [Google Scholar] [CrossRef] [Green Version]
- Tian, P.; Ball, J.M.; Zeng, C.Q.; Estes, M.K. Rotavirus protein expression is important for virus assembly and pathogenesis. Arch. Virol. Suppl. 1996, 12, 69–77. [Google Scholar] [CrossRef]
- Elsherbini, A.; Bieberich, E. Ceramide and exosomes: A novel target in cancer biology and therapy. Adv. Cancer Res. 2018, 140, 121–154. [Google Scholar] [CrossRef]
- Skotland, T.; Sandvig, K.; Llorente, A. Lipids in exosomes: Current knowledge and the way forward. Prog. Lipid Res. 2017, 66, 30–41. [Google Scholar] [CrossRef]




© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Iša, P.; Pérez-Delgado, A.; Quevedo, I.R.; López, S.; Arias, C.F. Rotaviruses Associate with Distinct Types of Extracellular Vesicles. Viruses 2020, 12, 763. https://doi.org/10.3390/v12070763
Iša P, Pérez-Delgado A, Quevedo IR, López S, Arias CF. Rotaviruses Associate with Distinct Types of Extracellular Vesicles. Viruses. 2020; 12(7):763. https://doi.org/10.3390/v12070763
Chicago/Turabian StyleIša, Pavel, Arianna Pérez-Delgado, Iván R. Quevedo, Susana López, and Carlos F. Arias. 2020. "Rotaviruses Associate with Distinct Types of Extracellular Vesicles" Viruses 12, no. 7: 763. https://doi.org/10.3390/v12070763
APA StyleIša, P., Pérez-Delgado, A., Quevedo, I. R., López, S., & Arias, C. F. (2020). Rotaviruses Associate with Distinct Types of Extracellular Vesicles. Viruses, 12(7), 763. https://doi.org/10.3390/v12070763




