The Role of Epidermal Growth Factor Receptor Signaling Pathway during Bovine Herpesvirus 1 Productive Infection in Cell Culture
Abstract
1. Introduction
2. Materials and Methods
2.1. Cells and Viruses
2.2. Antibodies and Reagents
2.3. Cell Viability Assay
2.4. Virus Replication Inhibition Assay
2.5. Western Blotting Analysis
3. Results
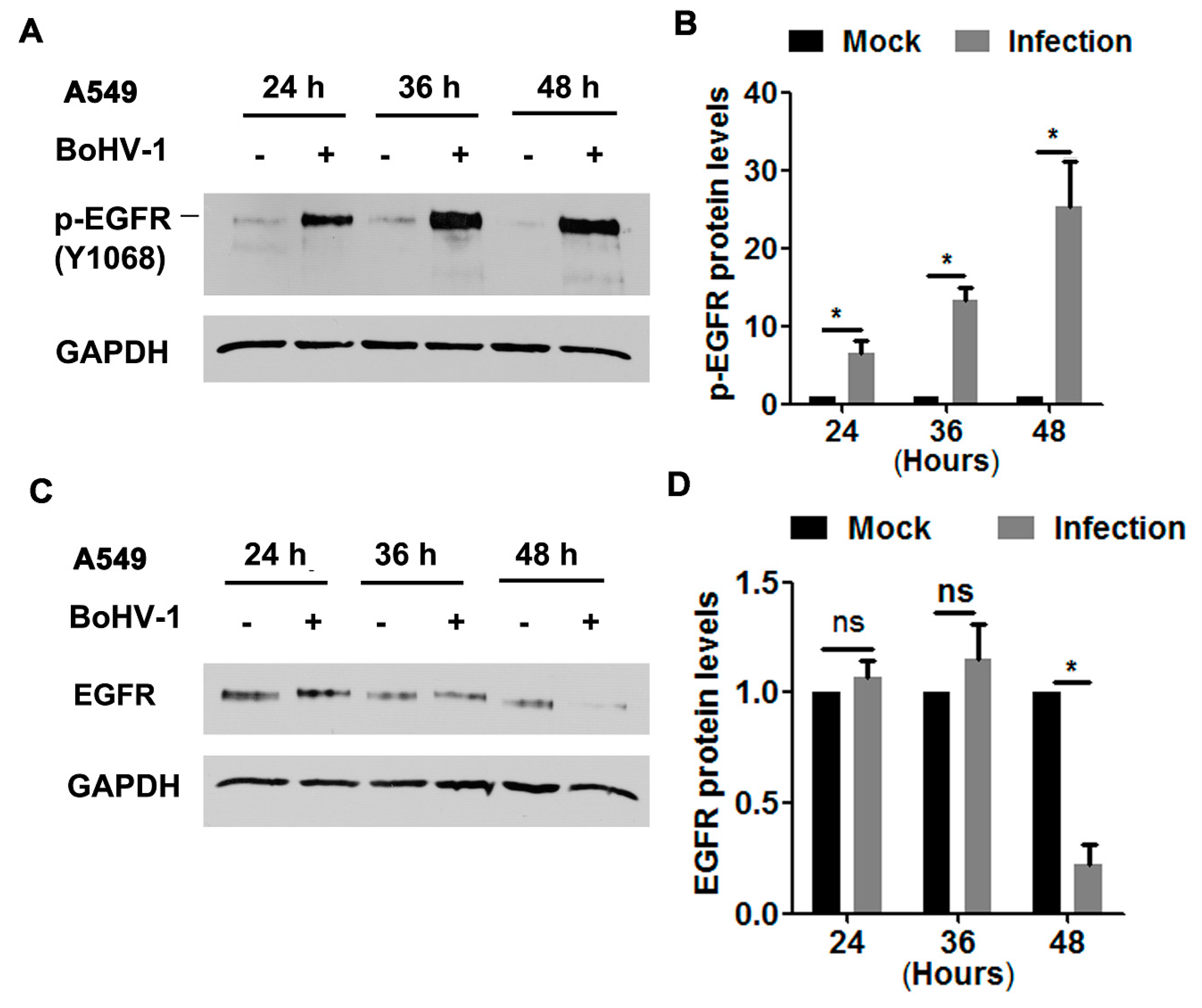
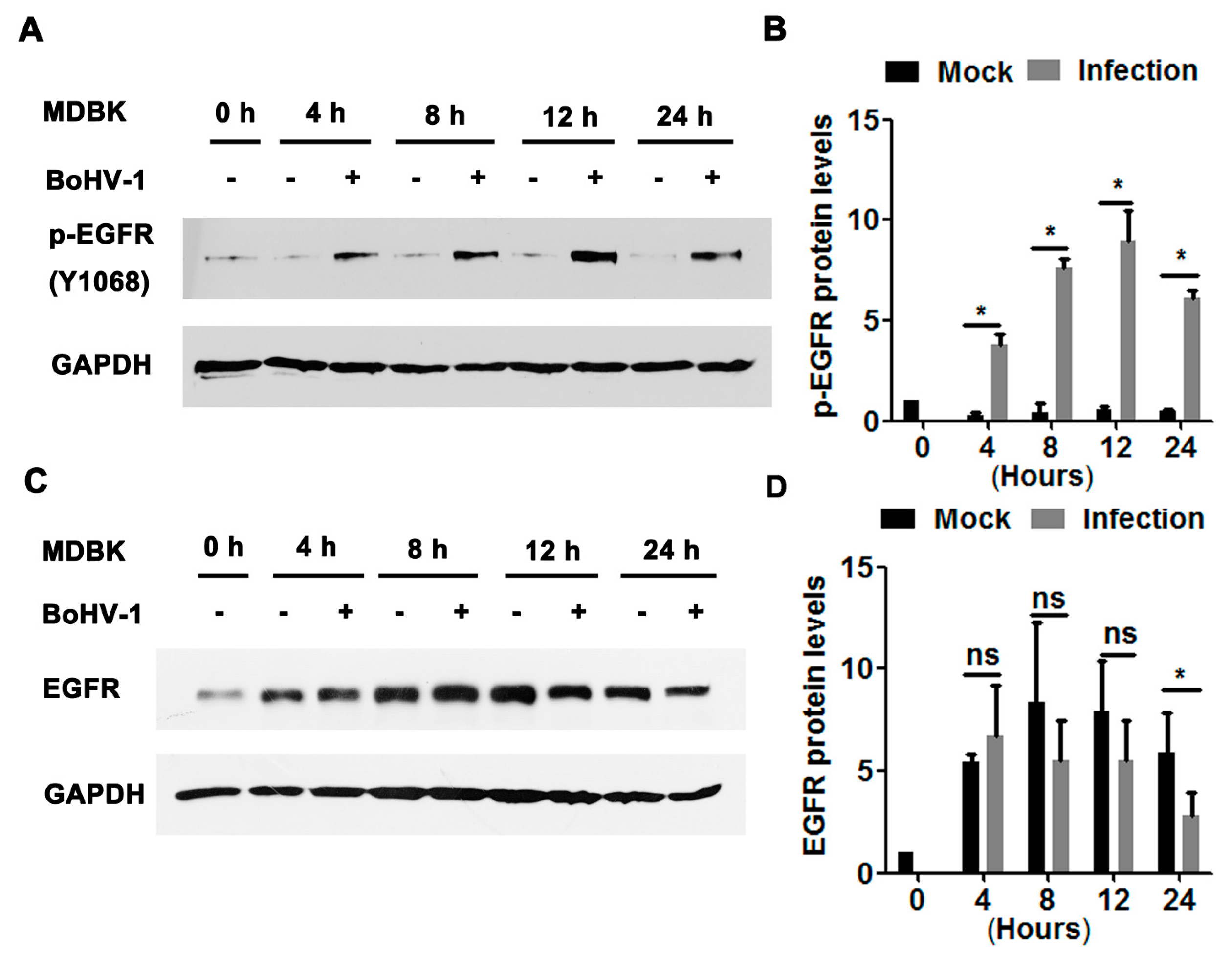
3.1. BoHV-1 Productive Infection in Cell Culture Leads to EGFR Activation
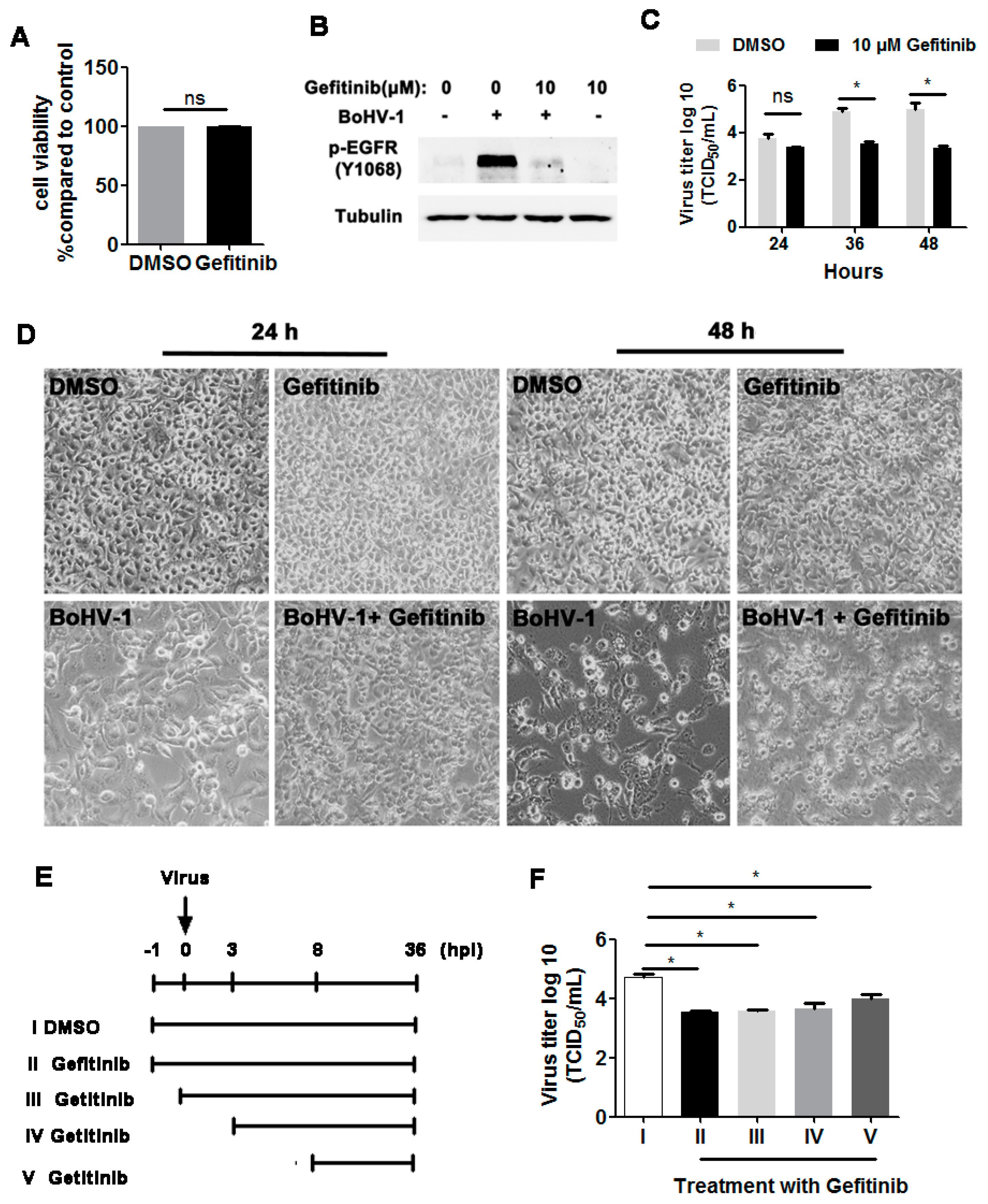
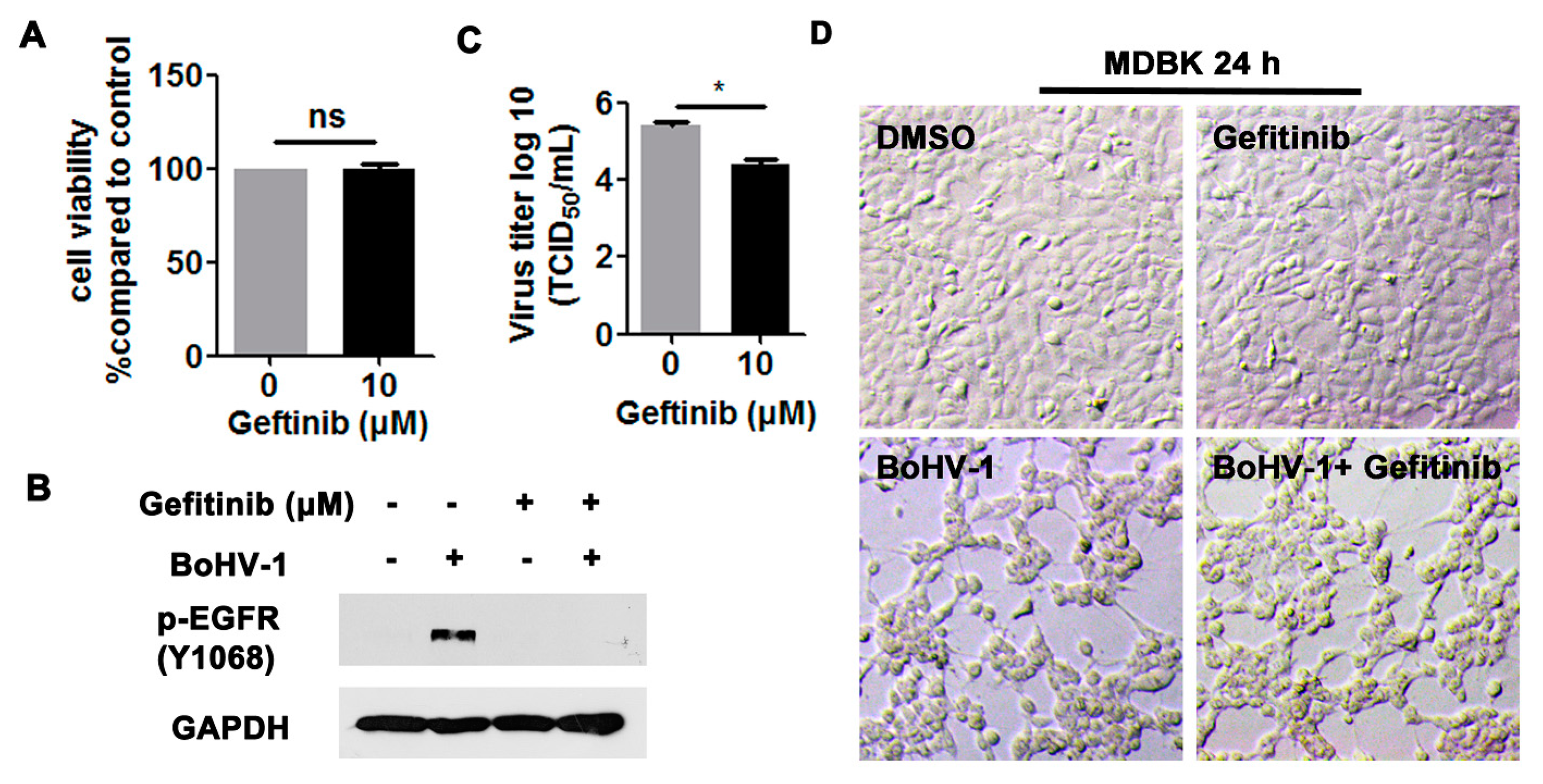
3.2. EGFR Inhibitor Decreased BoHV-1 Infection in Cell Culture
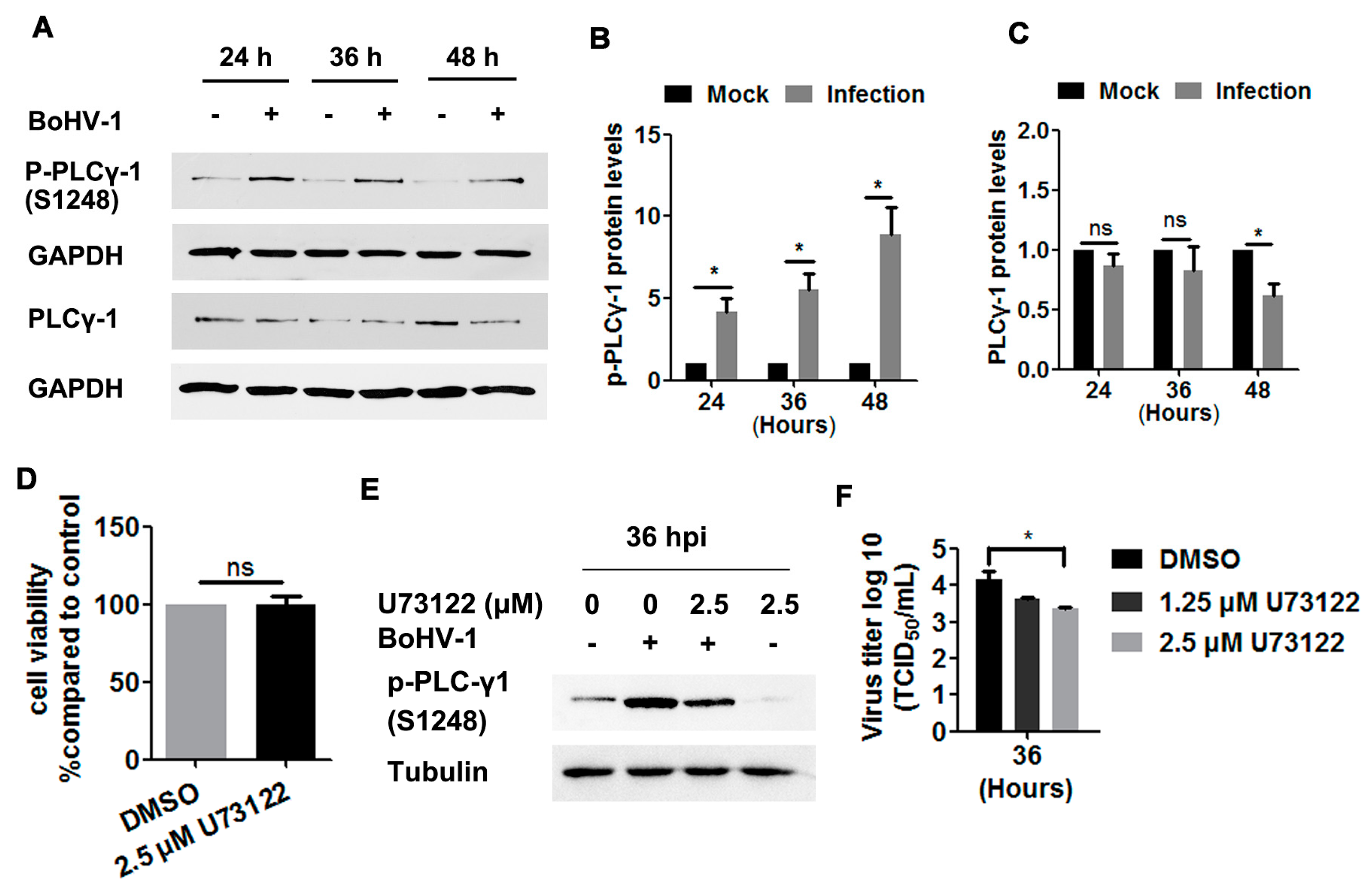
3.3. Potential Involvement of EGFR Signaling in the Activation of PLC-γ1 by BoHV-1 Infection in A549 Cells
3.4. The Activation of Akt Signaling in BoHV-1-Infected A549 Cells is Independent of EGFR
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Hodgson, P.D.; Aich, P.; Manuja, A.; Hokamp, K.; Roche, F.M.; Brinkman, F.S.; Potter, A.; Babiuk, L.A.; Griebel, P.J. Effect of stress on viral-bacterial synergy in bovine respiratory disease: Novel mechanisms to regulate inflammation. Comp. Funct. Genom. 2005, 6, 244–250. [Google Scholar] [CrossRef]
- Fulton, R.W.; d’Offay, J.M.; Landis, C.; Miles, D.G.; Smith, R.A.; Saliki, J.T.; Ridpath, J.F.; Confer, A.W.; Neill, J.D.; Eberle, R.; et al. Detection and characterization of viruses as field and vaccine strains in feedlot cattle with bovine respiratory disease. Vaccine 2016, 34, 3478–3492. [Google Scholar] [CrossRef]
- Jones, C.; Chowdhury, S. A review of the biology of bovine herpesvirus type 1 (bhv-1), its role as a cofactor in the bovine respiratory disease complex and development of improved vaccines. Anim. Health Res. Rev. 2007, 8, 187–205. [Google Scholar] [CrossRef]
- Jones, C. Bovine Herpesvirus 1 Counteracts Immune Responses and Immune-Surveillance to Enhance Pathogenesis and Virus Transmission. Front. Immunol. 2019, 10, 1008. [Google Scholar] [CrossRef]
- Griffin, D. Economic impact associated with respiratory disease in beef cattle. Vet. Clin. North Am. Food Anim. Pract. 1997, 13, 367–377. [Google Scholar] [CrossRef]
- Chase, C.C.L.; Fulton, R.W.; O’Toole, D.; Gillette, B.; Daly, R.F.; Perry, G.; Clement, T. Bovine herpesvirus 1 modified live virus vaccines for cattle reproduction: Balancing protection with undesired effects. Vet. Microbiol. 2017, 206, 69–77. [Google Scholar] [CrossRef]
- Rodrigues, R.; Cuddington, B.; Mossman, K. Bovine herpesvirus type 1 as a novel oncolytic virus. Cancer Gene Ther. 2010, 17, 344–355. [Google Scholar] [CrossRef]
- Thunuguntla, P.; El-Mayet, F.S.; Jones, C. Bovine herpesvirus 1 can efficiently infect the human (SH-SY5Y) but not the mouse neuroblastoma cell line (Neuro-2A). Virus Res. 2017, 232, 1–5. [Google Scholar] [CrossRef]
- Cardoso, T.C.; Rosa, A.C.; Ferreira, H.L.; Okamura, L.H.; Oliveira, B.R.; Vieira, F.V.; Silva-Frade, C.; Gameiro, R.; Flores, E.F. Bovine herpesviruses induce different cell death forms in neuronal and glial-derived tumor cell cultures. J. Neurovirol. 2016, 22, 725–735. [Google Scholar] [CrossRef]
- Steck, P.A.; Lee, P.; Hung, M.C.; Yung, W.K. Expression of an altered epidermal growth factor receptor by human glioblastoma cells. Cancer Res. 1988, 48, 5433–5439. [Google Scholar]
- Abou-Faycal, C.; Hatat, A.S.; Gazzeri, S.; Eymin, B. Splice Variants of the RTK Family: Their Role in Tumour Progression and Response to Targeted Therapy. Int. J. Mol. Sci. 2017, 18, 383. [Google Scholar] [CrossRef] [PubMed]
- Downward, J.; Parker, P.; Waterfield, M.D. Autophosphorylation sites on the epidermal growth factor receptor. Nature 1984, 311, 483–485. [Google Scholar] [CrossRef] [PubMed]
- Hackel, P.O.; Zwick, E.; Prenzel, N.; Ullrich, A. Epidermal growth factor receptors: Critical mediators of multiple receptor pathways. Curr. Opin. Cell Biol. 1999, 11, 184–189. [Google Scholar] [CrossRef]
- Buehler, J.; Zeltzer, S.; Reitsma, J.; Petrucelli, A.; Umashankar, M.; Rak, M.; Zagallo, P.; Schroeder, J.; Terhune, S.; Goodrum, F. Opposing Regulation of the EGF Receptor: A Molecular Switch Controlling Cytomegalovirus Latency and Replication. PLoS Pathog. 2016, 12, e1005655. [Google Scholar] [CrossRef] [PubMed]
- Eierhoff, T.; Hrincius, E.R.; Rescher, U.; Ludwig, S.; Ehrhardt, C. The epidermal growth factor receptor (EGFR) promotes uptake of influenza A viruses (IAV) into host cells. PLoS Pathog. 2010, 6, e1001099. [Google Scholar] [CrossRef] [PubMed]
- Iwamoto, M.; Saso, W.; Sugiyama, R.; Ishii, K.; Ohki, M.; Nagamori, S.; Suzuki, R.; Aizaki, H.; Ryo, A.; Yun, J.H.; et al. Epidermal growth factor receptor is a host-entry cofactor triggering hepatitis B virus internalization. Proc. Natl. Acad. Sci. USA 2019, 116, 8487–8492. [Google Scholar] [CrossRef]
- Kalinowski, A.; Ueki, I.; Min-Oo, G.; Ballon-Landa, E.; Knoff, D.; Galen, B.; Lanier, L.L.; Nadel, J.A.; Koff, J.L. EGFR activation suppresses respiratory virus-induced IRF1-dependent CXCL10 production. Am. J. Physiol. Lung Cell Mol. Physiol. 2014, 307, 186–196. [Google Scholar] [CrossRef]
- Liu, K.; Gualano, R.C.; Hibbs, M.L.; Anderson, G.P.; Bozinovski, S. Epidermal growth factor receptor signaling to Erk1/2 and STATs control the intensity of the epithelial inflammatory responses to rhinovirus infection. J. Biol. Chem. 2008, 283, 9977–9985. [Google Scholar] [CrossRef]
- Zhu, L.; Yuan, C.; Ding, X.; Jones, C.; Zhu, G. The role of phospholipase C signaling in bovine herpesvirus 1 infection. Vet. Res. 2017, 48, 45. [Google Scholar] [CrossRef]
- Zhu, L.; Yuan, C.; Huang, L.; Ding, X.; Wang, J.; Zhang, D.; Zhu, G. The activation of p38MAPK and JNK pathways in bovine herpesvirus 1 infected MDBK cells. Vet. Res. 2016, 47, 91. [Google Scholar] [CrossRef]
- Ohm, A.M.; Affandi, T.; Reyland, M.E. EGF receptor and PKCdelta kinase activate DNA damage-induced pro-survival and pro-apoptotic signaling via biphasic activation of ERK and MSK1 kinases. J. Biol. Chem. 2019, 294, 4488–4497. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Fu, X.; Yuan, C.; Jiang, X.; Zhang, G. Induction of Oxidative DNA Damage in Bovine Herpesvirus 1 Infected Bovine Kidney Cells (MDBK Cells) and Human Tumor Cells (A549 Cells and U2OS Cells). Viruses 2018, 10, 393. [Google Scholar] [CrossRef] [PubMed]
- Antonicelli, A.; Cafarotti, S.; Indini, A.; Galli, A.; Russo, A.; Cesario, A.; Lococo, F.M.; Russo, P.; Mainini, A.F.; Bonifati, L.G.; et al. EGFR-targeted therapy for non-small cell lung cancer: Focus on EGFR oncogenic mutation. Int. J. Med. Sci. 2013, 10, 320–330. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Lee, J.C.; Lin, L.; Olivas, V.; Au, V.; LaFramboise, T.; Abdel-Rahman, M.; Wang, X.; Levine, A.D.; Rho, J.K.; et al. Activation of the AXL kinase causes resistance to EGFR-targeted therapy in lung cancer. Nature Genet. 2012, 44, 852–860. [Google Scholar] [CrossRef] [PubMed]
- Ladanyi, M.; Pao, W. Lung adenocarcinoma: Guiding EGFR-targeted therapy and beyond. Mod. Pathol. 2008, 21, S16–S22. [Google Scholar] [CrossRef]
- Liu, T.C.; Jin, X.; Wang, Y.; Wang, K. Role of epidermal growth factor receptor in lung cancer and targeted therapies. Am. J. Cancer Res. 2017, 7, 187–202. [Google Scholar]
- Zhu, L.; Yu, Y.; Jiang, X.; Yuan, W.; Zhu, G. First report of bovine herpesvirus 1 isolation from bull semen samples in China. Acta Virol. 2017, 61, 483–486. [Google Scholar] [CrossRef]
- Becatti, M. Oxidative stress and high-mobility group box 1 (HMGB1) protein release in vitiligo. Br. J. Dermatol. 2017, 176, 1436–1437. [Google Scholar] [CrossRef]
- Zhu, L.; Ly, H.; Liang, Y. PLC-gamma1 signaling plays a subtype-specific role in postbinding cell entry of influenza A virus. J. Virol. 2014, 88, 417–424. [Google Scholar] [CrossRef]
- Kitazaki, T.; Oka, M.; Nakamura, Y.; Tsurutani, J.; Doi, S.; Yasunaga, M.; Takemura, M.; Yabuuchi, H.; Soda, H.; Kohno, S. Gefitinib, an EGFR tyrosine kinase inhibitor, directly inhibits the function of P-glycoprotein in multidrug resistant cancer cells. Lung Cancer 2005, 49, 337–343. [Google Scholar] [CrossRef]
- Hu, L.; Zaloudek, C.; Mills, G.B.; Gray, J.; Jaffe, R.B. In vivo and in vitro ovarian carcinoma growth inhibition by a phosphatidylinositol 3-kinase inhibitor (LY294002). Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2000, 6, 880–886. [Google Scholar]
- Fiorito, F.; Iovane, V.; Cantiello, A.; Marullo, A.; De Martino, L.; Iovane, G. MG-132 reduces virus release in Bovine herpesvirus-1 infection. Sci. Rep. 2017, 7, 13306. [Google Scholar] [CrossRef] [PubMed]
- Sprague, L.; Lee, J.M.; Hutzen, B.J.; Wang, P.Y.; Chen, C.Y.; Conner, J.; Braidwood, L.; Cassady, K.A.; Cripe, T.P. High Mobility Group Box 1 Influences HSV1716 Spread and Acts as an Adjuvant to Chemotherapy. Viruses 2018, 10, 132. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Yuan, C.; Ding, X.; Xu, S.; Yang, J.; Liang, Y.; Zhu, Q. PLC-gamma1 is involved in the inflammatory response induced by influenza A virus H1N1 infection. Virology 2016, 496, 131–137. [Google Scholar] [CrossRef] [PubMed]
- Xie, Z.; Chen, Y.; Liao, E.Y.; Jiang, Y.; Liu, F.Y.; Pennypacker, S.D. Phospholipase C-gamma1 is required for the epidermal growth factor receptor-induced squamous cell carcinoma cell mitogenesis. Biochem. Biophys. Res. Commun. 2010, 397, 296–300. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, B.; Tyagi, A.; Sharma, A.K.; Cai, L.; Ankem, M.; Damodaran, C. Molecular insights: Suppression of EGFR and AKT activation by a small molecule in non-small cell lung cancer. Genes Cancer 2017, 8, 713–724. [Google Scholar] [CrossRef]
- Zhu, L.; Ding, X.; Zhu, X.; Meng, S.; Wang, J.; Zhou, H.; Duan, Q.; Tao, J.; Schifferli, D.M.; Zhu, G. Biphasic activation of PI3K/Akt and MAPK/Erk1/2 signaling pathways in bovine herpesvirus type 1 infection of MDBK cells. Vet. Res. 2011, 42, 57. [Google Scholar] [CrossRef]
- Zheng, K.; Kitazato, K.; Wang, Y. Viruses exploit the function of epidermal growth factor receptor. Rev. Med. Virol. 2014, 24, 274–286. [Google Scholar] [CrossRef]
- Wang, X.; Huong, S.M.; Chiu, M.L.; Raab-Traub, N.; Huang, E.S. Epidermal growth factor receptor is a cellular receptor for human cytomegalovirus. Nature 2003, 424, 456–461. [Google Scholar] [CrossRef]
- Weller, M.L.; Amornphimoltham, P.; Schmidt, M.; Wilson, P.A.; Gutkind, J.S.; Chiorini, J.A. Epidermal growth factor receptor is a co-receptor for adeno-associated virus serotype 6. Nature Med. 2010, 16, 662–664. [Google Scholar] [CrossRef]
- Zheng, K.; Xiang, Y.; Wang, X.; Wang, Q.; Zhong, M.; Wang, S.; Wang, X.; Fan, J.; Kitazato, K.; Wang, Y. Epidermal growth factor receptor-PI3K signaling controls cofilin activity to facilitate herpes simplex virus 1 entry into neuronal cells. mBio 2014, 5, e00958-13. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.N.; Yamaguchi, H.; Hsu, J.M.; Hung, M.C. Nuclear trafficking of the epidermal growth factor receptor family membrane proteins. Oncogene 2010, 29, 3997–4006. [Google Scholar] [CrossRef] [PubMed]
- Faria, J.; de Andrade, C.; Goes, A.M.; Rodrigues, M.A.; Gomes, D.A. Effects of different ligands on epidermal growth factor receptor (EGFR) nuclear translocation. Biochem. Biophys. Res. Commun. 2016, 478, 39–45. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.Y.; Makino, K.; Xia, W.; Matin, A.; Wen, Y.; Kwong, K.Y.; Bourguignon, L.; Hung, M.C. Nuclear localization of EGF receptor and its potential new role as a transcription factor. Nat. Cell Biol. 2001, 3, 802–808. [Google Scholar] [CrossRef] [PubMed]
- Collier, J.B.; Whitaker, R.M.; Eblen, S.T.; Schnellmann, R.G. Rapid Renal Regulation of Peroxisome Proliferator-activated Receptor gamma Coactivator-1alpha by Extracellular Signal-Regulated Kinase 1/2 in Physiological and Pathological Conditions. J. Biol. Chem. 2016, 291, 26850–26859. [Google Scholar] [CrossRef] [PubMed]
- Vink, E.I.; Lee, S.; Smiley, J.R.; Mohr, I. Remodeling mTORC1 Responsiveness to Amino Acids by the Herpes Simplex Virus UL46 and Us3 Gene Products Supports Replication during Nutrient Insufficiency. J. Virol. 2018, 92. [Google Scholar] [CrossRef]
- Diehl, N.; Schaal, H. Make yourself at home: Viral hijacking of the PI3K/Akt signaling pathway. Viruses 2013, 5, 3192–3212. [Google Scholar] [CrossRef]
- Giard, D.J.; Aaronson, S.A.; Todaro, G.J.; Arnstein, P.; Kersey, J.H.; Dosik, H.; Parks, W.P. In vitro cultivation of human tumors: Establishment of cell lines derived from a series of solid tumors. J. Natl. Cancer Inst. 1973, 51, 1417–1423. [Google Scholar] [CrossRef]
- Zhu, L.; Yang, S.; Tong, W.; Zhu, J.; Yu, H.; Zhou, Y.; Morrison, R.B.; Tong, G. Control of the PI3K/Akt pathway by porcine reproductive and respiratory syndrome virus. Arch. Virol. 2013, 158, 1227–1234. [Google Scholar] [CrossRef]
- Siddiqui, S.; Fang, M.; Ni, B.; Lu, D.; Martin, B.; Maudsley, S. Central role of the EGF receptor in neurometabolic aging. Int. J. Endocrinol. 2012, 2012, 739428. [Google Scholar] [CrossRef]
- Cuddington, B.P.; Dyer, A.L.; Workenhe, S.T.; Mossman, K.L. Oncolytic bovine herpesvirus type 1 infects and kills breast tumor cells and breast cancer-initiating cells irrespective of tumor subtype. Cancer Gene Ther. 2013, 20, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Cuddington, B.P.; Mossman, K.L. Oncolytic bovine herpesvirus type 1 as a broad spectrum cancer therapeutic. Curr. Opin. Virol. 2015, 13, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Iradyan, M.; Iradyan, N.; Hulin, P.; Hambardzumyan, A.; Gyulkhandanyan, A.; de Alves Sousa, R.; Hessani, A.; Roussakis, C.; Bollot, G.; Bauvais, C.; et al. Targeting Degradation of EGFR through the Allosteric Site Leads to Cancer Cell Detachment-Promoted Death. Cancers Basel 2019, 11, 1094. [Google Scholar] [CrossRef] [PubMed]
- Herbst, R.S. Review of epidermal growth factor receptor biology. Int. J. Radiat. Oncol. Biol. Phys. 2004, 59, 21–26. [Google Scholar] [CrossRef] [PubMed]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Qiu, W.; Chang, L.; He, Y.; Zhu, L. The Role of Epidermal Growth Factor Receptor Signaling Pathway during Bovine Herpesvirus 1 Productive Infection in Cell Culture. Viruses 2020, 12, 927. https://doi.org/10.3390/v12090927
Qiu W, Chang L, He Y, Zhu L. The Role of Epidermal Growth Factor Receptor Signaling Pathway during Bovine Herpesvirus 1 Productive Infection in Cell Culture. Viruses. 2020; 12(9):927. https://doi.org/10.3390/v12090927
Chicago/Turabian StyleQiu, Wencai, Long Chang, Yongming He, and Liqian Zhu. 2020. "The Role of Epidermal Growth Factor Receptor Signaling Pathway during Bovine Herpesvirus 1 Productive Infection in Cell Culture" Viruses 12, no. 9: 927. https://doi.org/10.3390/v12090927
APA StyleQiu, W., Chang, L., He, Y., & Zhu, L. (2020). The Role of Epidermal Growth Factor Receptor Signaling Pathway during Bovine Herpesvirus 1 Productive Infection in Cell Culture. Viruses, 12(9), 927. https://doi.org/10.3390/v12090927




