Co-Circulation of Multiple Serotypes of Bluetongue Virus in Zambia
Abstract
:1. Introduction
2. Materials and Methods
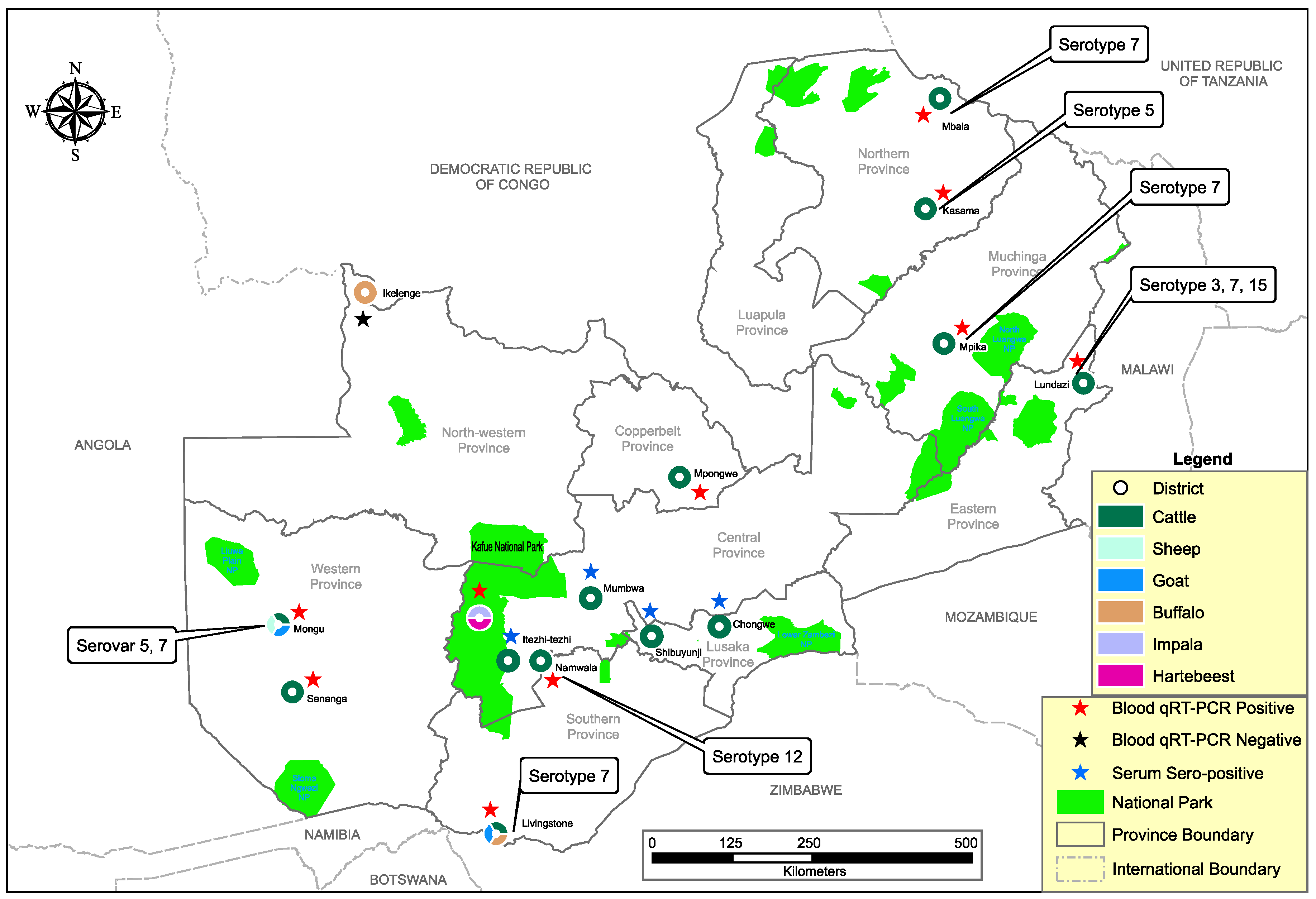
2.1. Study Area
2.2. Blood and Serum Samples
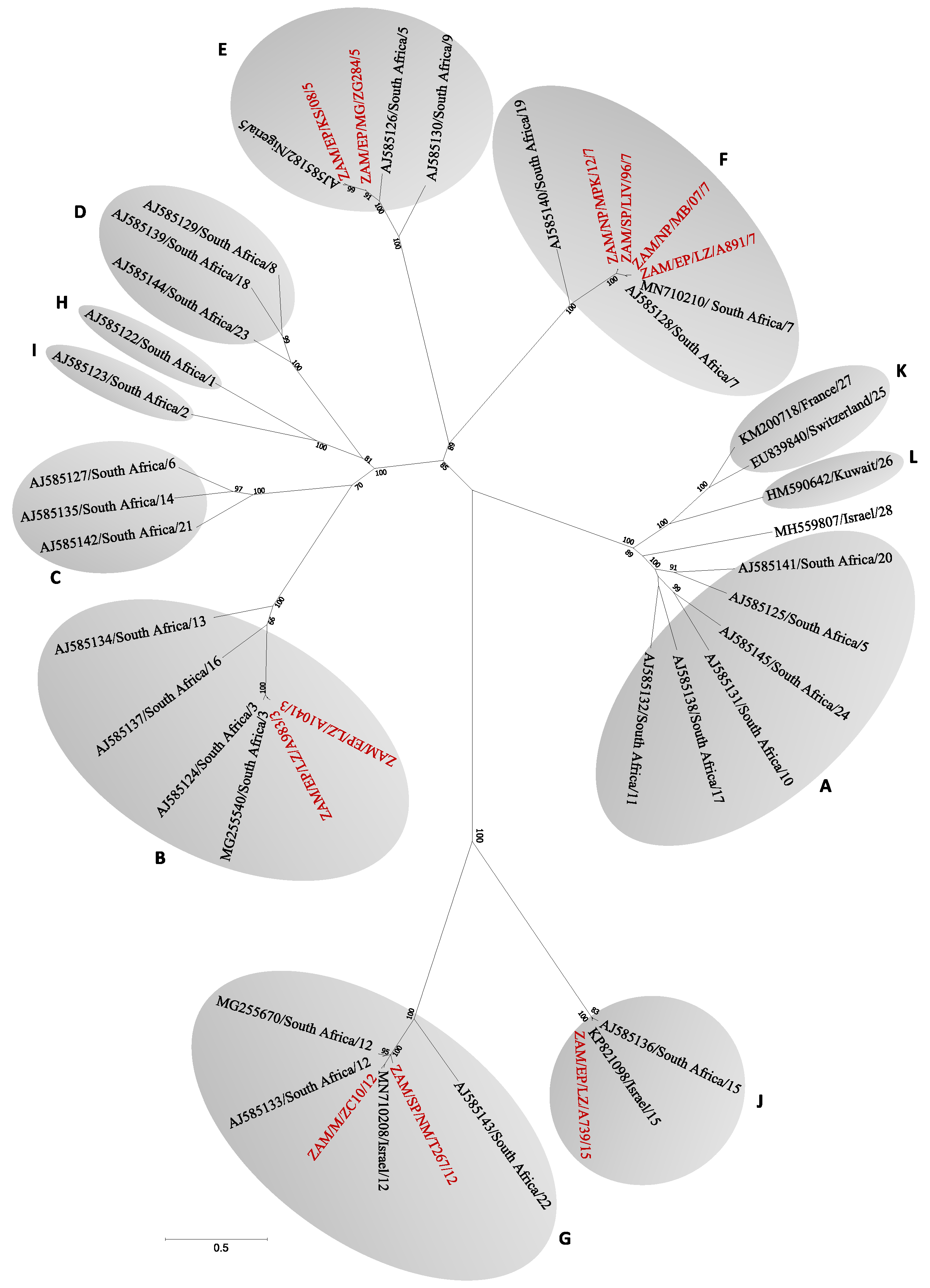
2.3. Sequencing and Phylogenetic Analysis
2.4. Serologic Analysis
3. Results
3.1. Molecular Screening and Phylogenetic Analysis
3.2. BTV Antibodies in Cattle
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Meyer, G.; Lacroux, C.; Léger, S.; Top, S.; Goyeau, K.; Deplanche, M.; Lemaire, M. Lethal bluetongue virus serotype 1 infection in llamas. Emerg. Infect. Dis. 2009, 15, 608–610. [Google Scholar] [CrossRef] [Green Version]
- Alexander, K.A.; MacLachlan, N.J.; Kat, P.W.; House, C.; O’Brien, S.J.; Lerche, N.W.; Sawyer, M.; Frank, L.G.; Holekamp, K.; Smale, L. Evidence of natural bluetongue virus infection among African carnivores. Am. J. Trop. Med. Hyg. 1994, 51, 568–576. [Google Scholar] [CrossRef] [Green Version]
- Erasmus, B.J. Bluetongue virus. Virus Infect. Ruminants 1990, 3, 227–237. [Google Scholar]
- Conraths, F.J.; Gethmann, J.M.; Staubach, C.; Mettenleiter, T.C.; Beer, M.; Hoffmann, B. Epidemiology of bluetongue virus serotype 8, Germany. Emerg. Infect. Dis. 2009, 15, 433–435. [Google Scholar] [CrossRef]
- Darpel, K.E.; Batten, C.A.; Veronesi, E.; Shaw, A.E.; Anthony, S.; Bachanek-Bankowska, K.; Kgosana, L.; bin-Tarif, A.; Carpenter, S.; Müller-Doblies, U.U.; et al. Clinical signs and pathology shown by British sheep and cattle infected with bluetongue virus serotype 8 derived from the 2006 outbreak in northern Europe. Vet. Rec. 2007, 161, 253–261. [Google Scholar] [CrossRef] [Green Version]
- Singer, R.S.; MacLachlan, N.J.; Carpenter, T.E. Maximal predicted duration of viremia in bluetongue virus-infected cattle. J. Vet. Diagnostic. Investig. 2001, 13, 43–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huismans, H.; Erasmus, B.J. Identification of the serotype-specific and group-specific antigens of bluetongue virus. Onderstepoort J. Vet. Res. 1981, 48, 51–58. [Google Scholar]
- Ratinier, M.; Caporale, M.; Golder, M.; Franzoni, G.; Allan, K.; Nunes, S.F.; Armezzani, A.; Bayoumy, A.; Rixon, F.; Shaw, A.; et al. Identification and characterization of a novel non-structural protein of bluetongue virus. PLoS Pathog. 2011, 7, e1002477. [Google Scholar] [CrossRef] [Green Version]
- Belhouchet, M.; Mohd Jaafar, F.; Firth, A.E.; Grimes, J.M.; Mertens, P.P.C.; Attoui, H. Detection of a fourth orbivirus non-structural protein. PLoS ONE 2011, 6, e25697. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maan, S.; Maan, N.S.; Samuel, A.R.; Rao, S.; Attoui, H.; Mertens, P.P.C. Analysis and phylogenetic comparisons of full-length VP2 genes of the 24 bluetongue virus serotypes. J. Gen. Virol. 2007, 88, 621–630. [Google Scholar] [CrossRef] [PubMed]
- Kahlon, J.; Sugiyama, K.; Roy, P. Molecular basis of bluetongue virus neutralization. J. Virol. 1983, 48, 627–632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bumbarov, V.; Golender, N.; Jenckel, M.; Wernike, K.; Beer, M.; Khinich, E.; Zalesky, O.; Erster, O. Characterization of bluetongue virus serotype 28. Transbound. Emerg. Dis. 2020, 67, 171–182. [Google Scholar] [CrossRef] [PubMed]
- Schulz, C.; Bréard, E.; Sailleau, C.; Jenckel, M.; Viarouge, C.; Vitour, D.; Palmarini, M.; Gallois, M.; Höper, D.; Hoffmann, B.; et al. Bluetongue virus serotype 27: Detection and characterization of two novel variants in Corsica, France. J. Gen. Virol. 2016, 97, 2073–2083. [Google Scholar] [CrossRef] [PubMed]
- Maan, S.; Maan, N.S.; van Rijn, P.A.; van Gennip, R.G.P.; Sanders, A.; Wright, I.M.; Batten, C.; Hoffmann, B.; Eschbaumer, M.; Oura, C.A.L.; et al. Full genome characterisation of bluetongue virus serotype 6 from the Netherlands 2008 and comparison to other field and vaccine strains. PLoS ONE 2010, 5, e10323. [Google Scholar] [CrossRef] [PubMed]
- Maan, S.; Maan, N.S.; Nomikou, K.; Veronesi, E.; Bachanek-Bankowska, K.; Belaganahalli, M.N.; Attoui, H.; Mertens, P.P.C. Complete genome characterisation of a novel 26th bluetongue virus serotype from Kuwait. PLoS ONE 2011, 6, e26147. [Google Scholar] [CrossRef] [Green Version]
- Mellor, P.S. The replication of bluetongue virus in Culicoides vectors. Curr. Top. Microbiol. Immunol. 1990, 162, 143–161. [Google Scholar] [CrossRef] [PubMed]
- Venter, G.J.; Paweska, J.T.; Van Dijk, A.A.; Mellor, P.S.; Tabachnick, W.J. Vector competence of Culicoides bolitinos and C. imicola for South African bluetongue virus serotypes 1, 3 and 4. Med. Vet. Entomol. 1998, 12, 378–385. [Google Scholar] [CrossRef]
- Coetzee, P.; Stokstad, M.; Venter, E.H.; Myrmel, M.; Van Vuuren, M. Bluetongue: A historical and epidemiological perspective with the emphasis on South Africa. Virol. J. 2012, 9, 198. [Google Scholar] [CrossRef] [Green Version]
- Haresnape, J.M.; Taylor, W.P.; Lungu, S.A. The epidemiology of bluetongue in Malawi. Epidemiol. Infect. 1988, 100, 493–499. [Google Scholar] [CrossRef] [Green Version]
- Krauss, H.; Roettcher, D.; Weiss, R.; Danner, K.; Hubschle, O.J.B. Wildtiere als Infectionpotential für Nutztiere: Untersuchungen an Wild in Sambia. Giessener Beitrage zur Entwicklungsforschung. R. I 1984, 10, 133–149. [Google Scholar]
- Mweene, A.S.; Pandey, G.S.; Sinyangwe, P.; Nambota, A.; Samui, K.; Kida, H. Viral diseases of livestock in Zambia. Jpn. J. Vet. Res. 1996, 44, 89–105. [Google Scholar] [PubMed]
- Ghirotti, M.; Semproni, G.; De Meneghi, D.; Mungaba, F.N.; Nannini, D.; Calzetta, G.; Paganico, G. Sero-prevalences of selected cattle diseases in the Kafue flats of Zambia. Vet. Res. Commun. 1991, 15, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Kitaoka, S.; Zulu, F.P. Species composition of Culicoides (Diptera: Ceratopogonidae) found at Chilanga near Lusaka, Zambia. Niigata Sangyo Univ. Bull. 1990, 4, 197–206. [Google Scholar]
- Calkins, C.M.; Scasta, J.D. Transboundary Animal Diseases (TADs) affecting domestic and wild African ungulates: African swine fever, foot and mouth disease, Rift Valley fever (1996-2018). Res. Vet. Sci. 2020, 131, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Simulundu, E.; Chambaro, H.M.; Sinkala, Y.; Kajihara, M.; Ogawa, H.; Mori, A.; Ndebe, J.; Dautu, G.; Mataa, L.; Lubaba, C.H.; et al. Co-circulation of multiple genotypes of African swine fever viruses among domestic pigs in Zambia (2013-2015). Transbound. Emerg. Dis. 2018, 65, 114–122. [Google Scholar] [CrossRef]
- Sinkala, Y.; Simuunza, M.; Muma, J.B.; Pfeiffer, D.U.; Kasanga, C.J.; Mweene, A. Foot and mouth disease in Zambia: Spatial and temporal distributions of outbreaks, assessment of clusters and implications for control. Onderstepoort J. Vet. Res. 2014, 81, E1–E6. [Google Scholar] [CrossRef]
- Flannery, J.; Rajko-Nenow, P.; Hicks, H.; Hill, H.; Gubbins, S.; Batten, C. Evaluating the most appropriate pooling ratio for EDTA blood samples to detect Bluetongue virus using real-time RT-PCR. Vet. Microbiol. 2018, 217, 58–63. [Google Scholar] [CrossRef]
- Maan, N.S.; Maan, S.; Belaganahalli, M.; Pullinger, G.; Montes, A.J.A.; Gasparini, M.R.; Guimera, M.; Nomikou, K.; Mertens, P.P.C. A quantitative real-time reverse transcription PCR (qRT-PCR) assay to detect genome segment 9 of all 26 bluetongue virus serotypes. J. Virol. Methods 2015, 213, 118–126. [Google Scholar] [CrossRef]
- Maan, N.S.; Maan, S.; Belaganahalli, M.N.; Ostlund, E.N.; Johnson, D.J.; Nomikou, K.; Mertens, P.P.C. Identification and differentiation of the twenty six bluetongue virus serotypes by RT-PCR amplification of the serotype-specific genome segment 2. PLoS ONE 2012, 7, e32601. [Google Scholar] [CrossRef]
- Steyn, J.; Venter, E.H. Sequence analysis and evaluation of the NS3/A gene region of bluetongue virus isolates from South Africa. Arch. Virol. 2016, 161, 947–957. [Google Scholar] [CrossRef]
- Orba, Y.; Hang’ombe, B.M.; Mweene, A.S.; Wada, Y.; Anindita, P.D.; Phongphaew, W.; Qiu, Y.; Kajihara, M.; Mori-Kajihara, A.; Eto, Y.; et al. First isolation of West Nile virus in Zambia from mosquitoes. Transbound. Emerg. Dis. 2018, 65, 933–938. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.E.; Gorman, B.M.; Tesh, R.B.; Knudson, D.L. Isolation of bluetongue and epizootic hemorrhagic disease viruses from mosquitoes collected in Indonesia. Vet. Microbiol. 1992, 32, 241–252. [Google Scholar] [CrossRef]
- Gerdes, G.H. A South African overview of the virus, vectors, surveillance and unique features of bluetongue. Vet. Ital. 2004, 40, 39–42. [Google Scholar]
- Toye, P.; Batten, C.; Kiara, H.; Henstock, M.; Edwards, L.; Thumbi, S.; Poole, E.J.; Handel, I.; Bronsvoort, B.D.C.; Hanotte, O.; et al. Bluetongue and Epizootic Haemorrhagic Disease virus in local breeds of cattle in Kenya. Res. Veter-Sci. 2013, 94, 769–773. [Google Scholar] [CrossRef] [Green Version]
- Andriamandimby, S.F.; Viarouge, C.; Ravalohery, J.-P.; Reynes, J.-M.; Sailleau, C.; Tantely, M.L.; Elissa, N.; Cardinale, E.; Sall, A.A.; Zientara, S.; et al. Detection in and circulation of Bluetongue virus among domestic ruminants in Madagascar. Vet. Microbiol. 2015, 176, 268–273. [Google Scholar] [CrossRef] [PubMed]
- Anderson, E.C.; Rowe, L.W. The prevalence of antibody to the viruses of bovine virus diarrhoea, bovine herpes virus 1, rift valley fever, ephemeral fever and bluetongue and to Leptospira sp in free-ranging wildlife in Zimbabwe. Epidemiol. Infect. 1998, 121, 441–449. [Google Scholar] [CrossRef]
- Simpson, V.R. Serological evidence of bluetongue in game animals in Botswana. Trop. Anim. Health Prod. 1978, 10, 55–60. [Google Scholar] [CrossRef]
- Fernández-Pacheco, P.; Fernández-Pinero, J.; Agüero, M.; Jiménez-Clavero, M.A. Bluetongue virus serotype 1 in wild mouflons in Spain. Vet. Rec. 2008, 162, 659–660. [Google Scholar] [CrossRef]
- Maree, F.F.; Kasanga, C.J.; Scott, K.A.; Opperman, P.A.; Melanie, C.; Sangula, A.K.; Raphael, S.; Yona, S.; Wambura, P.N.; King, D.P.; et al. Challenges and prospects for the control of foot-and-mouth disease: An African perspective. Veter Med. Res. Rep. 2014, 5, 119–138. [Google Scholar] [CrossRef] [Green Version]

| Province | District | Species | Blood † | Sera | Year (Season) |
|---|---|---|---|---|---|
| Copperbelt | Mpongwe | Cattle | 100 (10) | - | 2018 (Dry) |
| Eastern | Lundazi | Cattle | 350 (35) | - | 2018 (Wet) |
| Northern | Mbala | Cattle | 200 (20) | - | 2016 (Wet) |
| Kasama | Cattle | 243 (25) | - | 2016 (Wet) | |
| Mpika | Cattle | 200 (20) | - | 2016 (Wet) | |
| North-Western ‡ | Ikelenge | Buffalo | 5(1) | - | 2017 (Dry) |
| Western | Mongu | Cattle | 210 (21) | - | 2018 (Dry) |
| Goats | 84 (9) | - | 2018 (Dry) | ||
| Sheep | 6 (1) | - | 2018 (Dry) | ||
| Senanga | Cattle | 350 (35) | - | 2017 (Dry) | |
| Southern | Livingstone | Cattle | 104 (11) | - | 2019 (Dry) |
| Livingstone | Goats | 99 (10) | - | 2019 (Dry) | |
| Livingstone | Buffalo | 11 (5) | - | 2017 (Dry) | |
| Namwala | Cattle | 188 (19) | - | 2017 (Wet) | |
| Kafue National Park | Mumbwa | Impala | 98 (21) | - | 2017 (Dry) |
| Hartebeest | 29 (8) | - | 2017 (Dry) | ||
| Lusaka ‡ | Chongwe | Hartebeest | 76 (8) | - | 2018 (Dry) |
| Central | Shibuyunji | Cattle | 110 | 2017 (Dry) | |
| Mumbwa | Cattle | 115 | 2017 (Wet) | ||
| Itezhi tezhi | Cattle | 114 | 2017 (Wet) | ||
| Lusaka | Chongwe | Cattle | 110 | 2017 (Wet) | |
| Total | 2353 (259) | 449 |
| Province | District | Species | Pools Tested | qRT-PCR † | Season |
|---|---|---|---|---|---|
| Copperbelt | Mpongwe | Cattle | 10 | 1 (10) | Dry |
| Eastern | Lundazi | Cattle | 35 | 16 (45.7) | Wet |
| Northern | Mbala | Cattle | 20 | 16 (80) | Wet |
| Kasama | Cattle | 25 | 18 (72) | Wet | |
| Mpika | Cattle | 20 | 11 (55) | Wet | |
| North-Western ‡ | Ikelenge | Buffalo | 1 | 0 (0) | Dry |
| Western | Mongu | Cattle | 21 | 4 (19) | Dry |
| Goats | 9 | 2 (22.2) | Dry | ||
| Sheep | 1 | 0 (0) | Dry | ||
| Senanga | Cattle | 35 | 2 (5.7) | Dry | |
| Southern | Livingstone | Cattle | 11 | 6 (54.5) | Dry |
| Goats | 10 | 8 (80) | Dry | ||
| Buffalo | 5 | 0 (0) | Dry | ||
| Namwala | Cattle | 19 | 2 (10.5) | Wet | |
| Kafue National Park | Mumbwa | Impala | 21 | 0 (0) | Dry |
| Hartebeest | 8 | 1 (12.5) | Dry | ||
| Lusaka ‡ | Chongwe | Hartebeest | 8 | 0 (0) | Wet |
| Total | 259 | 87 (33.6) |
| Species | Sampling Season | Pools † | Pooled Prevalence ‡ |
|---|---|---|---|
| Cattle, goats, buffalo, impala, hartebeest | Wet, Dry | 87/259 (33.6) | 4.4 (3.6–5.4) |
| Cattle, goats, buffalo, impala, hartebeest | Dry | 17/99 (17.2) | 2.2 (1.4–3.2) |
| Cattle | Wet and Dry | 76/196 (38.8) | 4.8 (3.8–6.0) |
| Cattle | Wet | 63/119 (52.9) | 7.3 (5.6–9.3) |
| Cattle | Dry | 13/77 (16.9) | 1.8 (1.0–3.1) |
| Goats | Wet | - | - |
| Goats | Dry | 10/19 (52.6) | 7.2 (3.3–13.1) |
| Cattle and Goats | Dry | 23/96 (24.0) | 2.7 (1.7–4.0) |
| Wildlife | Dry | 1/43 (2.3) | 0.4% (0.0–1.8]) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chambaro, H.M.; Sasaki, M.; Simulundu, E.; Silwamba, I.; Sinkala, Y.; Gonzalez, G.; Squarre, D.; Fandamu, P.; Lubaba, C.H.; Munyeme, M.; et al. Co-Circulation of Multiple Serotypes of Bluetongue Virus in Zambia. Viruses 2020, 12, 963. https://doi.org/10.3390/v12090963
Chambaro HM, Sasaki M, Simulundu E, Silwamba I, Sinkala Y, Gonzalez G, Squarre D, Fandamu P, Lubaba CH, Munyeme M, et al. Co-Circulation of Multiple Serotypes of Bluetongue Virus in Zambia. Viruses. 2020; 12(9):963. https://doi.org/10.3390/v12090963
Chicago/Turabian StyleChambaro, Herman M., Michihito Sasaki, Edgar Simulundu, Isaac Silwamba, Yona Sinkala, Gabriel Gonzalez, David Squarre, Paul Fandamu, Caesar H. Lubaba, Musso Munyeme, and et al. 2020. "Co-Circulation of Multiple Serotypes of Bluetongue Virus in Zambia" Viruses 12, no. 9: 963. https://doi.org/10.3390/v12090963







