Alternaria alternata Accelerates Loss of Alveolar Macrophages and Promotes Lethal Influenza A Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mice
2.2. Fungal Filtrate Challenge and Inf A Infection
2.3. Virus Titration by qPCR
2.4. Lung Wet-to-Dry Measurements
2.5. Lung Histopathology and Immunohistochemistry
2.6. Immunoreactive Cytokines in BAL Fluid
2.7. Flow Cytometry
2.8. Mouse Tracheal Epithelial Cell Cultures
2.9. Protease Activity Assays
2.10. Ribonuclease Activity Assays
2.11. Bacterial Colony Forming Units (CFU)
2.12. Administration of Lactobacillus plantarum to the Respiratory Tract
2.13. Statistical Evaluation
3. Results and Discussion
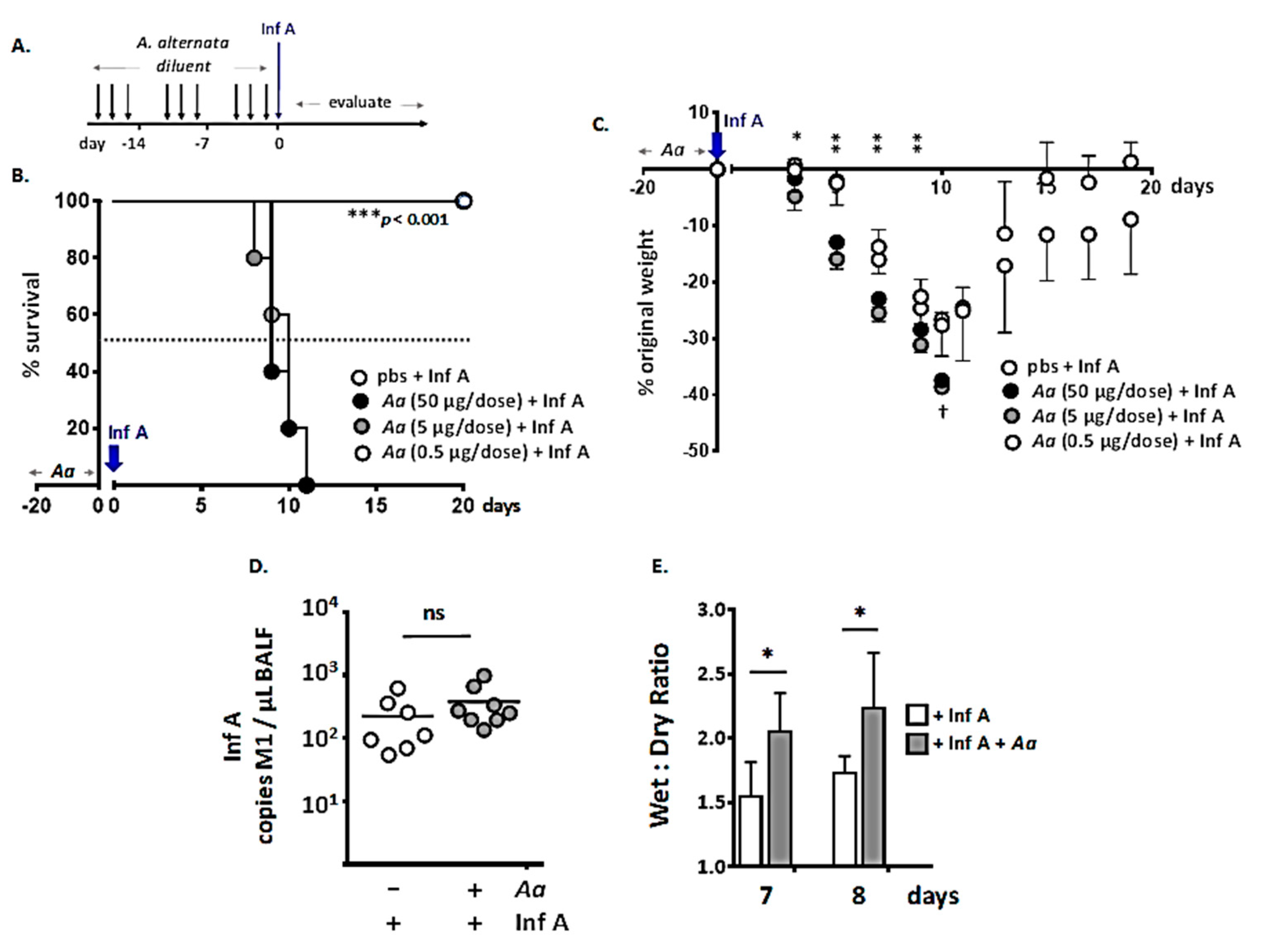
3.1. Repetitive Administration of A. alternata Increases Susceptibility to Lethal Inf A Infection
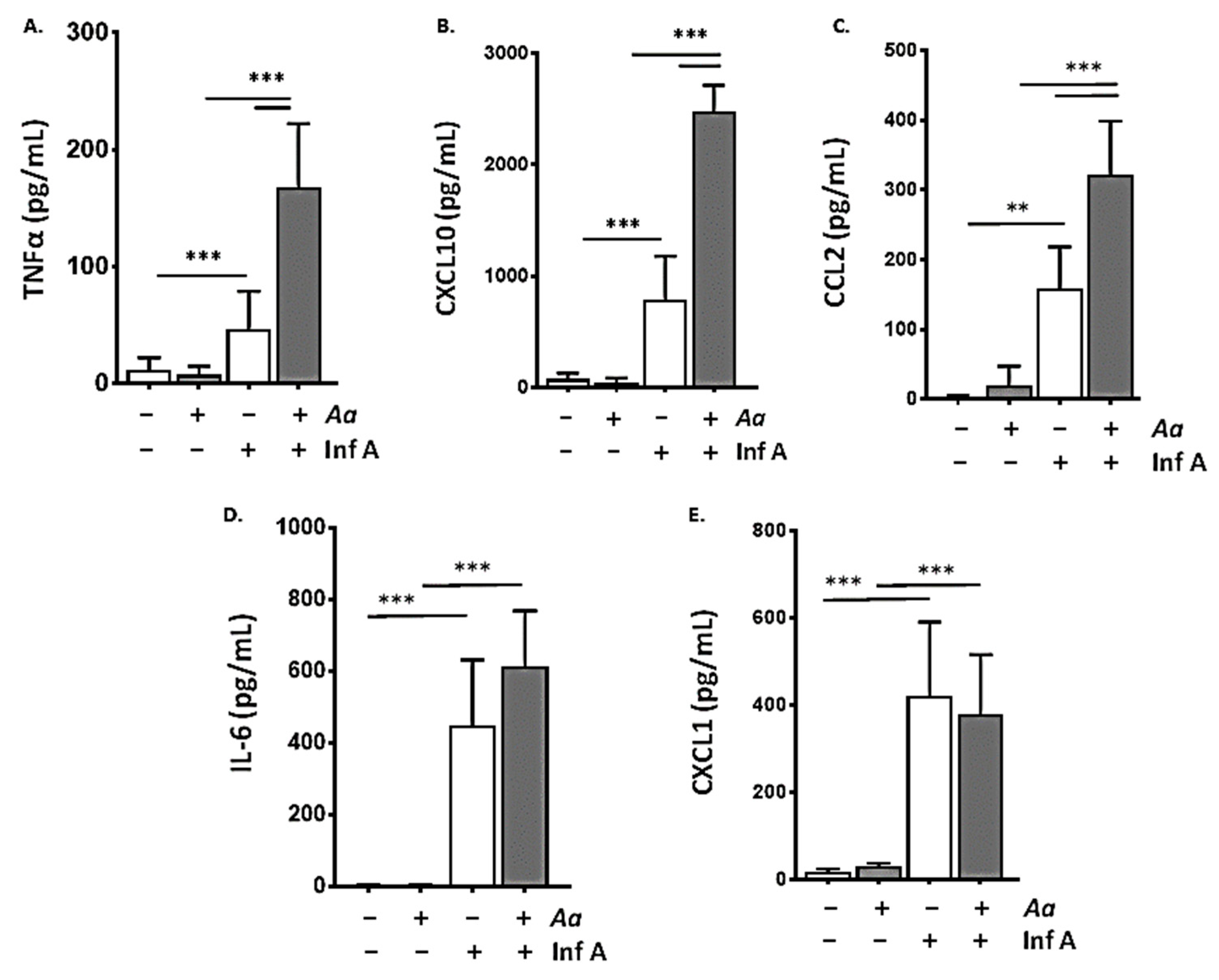
3.2. Repetitive Administration of A. alternata Amplifies the Biochemical Inflammatory Response to Infection with Inf A
3.3. Repetitive Administration of A. alternata Accelerates Depletion of Alveolar Macrophages in Response to Infection with Inf A
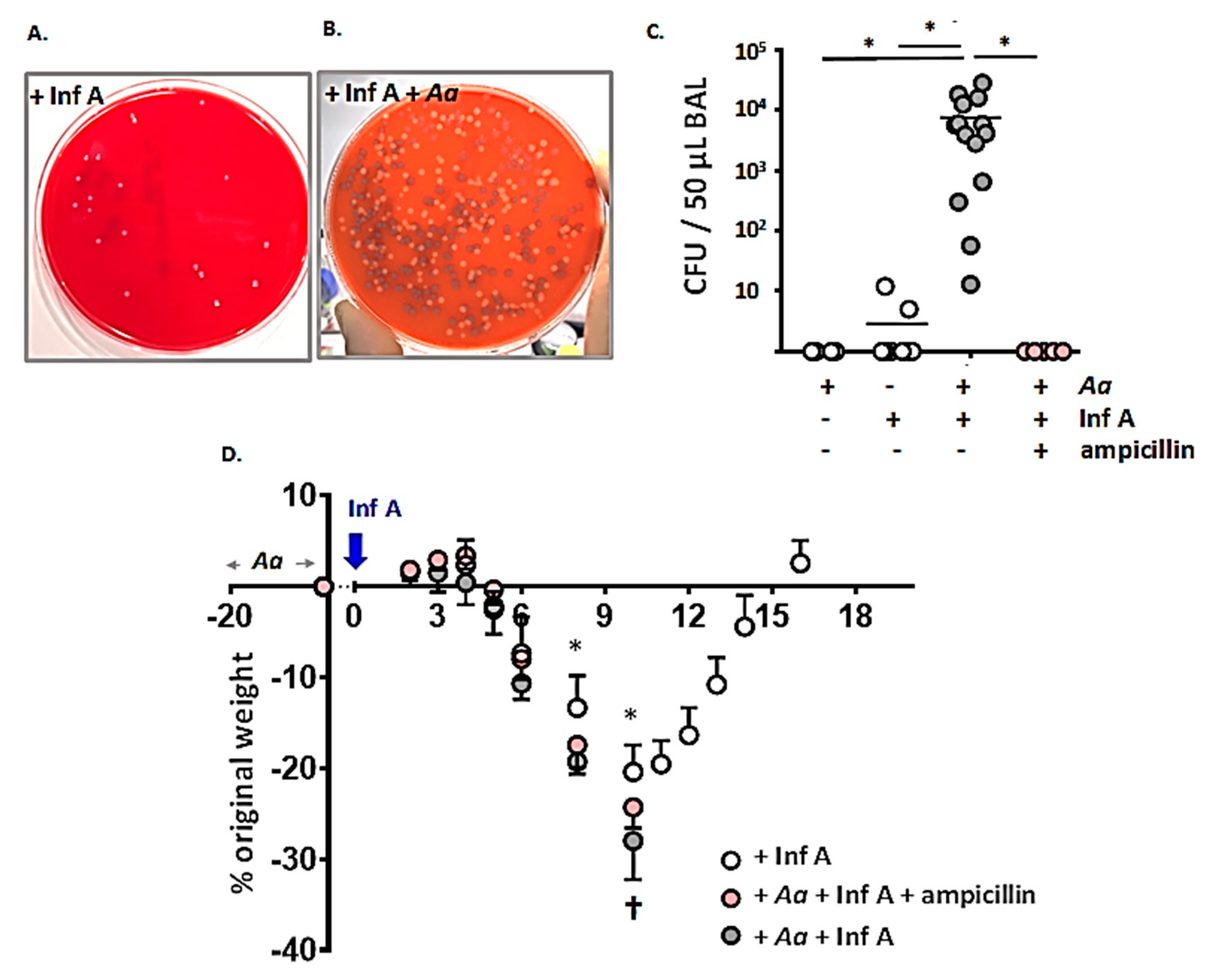
3.4. Spontaneous Accumulation of Bacteria in the Airways of A. alternata-Challenged Inf A-Infected Mice
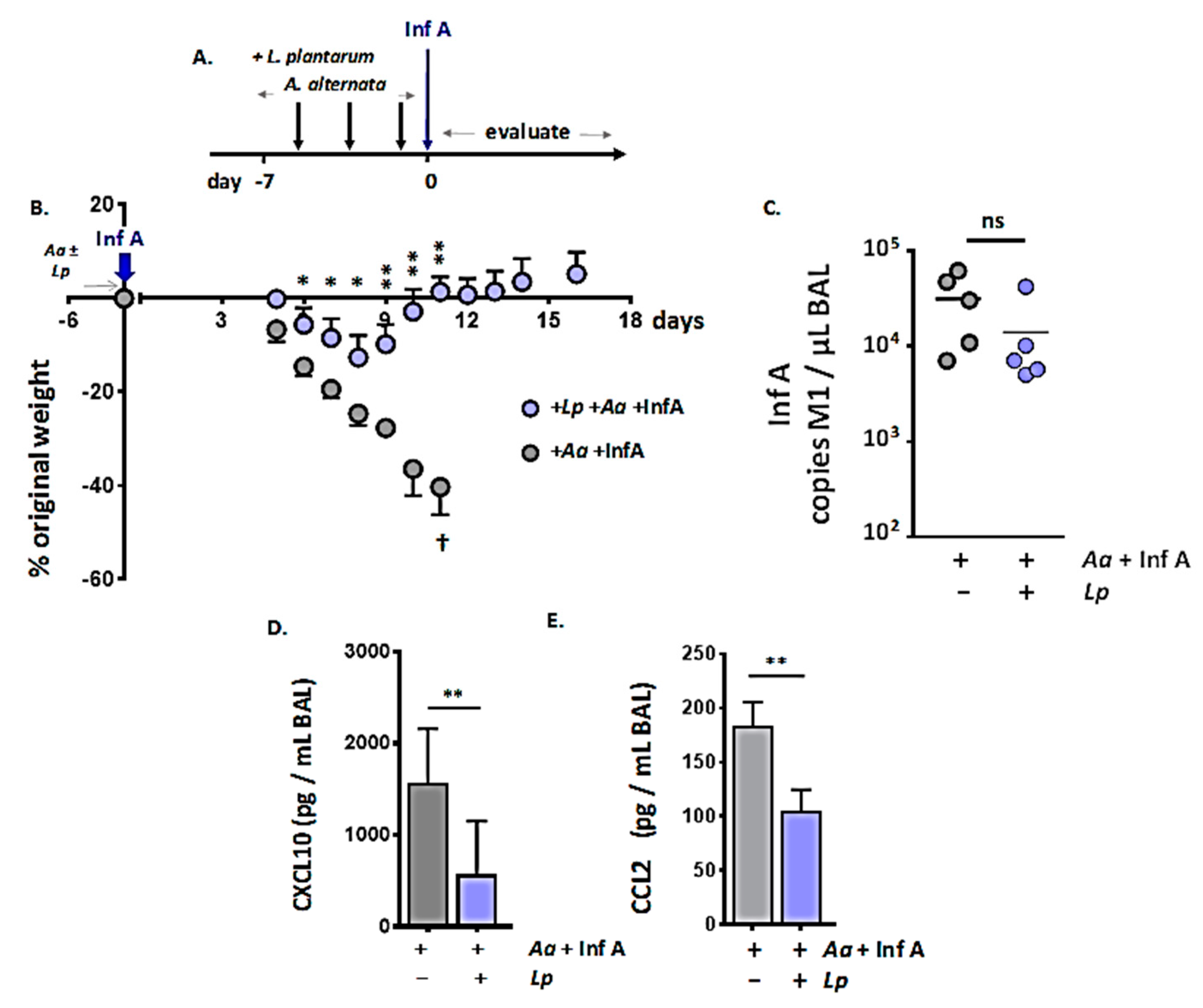
3.5. Administration of Immunobiotic Lactobacillus plantarum Directly to the Respiratory Mucosa Protects against the Lethal Impact of A. alternata
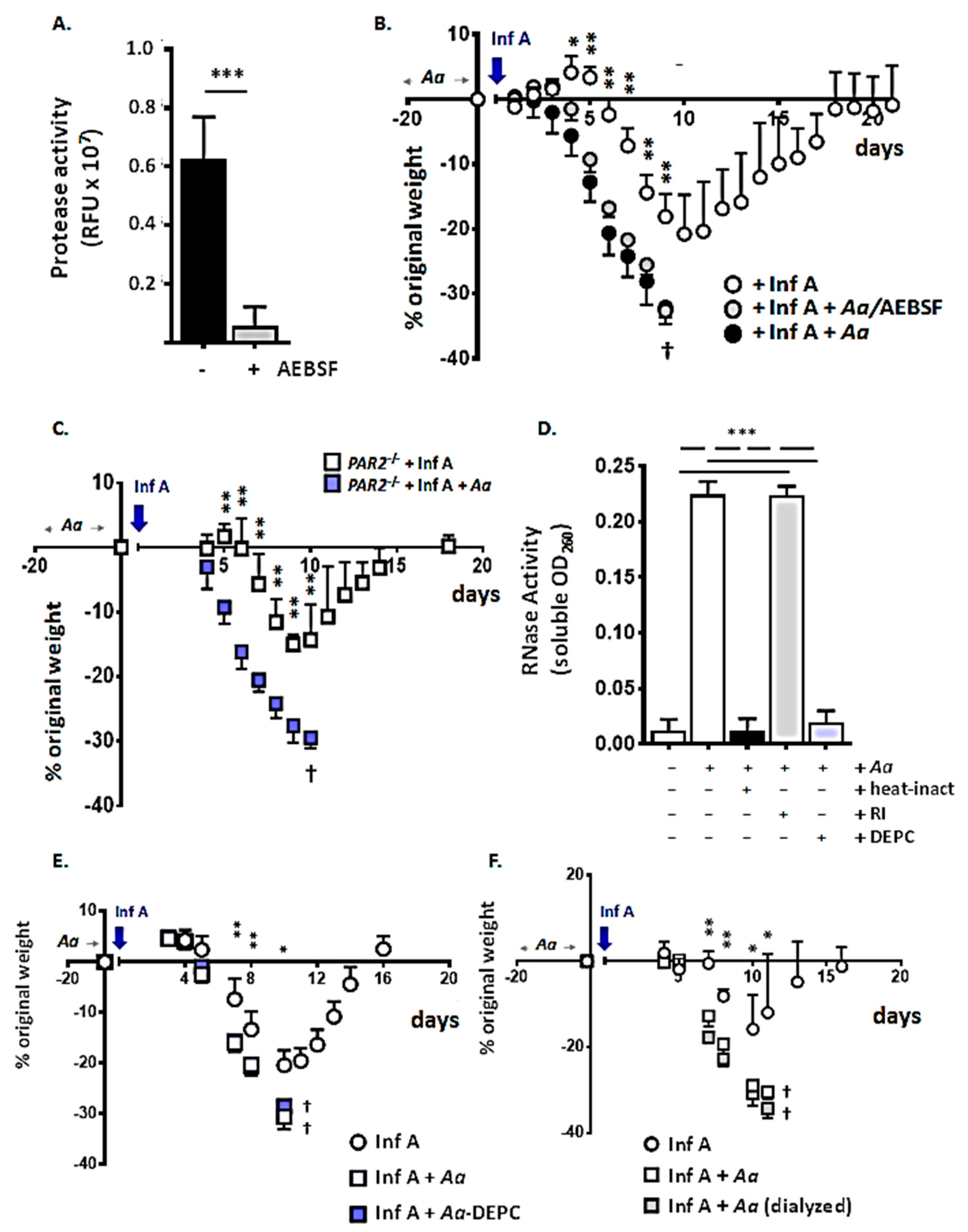
3.6. Proinflammatory Components of the A. alternata Filtrate: Serine Proteases, RNases, and Low Molecular Weight Biomolecules
3.7. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- U.S. Centers for Disease Control and Prevention. Disease Burden of Influenza. Available online: https://www.cdc.gov/flu/about/burden/index.html (accessed on 25 July 2020).
- Kalil, A.C.; Thomas, P.G. Influenza virus-related critical illness: Pathophysiology and epidemiology. Crit. Care 2019, 23, 258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baxi, S.N.; Portnoy, J.M.; Larenas-Linnemann, D.; Phipatanakui, W. Environmental Allergens Workgroup. Exposure and health effects of fungi on humans. J. Allergy Clin. Immunol. Pract. 2016, 4, 396–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartemes, K.R.; Kita, H. Innate and adaptive immune responses to fungi in the airway. J. Allergy Clin Immunol. 2018, 142, 353–363. [Google Scholar] [CrossRef] [Green Version]
- Bush, R. The Role of Fungi (Molds) in Human Disease; Wolters Kluwer: Alphen aan den Rijn, The Netherlands, 2019; Available online: http://www.uptodate.com (accessed on 25 July 2020).
- O’Hollaren, M.T.; Yunginger, J.W.; Offord, K.P.; Somers, M.J.; O’Connell, E.J.; Ballard, D.J.; Sachs, M.I. Exposure to an aeroallergen as a possible precipitating factor in respiratory arrest in young patients with asthma. N. Engl. J. Med. 1991, 324, 359–363. [Google Scholar] [CrossRef] [PubMed]
- Fukutomi, Y.; Taniguchi, M. Sensitization to fungal allergens: Resolved and unresolved issues. Allergol. Int. 2015, 64, 321–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasser, S.M.; Pulimood, T.B. Allergens and thunderstorm asthma. Curr. Allergy Asthma Rep. 2009, 9, 384–390. [Google Scholar] [CrossRef]
- Debeuf, N.; Haspeslagh, E.; van Helden, M.; Hammad, H.; Lambrecht, B.N. Mouse models of asthma. Curr. Protoc. Mouse Biol. 2016, 6, 169–184. [Google Scholar] [CrossRef]
- Bartemes, K.R.; Iijima, K.; Kobayashi, T.; Kephart, G.M.; McKenzie, A.N.; Kita, H. IL-33-resposive lineage-CD25+CD44hi lymphoid cells mediate innate type 2 immunity and allergy inflammation in the lungs. J. Immunol. 2012, 188, 1503–1513. [Google Scholar] [CrossRef] [Green Version]
- Iijima, K.; Kobayashi, T.; Hara, K.; Kephart, G.M.; Ziegler, S.F.; McKenzie, A.N.; Kita, H. IL-33 and thymic stromal lymphopoietin mediate immune pathology in response to chronic airborne allergen exposure. J. Immunol. 2014, 193, 1549–1559. [Google Scholar] [CrossRef]
- Boitano, S.; Flynn, A.N.; Sherwood, C.L.; Schulz, S.M.; Hoffman, J.; Gruzinova, I.; Daines, M.O. Alternaria alternata serine proteases induce lung inflammation and airway epithelial cell activation via PAR2. Am. J. Physiol. Lung Cell Mol. Physiol. 2011, 300, L605–L614. [Google Scholar] [CrossRef] [Green Version]
- Yee, M.C.; Nichols, H.L.; Polley, D.; Saifeddine, M.; Pal, K.; Lee, K.; Wilson, E.H.; Daines, M.O.; Hollenberg, M.D.; Boitano, S.; et al. Protease activated receptor-2 signaling through beta-arrestin-2 mediates Alternaria alkaline serine protease-induced airway inflammation. Am. J. Phsiol. Lung Cell Mol. Physiol. 2018, 315, L1042–L1057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Babiceanu, M.C.; Howard, B.A.; Rumore, A.C.; Kita, H.; Lawrence, C.B. Analysis of global gene expression changes in human bronchial epithelial cells exposed to spores of the allergenic fungus, Alternaria alternata. Front. Microbiol. 2013, 4, 196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daines, M.; Zhu, L.; Pereira, R.; Zhou, X.; Bondy, C.; Pryor, B.M.; Zhou, J.; Chen, Y. Alternaria induces airway epithelial cytokine expression independent of protease-activated receptor. Respirology 2020, 25, 502–510. [Google Scholar] [CrossRef] [PubMed]
- Ma, M.; Redes, J.L.; Percopo, C.M.; Druey, K.M.; Rosenberg, H.F. Alternaria alternata at the nasal mucosa results in eosinophilic inflammation and increased susceptibility to influenza virus infection. Clin. Exp. Allergy 2018, 48, 691–702. [Google Scholar] [CrossRef] [PubMed]
- Percopo, C.M.; Dyer, K.D.; Ochkur, S.I.; Luo, J.L.; Fischer, E.R.; Lee, J.J.; Lee, N.A.; Domachowske, J.B.; Rosenberg, H.F. Activated mouse eosinophils protect against lethal respiratory virus infection. Blood 2014, 123, 743–752. [Google Scholar] [CrossRef]
- Phipps, S.; Lam, C.E.; Mahalingam, S.; Newhouse, M.; Ramirez, R.; Rosenberg, H.F.; Foster, P.S.; Matthaei, K.I. Eosinophils contribute to innate antiviral immunity and promote clearance of respiratory syncytial virus. Blood 2007, 110, 1578–1586. [Google Scholar] [CrossRef] [Green Version]
- Adamko, D.J.; Yost, B.L.; Gleich, G.J.; Fryer, A.D.; Jacoby, D.B. Ovalbumin sensitization changes the inflammatory response to subsequent parainfluenza infection. Eosinophils mediate airway hyperresponsiveness, m(2) muscarinic receptor dysfunction, and antiviral effects. J. Exp. Med. 1991, 190, 1465–1478. [Google Scholar] [CrossRef] [Green Version]
- Samarasinghe, A.E.; Melo, R.C.; Duan, S.; LeMessurier, K.S.; Liedmann, S.; Surman, S.L.; Lee, J.J.; Hurwitz, J.L.; Thomas, P.G.; McCullers, J.A. Eosinophils promote antiviral immunity in mice infected with Influenza A virus. J. Immunol. 2017, 198, 3214–3226. [Google Scholar] [CrossRef]
- Southam, D.S.; Dolovich, M.; O’Byrne, P.M.; Inman, M.D. Distribution of intranasal instillations in mice: Effects of volume, time, body position and anesthesia. Am. J. Physiol Lung Cell Mol. Physiol. 2002, 282, L833–L834. [Google Scholar] [CrossRef] [Green Version]
- Percopo, C.M.; Dyer, K.D.; Karpe, K.A.; Domachowske, J.B.; Rosenberg, H.F. Eosinophils and respiratory virus infection: A dual-standard curve qRT-PCR-based method for determining virus recovery from mouse lung tissue. Methods Mol. Biol. 2014, 1178, 257–266. [Google Scholar]
- Percopo, C.M.; Ma, M.; Brenner, T.A.; Krumholz, J.O.; Break, T.J.; Laky, K.; Rosenberg, H.F. Critical adverse impact of IL-6 in acute pneumovirus infection. J. Immunol. 2019, 202, 871–882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsuyama, H.; Amaya, F.; Hashimoto, S.; Ueno, H.; Beppu, S.; Mizuta, M.; Shime, N.; Ishizaka, A.; Hashimoto, A. Acute lung inflammation and ventilator-induced lung injury caused by ATP via the P2Y receptors: An experimental study. Respir Res. 2008, 9, 79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- You, Y.; Brody, S.L. Chapter 9: Culture and differentiation of mouse tracheal epithelial cells. Methods Mol. Biol. 2012, 945, 123–143. [Google Scholar]
- Brown, E.G.; Bailly, J.E. Genetic analysis of mouse-adapted influenza A virus identifies roles for the NA, PB1 and PB2 genes in virulence. Virus Res. 1999, 61, 63–76. [Google Scholar] [CrossRef]
- Rosenberg, H.F.; Domachowske, J.B. Assays for detection of RNase A superfamily ribonucleases. Methods Mol. Biol. 2001, 160, 355–362. [Google Scholar] [PubMed]
- Gabryszewski, S.J.; Bachar, O.; Dyer, K.D.; Percopo, C.M.; Killoran, K.E.; Domachowske, J.B.; Rosenberg, H.F. Lactobacillus-mediated priming of the respiratory mucosa protects against lethal pneumovirus infection. J. Immunol. 2011, 186, 1151–1161. [Google Scholar] [CrossRef] [Green Version]
- Kauffmann, H.F.; Tomee, J.F.; van de Riet, M.A.; Timmerman, A.J.; Borger, P. Protease-dependent activation of epithelial cells by fungal allergens leads to morphologic changes and cytokine production. J. Allergy Clin. Immunol. 2000, 105, 1185–1193. [Google Scholar] [CrossRef]
- McCusker, C.; Chicoine, M.; Hamid, Q.; Mazer, B. Site-specific sensitization in a murine model of allergic rhinitis: Role of the upper airway in lower disease. J. Allergy Clin. Immunol. 2002, 110, 891–898. [Google Scholar] [CrossRef]
- Giovannini-Chami, L.; Paquet, A.; Sanfiorenzo, C.; Pons, N.; Cazareth, J.; Magnone, V.; Lebrigand, K.; Chevalier, B.; Vallauri, A.; Julia, V.; et al. The “one airway, one disease” concept in light of Th2 inflammation. Eur. Respir. J. 2018, 52, 1800437. [Google Scholar] [CrossRef]
- de Benedictis, F.M.; Bush, A. Janus looks both ways: How do the upper and lower airways interact? Paediatric. Respir. Rev. 2020, 34, 59–66. [Google Scholar] [CrossRef]
- McCusker, C.T. Use of mouse models of allergic rhinitis to study the upper and lower airway link. Curr. Opin. Allergy Clin. Immunol. 2004, 4, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Samitas, K.; Carter, A.; Kariyawasam, H.H.; Xanthou, G. Upper and lower airway remodeling mechanisms in asthma, allergic rhinitis and chronic rhinosinusitis: The one airway concept revisited. Allergy 2018, 73, 993–1002. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halstead, E.S.; Chroneos, Z.C. Lethal influenza infection: Is a macrophage to blame? Expert Rev. Anti Infect. Ther. 2015, 13, 1425–1428. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghoneim, H.E.; Thomas, P.G.; McCullers, J.A. Depletion of alveolar macrophages during influenza infection facilitates bacterial superinfections. J. Immunol. 2013, 191, 1250–1259. [Google Scholar] [CrossRef] [Green Version]
- Purnama, C.; Ng, S.L.; Tetlak, P.; Setiagani, Y.A.; Kandasamy, M.; Baalasubramanian, S.; Karjalainen, K.; Ruedl, C. Transient ablation of alveolar macrophages leads to massive pathology of influenza infection without affecting cellular adaptive immunity. Eur. J. Immunol. 2014, 44, 2003–2012. [Google Scholar] [CrossRef]
- Schneider, C.; Nobs, S.P.; Heer, A.K.; Kurrer, M.; Klinke, G.; van Rooijen, N.; Vogel, J.; Kopf, M. Alveolar macrophages are essential for protection from respiratory failure and associated morbidity following influenza virus infection. PLoS Pathog. 2014, 10, e1004053. [Google Scholar] [CrossRef] [Green Version]
- Huang, F.F.; Barnes, P.F.; Feng, Y.; Donis, R.; Chroneos, Z.C.; Idell, S.; Allen, T.; Perez, D.R.; Whitsett, J.A.; Dunissi-Joannopoulos, K.; et al. GM-CSF in the lung protects against lethal influenza infection. Am. J. Respir Crit. Care Med. 2011, 184, 259–268. [Google Scholar] [CrossRef]
- Magnen, M.; Gueugnon, F.; Petit-Courty, A.; Baranek, T.; Sizaret, D.; Brewah, Y.A.; Humbles, A.A.; Si-Tahar, M.; Courty, Y. Tissue kallikrein regulates alveolar macrophage apoptosis early in influenza virus infection. Am. J. Physiol. Lung Cell Mol. Physiol. 2019, 316, L1127–L1140. [Google Scholar] [CrossRef]
- Solhaug, A.; Vines, L.L.; Ivanova, L.; Spilsberg, B.; Holme, J.A.; Pestka, J.; Collins, A.; Eriksen, G.S. Mechanisms involved in alternariol-induced cell cycle arrest. Mutat. Res. 2012, 738-739, 1–11. [Google Scholar] [CrossRef]
- Solhaug, A.; Wisbech, C.; Christoffersen, T.E.; Hult, L.O.; Lea, T.; Eriksen, G.S.; Holme, J.A. The mycotoxin alternariol induces DNA damage and modify macrophage phenotype and inflammatory responses. Toxicol. Lett. 2015, 239, 9–21. [Google Scholar] [CrossRef] [Green Version]
- Martin-Loeches, I.; Someren Gréve, F.; Schultz, M.J. Bacterial pneumonia as an influenza complication. Curr. Opin. Infect. Dis. 2017, 30, 201–207. [Google Scholar] [CrossRef] [PubMed]
- Rynda-Apple, A.; Robinson, K.M.; Alcorn, J.F. Influenza and bacterial superinfection: Illuminating the immunologic mechanisms of disease. Infect. Immun. 2015, 83, 3764–3770. [Google Scholar] [CrossRef] [Green Version]
- Jochems, S.P.; Marcon, F.; Carniel, B.F.; Holloway, M.; Mitsi, E.; Smith, E.; Gritzfeld, J.F.; Solórzano, C.; Reiné, J.; Pojar, S.; et al. Inflammation induced by influenza virus impairs human innate immune controls of pneumococcus. Nat. Immunol. 2018, 19, 1299–1308. [Google Scholar] [CrossRef]
- Bansal, S.; Yajjala, V.K.; Bauer, C.; Sun, K. IL-1 signaling prevents alveolar macrophage depletion during influenza and Streptococcus pneumoniae co-infection. J. Immunol. 2018, 200, 1425–1433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, L.N.; Dias, P.; Han, D.; Yoon, S.; Shea, A.; Zakharov, V.; Parham, D.; Sarawar, S.R. A mouse model of lethal synergism between influenza virus and Haemophilus influenzae. Am. J. Pathol. 2010, 176, 800–811. [Google Scholar] [CrossRef] [PubMed]
- Gopal, R.; Lee, B.; McHugh, K.J.; Rich, H.E.; Ramanan, K.; Mandalapu, S.; Clay, M.E.; Seger, P.J.; Enelow, R.I.; Manni, M.L.; et al. STAT2 signaling regulates macrophage phenotype during influenza and bacterial super-infection. Front. Immunol. 2018, 9, 2151. [Google Scholar] [CrossRef] [Green Version]
- Klonoski, J.M.; Hurtig, H.R.; Juber, B.A.; Schuneman, M.J.; Bickett, T.E.; Svendsen, J.M.; Burum, B.; Penfound, T.A.; Sereda, G.; Dale, J.B.; et al. Vaccination against the M protein of Streptococcus pyogenes prevents death after influenza virus: S. pyogenes super-infection. Vaccine 2014, 32, 5241–5249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, S.M.; Bernui, M.; Shen, H.; Akerley, B.J. Genome-wide fitness profiling reveals adaptations required by Haemophilus in coinfection with influenza A virus in the murine lung. Proc. Natl. Acad. Sci. USA 2013, 11, 15413–15418. [Google Scholar] [CrossRef] [Green Version]
- Frank, J.A.; Reich, C.I.; Sharma, S.; Weisbaum, J.S.; Wilson, B.A.; Olsen, G.J. Critical evaluation of two primers commonly used for amplification of bacterial 16S rRNA genes. Appl. Environ. Microbiol. 2008, 74, 2461–2470. [Google Scholar] [CrossRef] [Green Version]
- Izumo, T.; Maekawa, T.; Ida, M.; Noguchi, A.; Kitagawa, Y.; Shibata, H.; Yasui, H.; Kiso, Y. Effect of intranasal administration of Lactobacillus pentosus S-PT84 on influenza virus infection in mice. Int. Immunopharmacol. 2010, 10, 1101–1106. [Google Scholar] [CrossRef]
- Tomosada, Y.; Chiba, E.; Zelaya, H.; Takahashi, T.; Tsukida, K.; Kitazawa, H.; Alvarez, S.; Villena, J. Nasally administered Lactobacillus rhamnosus strains differentially modulate respiratory antiviral immune responses and induce protection against respiratory syncytial virus infection. BMC Immunol. 2013, 14, 40. [Google Scholar] [CrossRef] [Green Version]
- Park, M.K.; Ngo, V.; Kwon, Y.M.; Lee, Y.T.; Yoo, S.; Cho, Y.H.; Hong, S.M.; Hwang, H.S.; Ko, E.J.; Jung, Y.J.; et al. Lactobacillus plantarum DK119 as a probiotic confers protection against influenza virus by modulating innate immunity. PLoS ONE 2013, 8, e75368. [Google Scholar] [CrossRef] [Green Version]
- Percopo, C.M.; Rice, T.A.; Brenner, T.A.; Dyer, K.D.; Luo, J.L.; Kanakabandi, K.; Sturdevant, D.E.; Porcella, S.F.; Domachowske, J.B.; Keicher, J.D.; et al. Immunobiotic Lactobacillus administered post-exposure averts the lethal sequelae of respiratory virus infection. Antiviral. Res. 2015, 121, 109–119. [Google Scholar] [CrossRef] [Green Version]
- Percopo, C.M.; Ma, M.; Rosenberg, H.F. Administration of immunobiotic Lactobacillus plantarum delays but does not prevent lethal pneumovirus infection in Rag1-/- mice. J. Leukoc. Biol. 2017, 102, 905–913. [Google Scholar] [CrossRef]
- Percopo, C.M.; Dyer, K.D.; Garcia-Crespo, K.E.; Gabryszewski, S.J.; Shaffer, A.L., 3rd; Domachowske, J.B.; Rosenberg, H.F. B cells are not essential for Lactobacillus-mediated protection against lethal pneumovirus infection. J. Immunol. 2014, 192, 5265–5272. [Google Scholar] [CrossRef] [Green Version]
- Rice, T.A.; Brenner, T.A.; Percopo, C.M.; Ma, M.; Keicher, J.D.; Domachowske, J.B.; Rosenberg, H.F. Signaling via pattern recognition receptors NOD2 and TLR2 contributes to immunomodulatory control of lethal pneumovirus infection. Antiviral. Res. 2016, 132, 131–140. [Google Scholar] [CrossRef] [Green Version]
- Snelgrove, R.J.; Gregory, L.G.; Peiro, T.; Akthar, S.; Campbell, G.A.; Walker, S.A.; Lloyd, C.M. Alternaria-derived serine protease activity drives IL-33 mediated asthma exacerbations. J. Allergy Clin. Immunol. 2014, 134, 583–592. [Google Scholar] [CrossRef] [Green Version]
- Balenga, N.A.; Klichinsky, M.; Xie, Z.; Chan, E.C.; Zhao, M.; Jude, J.; Laviolette, M.; Panettieri, R.A., Jr.; Druey, K.M. A fungal protease allergen provokes airway hyper-responsiveness in asthma. Nat. Commun. 2015, 6, 6763. [Google Scholar] [CrossRef]
- Yike, I. Fungal proteases and their pathophysiological effects. Mycopathologia 2011, 171, 299–323. [Google Scholar] [CrossRef]
- Dietz, C.J.; Sun, H.; Yao, W.C.; Citardi, M.J.; Corry, D.B.; Luong, A.U. Aspergillus fumigatus induction of IL-33 expression in chronic rhinosinusitis is PAR2-dependent. Laryngoscope 2019, 129, 2230–2235. [Google Scholar] [CrossRef]
- O’Grady, S.M.; Patil, N.; Melkamu, T.; Maniak, P.J.; Lancto, C.; Kita, H. ATP release and Ca2+ signaling by human bronchial epithelial cells following Alternaria aeroallergen exposure. J. Physiol. 2013, 591, 4595–4609. [Google Scholar] [CrossRef] [PubMed]
- Denis, O.; Vincent, M.; Havaux, X.; De Prins, S.; Treutens, G.; Huygen, K. Induction of the specific allergic immune response is independent of proteases from the fungus Alternaria alternata. Eur. J. Immunol. 2013, 43, 907–917. [Google Scholar] [CrossRef] [PubMed]
- Teijaro, J.R. The role of cytokine responses during influenza virus pathogenesis and potential therapeutic options. Curr. Top. Microbiol. Immunol. 2015, 386, 3–22. [Google Scholar] [PubMed]
- Oldstone, M.B.; Rosen, H. Cytokine storm plays a direct role in the morbidity and mortality from influenza virus infection and is chemically treatable with a single sphingosine-1-phosphate agonist molecule. Curr. Top. Microbiol. Immunol. 2014, 378, 129–147. [Google Scholar]
- Luhtala, N.; Parker, R. T2 family ribonucleases: Ancient enzymes with diverse roles. Trends Biochem. Sci. 2010, 35, 253–259. [Google Scholar] [CrossRef] [Green Version]
- Deshpande, R.A.; Shankar, V. Ribonucleases from the T2 family. Crit. Revs. Microbiol. 2002, 28, 79–122. [Google Scholar] [CrossRef]
- Acquati, F.; Mortara, L.; De Vito, A.; Baci, D.; Albini, A.; Cippitelli, M.; Taramelli, R.; Noonan, D.M. Innate immune response regulation by the human RNASET2 tumor suppressor gene. Front. Immunol. 2019, 10, 2587. [Google Scholar] [CrossRef] [Green Version]
- Rosenberg, H.F. RNase A ribonucleases and host defense: An evolving story. J. Leukoc Biol. 2008, 83, 1079–1087. [Google Scholar] [CrossRef] [Green Version]
- Solhaug, A.; Eriksen, G.S.; Holme, J.A. Mechanisms of action and toxicity of the mycotoxin alternariol: A review. Basic Clin. Pharmacol. Toxicol. 2016, 119, 533–539. [Google Scholar] [CrossRef]
- Meena, M.; Swapnil, P.; Upadhyay, R.S. Isolation, characterization and toxicological potential of Alternaria-mycotoxins (TeA, AOH, and AME) in different Alternaria species from various regions of India. Sci. Rep. 2017, 7, 8777. [Google Scholar] [CrossRef] [Green Version]
- Tsuge, T.; Harimoto, Y.; Akimitsu, K.; Ohtani, K.; Kodama, M.; Akagi, Y.; Equsa, M.; Yamamoto, M.; Otani, H. Host-selective toxins produced by the plant pathogenic fungus Alternaria alternata. FEMS Microbiol. Rev. 2013, 37, 44–66. [Google Scholar] [CrossRef] [PubMed]
- Ostry, V. Alternaria mycotoxins: An overview of chemical characterization, producers, toxicity, analysis and occurrence in foodstuffs. World Mycotoxin J. 2008, 1, 175–188. [Google Scholar] [CrossRef]
- Solhaug, A.; Karlsøen, L.M.; Holme, J.A.; Kristoffersen, A.B.; Eriksen, G.S. Immunomodulatory effects of individual and combined mycotoxins in the THP-1 cell line. Toxicol. In Vitro 2016, 36, 120–132. [Google Scholar] [CrossRef] [Green Version]
- Stark, P.C.; Burge, H.A.; Ryan, L.M.; Milton, D.K.; Gold, D.R. Fungal levels in the home and lower respiratory tract illnesses in the first year of life. Am. J. Respir. Crit. Care Med. 2003, 168, 232–237. [Google Scholar] [CrossRef]
- Biagini, J.M.; LeMasters, G.K.; Ryan, P.H.; Levin, L.; Reponen, T.; Bernstein, D.I.; Villareal, M.; Khurana Hershey, G.K.; Burkel, J.; Lockey, J. Environmental risk factors of rhinitis in early infancy. Pediatr. Allergy Immunol. 2006, 17, 278–284. [Google Scholar] [CrossRef] [Green Version]
- Fisk, W.J.; Eliseeva, E.A.; Mendell, M. Association of residential dampness and mold with respiratory tract infections and bronchitis: A meta-analysis. Env. Health 2010, 9, 72. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.-J.; Ryu, S.-L.; Jung, S.-H.; Shim, J.W.; Kim, D.S.; Jung, H.L.; Park, M.S.; Shim, J.Y. Increased prevalence of H1N1-induced severe lower respiratory tract diseases in children with atopic sensitization. Allergy Asthma Immunol. Res. 2012, 4, 277–283. [Google Scholar] [CrossRef] [Green Version]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Percopo, C.M.; Ma, M.; Mai, E.; Redes, J.L.; Kraemer, L.S.; Minai, M.; Moore, I.N.; Druey, K.M.; Rosenberg, H.F. Alternaria alternata Accelerates Loss of Alveolar Macrophages and Promotes Lethal Influenza A Infection. Viruses 2020, 12, 946. https://doi.org/10.3390/v12090946
Percopo CM, Ma M, Mai E, Redes JL, Kraemer LS, Minai M, Moore IN, Druey KM, Rosenberg HF. Alternaria alternata Accelerates Loss of Alveolar Macrophages and Promotes Lethal Influenza A Infection. Viruses. 2020; 12(9):946. https://doi.org/10.3390/v12090946
Chicago/Turabian StylePercopo, Caroline M., Michelle Ma, Eric Mai, Jamie L. Redes, Laura S. Kraemer, Mahnaz Minai, Ian N. Moore, Kirk M. Druey, and Helene F. Rosenberg. 2020. "Alternaria alternata Accelerates Loss of Alveolar Macrophages and Promotes Lethal Influenza A Infection" Viruses 12, no. 9: 946. https://doi.org/10.3390/v12090946
APA StylePercopo, C. M., Ma, M., Mai, E., Redes, J. L., Kraemer, L. S., Minai, M., Moore, I. N., Druey, K. M., & Rosenberg, H. F. (2020). Alternaria alternata Accelerates Loss of Alveolar Macrophages and Promotes Lethal Influenza A Infection. Viruses, 12(9), 946. https://doi.org/10.3390/v12090946



