Aedes aegypti from Amazon Basin Harbor High Diversity of Novel Viral Species
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mosquitoes Collection
2.2. Sample Processing and Next Generation Sequencing (NGS)
2.3. Phylogeny and Viral Annotation
3. Results
3.1. Sobemo-Related Virus
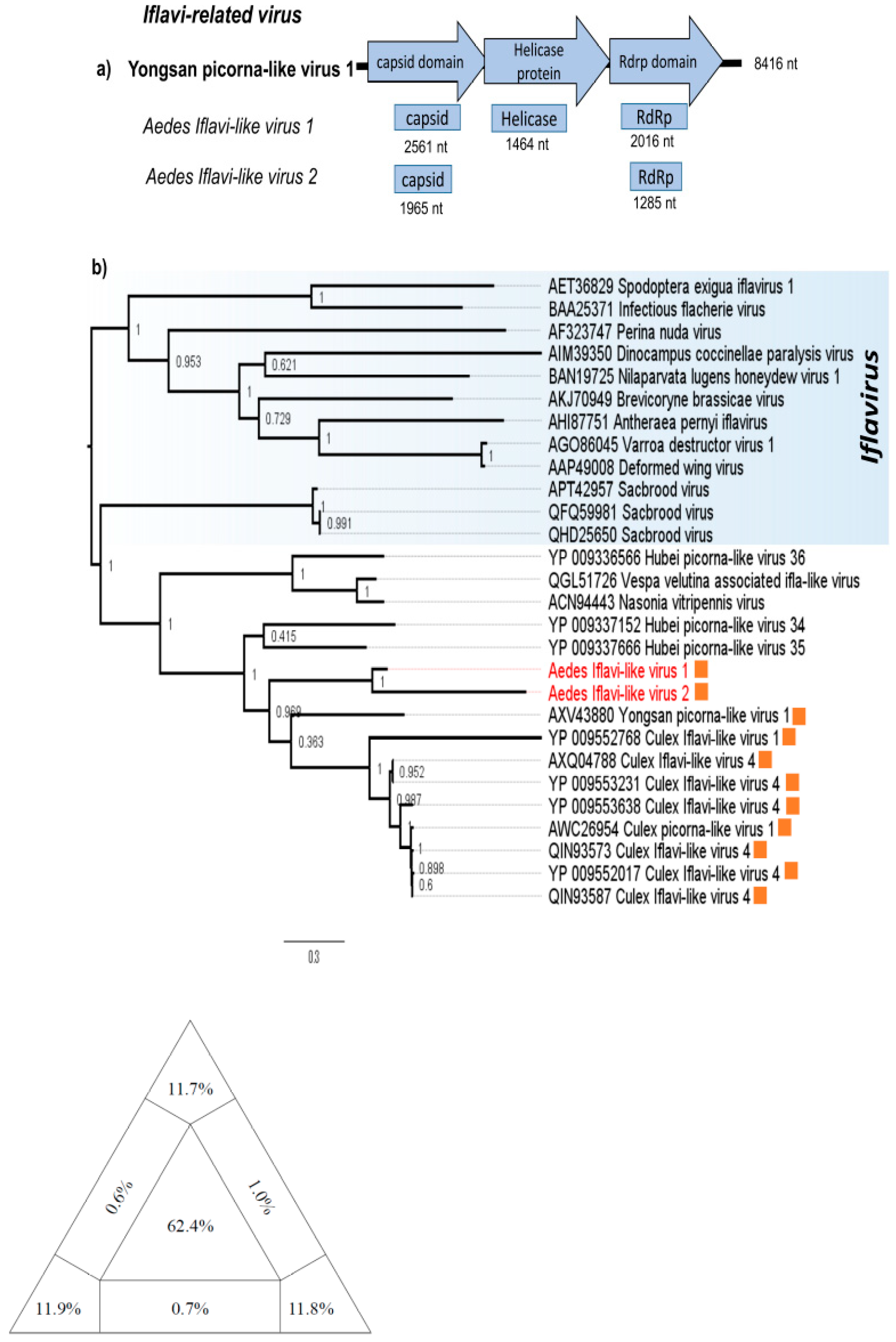
3.2. Iflavi-Related Virus
3.3. Permutotetra-Like Virus
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shi, M.; Lin, X.D.; Tian, J.H.; Chen, L.J.; Chen, X.; Li, C.X.; Qin, X.C.; Li, J.; Cao, J.P.; Eden, J.S.; et al. Redefining the invertebrate RNA virosphere. Nature 2016, 540, 539–543. [Google Scholar] [CrossRef]
- Li, C.X.; Shi, M.; Tian, J.H.; Lin, X.D.; Kang, Y.J.; Chen, L.J.; Qin, X.C.; Xu, J.; Holmes, E.C.; Zhang, Y.Z. Unprecedented genomic diversity of RNA viruses in arthropods reveals the ancestry of negative-sense RNA viruses. eLife 2015, 4, e05378. [Google Scholar] [CrossRef]
- Sadeghi, M.; Altan, E.; Deng, X.; Barker, C.M.; Fang, Y.; Coffey, L.L.; Delwart, E. Virome of > 12 thousand Culex mosquitoes from throughout California. Virology 2018, 523, 74–88. [Google Scholar] [CrossRef]
- Kenney, J.L.; Solberg, O.D.; Langevin, S.A.; Brault, A.C. Characterization of a novel insect-specific flavivirus from Brazil: Potential for inhibition of infection of arthropod cells with medically important flaviviruses. J. Gen. Virol. 2014, 95, 2796. [Google Scholar] [CrossRef]
- Nasar, F.; Erasmus, J.H.; Haddow, A.D.; Tesh, R.B.; Weaver, S.C. Eilat virus induces both homologous and heterologous interference. Virology 2015, 484, 51–58. [Google Scholar] [CrossRef] [Green Version]
- Öhlund, P.; Lundén, H.; Blomström, A.L. Insect-specific virus evolution and potential effects on vector competence. Virus Genes 2019, 55, 127–137. [Google Scholar]
- Romo, H.; Kenney, J.L.; Blitvich, B.J.; Brault, A.C. Restriction of Zika virus infection and transmission in Aedes aegypti mediated by an insect-specific flavivirus. Emerg. Microbes Infect. 2018, 7, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Vasilakis, N.; Tesh, R.B. Insect-specific viruses and their potential impact on arbovirus transmission. Curr. Opin. Virol. 2015, 15, 69–74. [Google Scholar] [CrossRef] [Green Version]
- Halbach, R.; Junglen, S.; van Rij, R.P. Mosquito-specific and mosquito-borne viruses: Evolution, infection, and host defense. Curr. Opin. Insect Sci. 2017, 22, 16–27. [Google Scholar] [CrossRef]
- Marklewitz, M.; Zirkel, F.; Kurth, A.; Drosten, C.; Junglen, S. Evolutionary and phenotypic analysis of live virus isolates suggests arthropod origin of a pathogenic RNA virus family. Proc. Natl. Acad. Sci. USA 2015, 112, 7536–7541. [Google Scholar] [CrossRef] [Green Version]
- Vasconcelos, P.F.; Travassos da Rosa, A.P.; Rodrigues, S.G.; Travassos da Rosa, E.S.; Dégallier, N.; Travassos da Rosa, J.F. Inadequate management of natural ecosystem in the Brazilian Amazon region results in the emergence and reemergence of arboviruses. Cad. Saúde Pública/Ministério da Saúde Fundação Oswaldo Cruz Esc. Nac. Saúde Pública 2001, 17, 155–164. [Google Scholar] [CrossRef]
- Donalisio, M.R.; Freitas, A.R.R.; Zuben, A.P.B. Von Arboviruses emerging in Brazil: Challenges for clinic and implications for public health. Rev. Saude Publica 2017, 51, 30. [Google Scholar] [CrossRef]
- Brown, J.E.; Evans, B.R.; Zheng, W.; Obas, V.; Barrera-Martinez, L.; Egizi, A.; Zhao, H.; Caccone, A.; Powell, J.R. Human impacts have shaped historical and recent evolution in Aedes aegypti, the dengue and yellow fever mosquito. Evolution (N. Y.) 2014, 68, 514–525. [Google Scholar] [CrossRef] [Green Version]
- Guagliardo, S.A.J.; Lee, Y.; Pierce, A.A.; Wong, J.; Chu, Y.Y.; Morrison, A.C.; Astete, H.; Brosi, B.; Vazquez-Prokopec, G.; Scott, T.W.; et al. The genetic structure of Aedes aegypti populations is driven by boat traffic in the Peruvian Amazon. PLoS Negl. Trop. Dis. 2019, 13. [Google Scholar] [CrossRef] [Green Version]
- Paixão, E.S.; Teixeira, M.G.; Rodrigues, L.C. Zika, chikungunya and dengue: The causes and threats of new and re-emerging arboviral diseases. BMJ Glob. Health 2017, 3, e000530. [Google Scholar] [CrossRef] [Green Version]
- Monteiro, F.J.C.; Mourão, F.R.P.; Ribeiro, E.S.D.; da Rêgo, M.O.S.; da Frances, P.A.C.; Souto, R.N.P.; Façanha, M.D.S.; Tahmasebi, R.; da Costa, A.C. Prevalence of dengue, zika and chikungunya viruses in aedes (Stegomyia) aegypti (diptera: Culicidae) in a medium-sized city, Amazon, Brazil. Rev. Inst. Med. Trop. Sao Paulo 2020, 62. [Google Scholar] [CrossRef] [Green Version]
- Fernandes, L.N.; Coletti, T.M.; Monteiro, F.J.C.; Rego, M.O.S.; Ribeiro, E.S.D.; Ribeiro, G.O.; Marinho, R.S.S.; Komninakis, S.V.; Witkin, S.S.; Deng, X.; et al. A novel highly divergent strain of cell fusing agent virus (Cfav) in mosquitoes from the Brazilian Amazon region. Viruses 2018, 10, 666. [Google Scholar] [CrossRef] [Green Version]
- de Ribeiro, G.O.; Monteiro, F.J.C.; da Rego, M.O.S.; Ribeiro, E.S.D.; de Castro, D.F.; Caseiro, M.M.; Marinho, S.; dos Santos, R.; Komninakis, S.V.; Witkin, S.S. Detection of RNA-Dependent RNA Polymerase of Hubei Reo-Like Virus 7 by Next-Generation Sequencing in Aedes aegypti and Culex quinquefasciatus Mosquitoes from Brazil. Viruses 2019, 11, 147. [Google Scholar] [CrossRef] [Green Version]
- Consoli, R.A.G.B.; de Oliveira, R.L. Principais Mosquitos de Importância Sanitária No Brasil, 1st ed.; Editora Fiocruz: Rio de Janeiro, Brasil, 1994; ISBN 85-85676-03-5. [Google Scholar]
- Deng, X.; Naccache, S.N.; Ng, T.; Federman, S.; Li, L.; Chiu, C.Y.; Delwart, E.L. An ensemble strategy that significantly improves de novo assembly of microbial genomes from metagenomic next-generation sequencing data. Nucleic Acids Res. 2015, 43, e46. [Google Scholar] [CrossRef]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 2017. [Google Scholar] [CrossRef] [Green Version]
- Guindon, S.; Dufayard, J.F.; Lefort, V.; Anisimova, M.; Hordijk, W.; Gascuel, O. New algorithms and methods to estimate maximum-likelihood phylogenies: Assessing the performance of PhyML 3.0. Syst. Biol. 2010, 59, 307–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Posada, D. jModelTest: Phylogenetic model averaging. Mol. Biol. Evol. 2008, 25, 1253–1256. [Google Scholar] [CrossRef]
- Quevillon, E.; Silventoinen, V.; Pillai, S.; Harte, N.; Mulder, N.; Apweiler, R.; Lopez, R. InterProScan: Protein domains identifier. Nucleic Acids Res. 2005, 33, W116–W120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marchler-Bauer, A.; Derbyshire, M.K.; Gonzales, N.R.; Lu, S.; Chitsaz, F.; Geer, L.Y.; Geer, R.C.; He, J.; Gwadz, M.; Hurwitz, D.I.; et al. CDD: NCBI’s conserved domain database. Nucleic Acids Res. 2015, 43, 222–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, C.; Beller, L.; Deboutte, W.; Yinda, K.C.; Delang, L.; Vega-Rúa, A.; Failloux, A.B.; Matthijnssens, J. Stable distinct core eukaryotic viromes in different mosquito species from Guadeloupe, using single mosquito viral metagenomics. Microbiome 2019, 7, 121. [Google Scholar] [CrossRef]
- Oers, M.M. Genomics and biology of lflaviruses. Insect Virol. 2010, 231–250. [Google Scholar]
- Hang, J.; Klein, T.A.; Kim, H.-C.; Yang, Y.; Jima, D.D.; Richardson, J.H.; Jarman, R.G. Genome sequences of five arboviruses in field-captured mosquitoes in a unique rural environment of South Korea. Genome Announc. 2016, 4. [Google Scholar] [CrossRef] [Green Version]
- Amoa-Bosompem, M.; Kobayashi, D.; Murota, K.; Faizah, A.N.; Itokawa, K.; Fujita, R.; Osei, J.H.N.; Agbosu, E.; Pratt, D.; Kimura, S. Entomological Assessment of the Status and Risk of Mosquito-borne Arboviral Transmission in Ghana. Viruses 2020, 12, 147. [Google Scholar] [CrossRef] [Green Version]
- Fujita, R.; Kato, F.; Kobayashi, D.; Murota, K.; Takasaki, T.; Tajima, S.; Lim, C.K.; Saijo, M.; Isawa, H.; Sawabe, K. Persistent viruses in mosquito cultured cell line suppress multiplication of flaviviruses. Heliyon 2018, 4, e00736. [Google Scholar] [CrossRef] [Green Version]
- Sadeghi, M.; Popov, V.; Guzman, H.; Phan, T.G.; Vasilakis, N.; Tesh, R.; Delwart, E. Genomes of viral isolates derived from different mosquitos species. Virus Res. 2017, 242, 49–57. [Google Scholar] [CrossRef] [Green Version]
- Shi, C.; Zhao, L.; Zeng, W.; Atoni, E.; Hu, X.; Matthijnssens, J.; Yuan, Z.; Xia, H. The conservation of a core virome in Aedes mosquitoes across different developmental stages and continents. bioRxiv 2020. [Google Scholar] [CrossRef]
- Saqib, M.; Wylie, S.J.; Jones, M.G.K. Serendipitous identification of a new Iflavirus-like virus infecting tomato and its subsequent characterization. Plant Pathol. 2015, 64, 519–527. [Google Scholar] [CrossRef] [Green Version]
- Valles, S.M.; Chen, Y.; Firth, A.E.; Guérin, D.M.A.; Hashimoto, Y.; Herrero, S.; De Miranda, J.R.; Ryabov, E. ICTV virus taxonomy profile: Iflaviridae. J. Gen. Virol. 2017, 98, 527–528. [Google Scholar] [CrossRef] [PubMed]
- Dalmon, A.; Gayral, P.; Decante, D.; Klopp, C.; Bigot, D.; Thomasson, M.; AHerniou, E.; Alaux, C.; Conte, Y. Le Viruses in the invasive hornet vespa velutina. Viruses 2019, 11, 1041. [Google Scholar] [CrossRef] [Green Version]
- Dudas, G.; Obbard, D.J. Are arthropods at the heart of virus evolution? eLife 2015, 4, e06837. [Google Scholar] [CrossRef] [Green Version]
- Cook, S.; Chung, B.Y.W.; Bass, D.; Moureau, G.; Tang, S.; McAlister, E.; Culverwell, C.L.; Glücksman, E.; Wang, H.; Brown, T.D.K.; et al. Novel virus discovery and genome reconstruction from field rna samples reveals highly divergent viruses in dipteran hosts. PLoS ONE 2013, 8, e80720. [Google Scholar] [CrossRef] [Green Version]
- Fort, P.; Albertini, A.; Van-Hua, A.; Berthomieu, A.; Roche, S.; Delsuc, F.; Pasteur, N.; Capy, P.; Gaudin, Y.; Weill, M. Fossil rhabdoviral sequences integrated into arthropod genomes: Ontogeny, evolution, and potential functionality. Mol. Biol. Evol. 2012, 29, 381–390. [Google Scholar] [CrossRef]
- Bolling, B.G.; Olea-Popelka, F.J.; Eisen, L.; Moore, C.G.; Blair, C.D. Transmission dynamics of an insect-specific flavivirus in a naturally infected Culex pipiens laboratory colony and effects of co-infection on vector competence for West Nile virus. Virology 2012, 427, 90–97. [Google Scholar] [CrossRef] [Green Version]
- Lequime, S.; Lambrechts, L. Discovery of flavivirus-derived endogenous viral elements in Anopheles mosquito genomes supports the existence of Anopheles-associated insect-specific flaviviruses. Virus Evol. 2017, 3. [Google Scholar] [CrossRef] [Green Version]
- Kuno, G.; Chang, G.-J.J. Biological transmission of arboviruses: Reexamination of and new insights into components, mechanisms, and unique traits as well as their evolutionary trends. Clin. Microbiol. Rev. 2005, 18, 608–637. [Google Scholar] [CrossRef] [Green Version]
- Faria, N.R.; Kraemer, M.U.G.; Hill, S.C.; De Jesus, J.G.; Aguiar, R.S.; Iani, F.C.M.; Xavier, J.; Quick, J.; Du Plessis, L.; Dellicour, S.; et al. Genomic and epidemiological monitoring of yellow fever virus transmission potential. Science 2018, 361, 894–899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scarpassa, V.M.; Debat, H.J.; Alencar, R.B.; Saraiva, J.F.; Calvo, E.; Arcà, B.; Ribeiro, J.M.C. An insight into the sialotranscriptome and virome of Amazonian anophelines. BMC Genom. 2019, 20, 166. [Google Scholar] [CrossRef] [PubMed]
- Blitvich, B.J.; Firth, A.E. Insect-specific flaviviruses: A systematic review of their discovery, host range, mode of transmission, superinfection exclusion potential and genomic organization. Viruses 2015, 7, 1927–1959. [Google Scholar] [CrossRef] [Green Version]
- Kuwata, R.; Isawa, H.; Hoshino, K.; Sasaki, T.; Kobayashi, M.; Maeda, K.; Sawabe, K. Analysis of mosquito-borne Flavivirus superinfection in Culex tritaeniorhynchus (Diptera: Culicidae) cells persistently infected with Culex Flavivirus (Flaviviridae). J. Med. Entomol. 2015, 52, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Käfer, S.; Paraskevopoulou, S.; Zirkel, F.; Wieseke, N.; Donath, A.; Petersen, M.; Jones, T.C.; Liu, S.; Zhou, X.; Middendorf, M.; et al. Re-assessing the diversity of negative strand RNA viruses in insects. PLoS Pathog. 2019, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Virus Name | Closely Related Viruses 1 | Gene | Length (nt) | Cover 1 | Amino Acid Identity 1 |
|---|---|---|---|---|---|
| Aedes Sobemo-like virus strain AP60-1 * | Wenzhou Sobemo-like virus 4 | Peptidase | 1719 | 42% | 36% |
| RdRp | 1308 | 88% | 72% | ||
| Aedes Sobemo-like virus strain AP60-2 * | Wenzhou Sobemo-like virus 4 | Peptidase | 2768 | 99% | 54% |
| RdRp | 1308 | 100% | 83% | ||
| Aedes Iflavi-like virus 1 | Yongsan picorna-like virus 1 | Capsid | 2561 | 60% | 49% |
| Helicase | 1464 | 100% | 46% | ||
| RdRp | 2016 | 97% | 48% | ||
| Aedes Iflavi-like virus 2 | Yongsan picorna-like virus 1 | Capsid | 1965 | 49% | 47% |
| RdRp | 1285 | 50% | 52% | ||
| Aedes permutotetra-like virus 1 | Culex Daeseongdong-like virus | RdRp | 3321 | 92% | 53% |
| Aedes permutotetra-like virus 2 strain AP59 * | Sarawak virus | Capsid | 1219 | 91% | 48% |
| Aedes permutotetra-like virus 2 strain AP60 * | Sarawak virus | Capsid | 882 | 94% | 49% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ribeiro, G.d.O.; Morais, V.S.; Monteiro, F.J.C.; Ribeiro, E.S.D.; Rego, M.O.d.S.; Souto, R.N.P.; Villanova, F.; Tahmasebi, R.; Hefford, P.M.; Deng, X.; et al. Aedes aegypti from Amazon Basin Harbor High Diversity of Novel Viral Species. Viruses 2020, 12, 866. https://doi.org/10.3390/v12080866
Ribeiro GdO, Morais VS, Monteiro FJC, Ribeiro ESD, Rego MOdS, Souto RNP, Villanova F, Tahmasebi R, Hefford PM, Deng X, et al. Aedes aegypti from Amazon Basin Harbor High Diversity of Novel Viral Species. Viruses. 2020; 12(8):866. https://doi.org/10.3390/v12080866
Chicago/Turabian StyleRibeiro, Geovani de Oliveira, Vanessa S. Morais, Fred Julio Costa Monteiro, Edcelha Soares D’Athaide Ribeiro, Marlisson Octavio da S Rego, Raimundo Nonato Picanço Souto, Fabiola Villanova, Roozbeh Tahmasebi, Philip Michael Hefford, Xutao Deng, and et al. 2020. "Aedes aegypti from Amazon Basin Harbor High Diversity of Novel Viral Species" Viruses 12, no. 8: 866. https://doi.org/10.3390/v12080866








