Acute RNA Viral Encephalomyelitis and the Role of Antibodies in the Central Nervous System
Abstract
:1. Introduction
1.1. Multiple Pathways for Viruses into the CNS
1.2. Recruitment of Antibody-Secreting Cells Predominant Over Antibodies Crossing the BBB
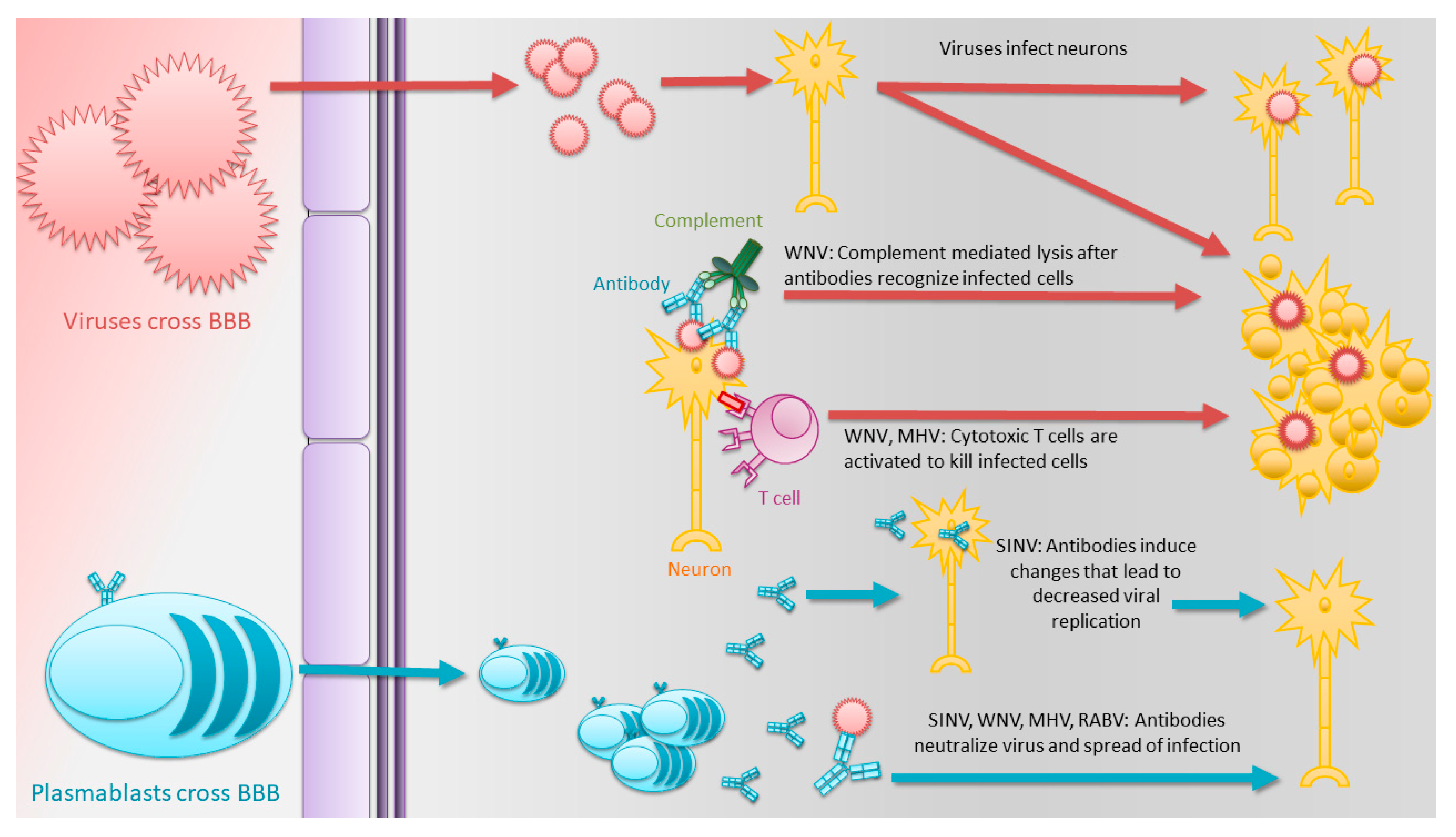
1.3. Antibodies Play Critical Roles in the Clearance of Many Acute Encephalomyelitis-Causing RNA Viruses
1.4. Alphaviruses
1.5. Flaviviruses
1.6. Coronaviruses
1.7. Rhabdoviruses
1.8. Clearance of Virus from the CNS without Antibody
1.9. Non-Canonical Functions of Antibodies in the CNS and Potential Future Avenues of Study
1.10. Antibody Synergism
1.11. Modifications and Auxiliary Features of Fc
1.12. Antibodies with Direct Microbe Killing or Proteolytic Activity
1.13. Antibodies Able to Suppress Virus Replication Intracellularly
2. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Glaser, C.A.; Honarmand, S.; Anderson, L.J.; Schnurr, D.P.; Forghani, B.; Cossen, C.K.; Schuster, F.L.; Christie, L.J.; Tureen, J.H. Beyond Viruses: Clinical Profiles and Etiologies Associated with Encephalitis. Clin. Infect. Dis. 2006, 43, 1565–1577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Metcalf, T.U.; Baxter, V.K.; Nilaratanakul, V.; Griffin, D.E. Recruitment and Retention of B Cells in the Central Nervous System in Response to Alphavirus Encephalomyelitis. J. Virol. 2012, 87, 2420–2429. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peters, C. Lymphocytic Choriomeningitis Virus—An Old Enemy up to New Tricks. N. Engl. J. Med. 2006, 354, 2208–2211. [Google Scholar] [CrossRef] [PubMed]
- Mahadevan, A.; Suja, M.S.; Mani, R.S.; Shankar, S.K. Perspectives in Diagnosis and Treatment of Rabies Viral Encephalitis: Insights from Pathogenesis. Neurotherapy 2016, 13, 477–492. [Google Scholar] [CrossRef] [PubMed]
- Metcalf, T.U.; Griffin, D.E. Alphavirus-Induced Encephalomyelitis: Antibody-Secreting Cells and Viral Clearance from the Nervous System. J. Virol. 2011, 85, 11490–11501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carter, M.J.; Mitchell, R.M.; Sauteur, P.M.M.; Kelly, D.F.; Trück, J. The Antibody-Secreting Cell Response to Infection: Kinetics and Clinical Applications. Front. Immunol. 2017, 8, 630. [Google Scholar] [CrossRef] [PubMed]
- Tschen, S.-I.; Bergmann, C.C.; Ramakrishna, C.; Morales, S.; Atkinson, R.; Stohlman, S.A. Recruitment kinetics and composition of antibody-secreting cells within the central nervous system following viral encephalomyelitis. J. Immunol. 2002, 168, 2922–2929. [Google Scholar] [CrossRef]
- Vogel, P.; Kell, W.M.; Fritz, D.L.; Parker, M.D.; Schoepp, R. Early Events in the Pathogenesis of Eastern Equine Encephalitis Virus in Mice. Am. J. Pathol. 2005, 166, 159–171. [Google Scholar] [CrossRef] [Green Version]
- Deresiewicz, R.L.; Thaler, S.J.; Hsu, L.; Zamani, A.A. Clinical and Neuroradiographic Manifestations of Eastern Equine Encephalitis. N. Engl. J. Med. 1997, 336, 1867–1874. [Google Scholar] [CrossRef]
- Phillips, A.T.; Rico, A.B.; Stauft, C.B.; Hammond, S.L.; Aboellail, T.A.; Tjalkens, R.B.; Olson, K.E. Entry Sites of Venezuelan and Western Equine Encephalitis Viruses in the Mouse Central Nervous System following Peripheral Infection. J. Virol. 2016, 90, 5785–5796. [Google Scholar] [CrossRef] [Green Version]
- Sharma, A.; Knollmann-Ritschel, B.E.C. Current Understanding of the Molecular Basis of Venezuelan Equine Encephalitis Virus Pathogenesis and Vaccine Development. Viruses 2019, 11, 164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De La Monte, S.M.; Castro, F.; Bonilla, N.J.; De Urdaneta, A.G.; Hutchins, G.M. The Systemic Pathology of Venezuelan Equine Encephalitis Virus Infection in Humans. Am. J. Trop. Med. Hyg. 1985, 34, 194–202. [Google Scholar] [CrossRef]
- Kimura, T.; Griffin, D.E. Extensive immune-mediated hippocampal damage in mice surviving infection with neuroadapted Sindbis virus. Virology 2003, 311, 28–39. [Google Scholar] [CrossRef] [Green Version]
- Jackson, A.C.; Moench, T.R.; Trapp, B.D.; Griffin, D.E. Basis of neurovirulence in Sindbis virus encephalomyelitis of mice. Lab. Investig. 1988, 58, 503–509. [Google Scholar] [PubMed]
- Fazakerley, J. Pathogenesis of Semliki Forest Virus Encephalitis. J. Neurovirol. 2002, 8 (Suppl. 2), 66–74. [Google Scholar] [CrossRef]
- Fazakerley, J.; Cotterill, C.L.; Lee, G.; Graham, A. Virus tropism, distribution, persistence and pathology in the corpus callosum of the Semliki Forest virus-infected mouse brain: A novel system to study virus-oligodendrocyte interactions. Neuropathol. Appl. Neurobiol. 2006, 32, 397–409. [Google Scholar] [CrossRef] [PubMed]
- Mehta, R.; Gerardin, P.; De Brito, C.A.A.; Soares, C.N.; Ferreira, M.L.B.; Solomon, T. The neurological complications of chikungunya virus: A systematic review. Rev. Med Virol. 2018, 28, e1978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Srivastava, R.; Kalita, J.; Khan, M.Y.; Gore, M.M.; Bondre, V.P.; Misra, U.K. Temporal changes of Japanese encephalitits virus in different brain regions of rat. Indian J. Med. Res. 2013, 138, 219–223. [Google Scholar]
- Figueiredo, C.P.; Barros-Aragão, F.G.D.Q.; Neris, R.L.S.; Frost, P.S.; Soares, C.; Souza, I.N.D.O.; Zeidler, J.D.; Zamberlan, D.C.; De Sousa, V.L.; Souza, A.S.; et al. Zika virus replicates in adult human brain tissue and impairs synapses and memory in mice. Nat. Commun. 2019, 10, 3890. [Google Scholar] [CrossRef] [Green Version]
- Butowt, R.; Bilinska, K. SARS-CoV-2: Olfaction, Brain Infection, and the Urgent Need for Clinical Samples Allowing Earlier Virus Detection. ACS Chem. Neurosci. 2020, 11, 1200–1203. [Google Scholar] [CrossRef] [Green Version]
- Koralnik, I.J.; Tyler, K.L. COVID -19: A Global Threat to the Nervous System. Ann. Neurol. 2020, 88, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Yokomori, K.; Asanaka, M.; Stohlman, S.A.; Makino, S.; Shubin, R.A.; Gilmore, W.; Weiner, L.P.; Wang, F.-I.; Mc Lai, M. Neuropathogenicity of mouse hepatitis virus JHM isolates differing in hemagglutinin-esterase protein expression. J. Neurovirol. 1995, 1, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Das Sarma, J.; Iacono, K.; Gard, L.; Marek, R.; Kenyon, L.; Koval, M.; Weiss, S.R. Demyelinating and Nondemyelinating Strains of Mouse Hepatitis Virus Differ in Their Neural Cell Tropism. J. Virol. 2008, 82, 5519–5526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Girard, S.; Couderc, T.; Destombes, J.; Thiesson, D.; Delpeyroux, F.; Blondel, B. Poliovirus Induces Apoptosis in the Mouse Central Nervous System. J. Virol. 1999, 73, 6066–6072. [Google Scholar] [CrossRef] [Green Version]
- Gerhauser, I.; Hansmann, F.; Ciurkiewicz, M.; Löscher, W.; Beineke, A. Facets of Theiler’s Murine Encephalomyelitis Virus-Induced Diseases: An Update. Int. J. Mol. Sci. 2019, 20, 448. [Google Scholar] [CrossRef] [Green Version]
- Munster, V.; Prescott, J.B.; Bushmaker, T.; Long, D.; Rosenke, R.; Thomas, T.; Scott, D.; Fischer, E.R.; Feldmann, H.; De Wit, E. Rapid Nipah virus entry into the central nervous system of hamsters via the olfactory route. Sci. Rep. 2012, 2, 736. [Google Scholar] [CrossRef]
- Albertini, A.A.; Baquero, E.; Ferlin, A.; Gaudin, Y. Molecular and Cellular Aspects of Rhabdovirus Entry. Viruses 2012, 4, 117–139. [Google Scholar] [CrossRef] [Green Version]
- Griffin, D.E. Viral Encephalomyelitis. PLoS Pathog. 2011, 7, e1002004. [Google Scholar] [CrossRef] [Green Version]
- Carson, M.J.; Doose, J.M.; Melchior, B.; Schmid, C.D.; Ploix, C.C. CNS immune privilege: Hiding in plain sight. Immunol. Rev. 2006, 213, 48–65. [Google Scholar] [CrossRef]
- Hickey, W.F.; Hsu, B.L.; Kimura, H. T-lymphocyte entry into the central nervous system. J. Neurosci. Res. 1991, 28, 254–260. [Google Scholar] [CrossRef]
- Anthony, I.C.; Crawford, D.H.; Bell, J.E. B lymphocytes in the normal brain: Contrasts with HIV-associated lymphoid infiltrates and lymphomas. Brain 2003, 126, 1058–1067. [Google Scholar] [CrossRef] [PubMed]
- Knopf, P.M.; Harling-Berg, C.J.; Cserr, H.F.; Basu, D.; Sirulnick, E.J.; Nolan, S.C.; Park, J.T.; Keir, G.; Thompson, E.J.; Hickey, W.F. Antigen-dependent intrathecal antibody synthesis in the normal rat brain: Tissue entry and local retention of antigen-specific B cells. J. Immunol. 1998, 161, 692. [Google Scholar] [PubMed]
- Phares, T.W.; Stohlman, S.A.; Bergmann, C.C. Intrathecal Humoral Immunity to Encephalitic RNA Viruses. Viruses 2013, 5, 732–752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, R.S.; Lin, E.; Zhang, B.; Luster, A.D.; Tollett, J.; Samuel, M.A.; Engle, M.; Diamond, M.S. Neuronal CXCL10 Directs CD8+ T-Cell Recruitment and Control of West Nile Virus Encephalitis. J. Virol. 2005, 79, 11457–11466. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Appolinario, C.M.; Allendorf, S.; Fonseca, C.R.; Ribeiro, B.D.; Vicente, A.F.; Antunes, J.M.A.D.P.; Peres, M.G.; Megid, J. Profile of Cytokines and Chemokines Triggered by Wild-Type Strains of Rabies Virus in Mice. Am. J. Trop. Med. Hyg. 2016, 94, 378–383. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chai, Q.; She, R.; Huang, Y.; Fu, Z. Expression of Neuronal CXCL10 Induced by Rabies Virus Infection Initiates Infiltration of Inflammatory Cells, Production of Chemokines and Cytokines, and Enhancement of Blood-Brain Barrier Permeability. J. Virol. 2014, 89, 870–876. [Google Scholar] [CrossRef] [Green Version]
- Ebinger, G.; Matthyssens, G. Cerebrospinal fluid measles antibody titer and γ globulins in subacute sclerosing panencephalitis. Zeitschrift für Neurologie 1971, 200, 1–5. [Google Scholar] [CrossRef]
- Nilaratanakul, V.; Chen, J.; Tran, O.; Baxter, V.K.; Troisi, E.M.; Yeh, J.X.; Griffin, D.E. Germ Line IgM Is Sufficient, but Not Required, for Antibody-Mediated Alphavirus Clearance from the Central Nervous System. J. Virol. 2018, 92, e02081-17. [Google Scholar] [CrossRef] [Green Version]
- Tyor, W.R.; Wesselingh, S.; Levine, B.; Griffin, D.E. Long term intraparenchymal Ig secretion after acute viral encephalitis in mice. J. Immunol. 1992, 149, 4016–4020. [Google Scholar]
- Griffin, D.E. Immunoglobulins in the cerebrospinal fluid: Changes during acute viral encephalitis in mice. J. Immunol. 1981, 126, 27–31. [Google Scholar]
- Tyor, W.R.; Moench, T.R.; Griffin, D.E. Characterization of the local and systemic B cell response of normal and athymic nude mice with Sindbis virus encephalitis. J. Neuroimmunol. 1989, 24, 207–215. [Google Scholar] [CrossRef]
- Griffin, D.E. Immune responses to RNA-virus infections of the CNS. Nat. Rev. Immunol. 2003, 3, 493–502. [Google Scholar] [CrossRef] [PubMed]
- Fragkoudis, R.; Dixon-Ballany, C.M.; Zagrajek, A.; Kedzierski, L.; Fazakerley, J. Following Acute Encephalitis, Semliki Forest Virus is Undetectable in the Brain by Infectivity Assays but Functional Virus RNA Capable of Generating Infectious Virus Persists for Life. Viruses 2018, 10, 273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parsons, L.M.; Webb, H.E. Identification of immunoglobulin-containing cells in the central nervous system of the mouse following infection with the demyelinating strain of Semliki Forest virus. Br. J. Exp. Pathol. 1989, 70, 247–255. [Google Scholar]
- Smith-Norowitz, T.A.; Sobel, R.A.; Mokhtarian, F. B Cells and Antibodies in the Pathogenesis of Myelin Injury in Semliki Forest Virus Encephalomyelitis. Cell Immunol. 2000, 200, 27–35. [Google Scholar] [CrossRef]
- Winkelmann, E.R.; Luo, H.; Wang, T. West Nile Virus Infection in the Central Nervous System. F1000 Res. 2016, 5, 105. [Google Scholar] [CrossRef] [Green Version]
- Diamond, M.; Sitati, E.M.; Friend, L.D.; Higgs, S.; Shrestha, B.; Engle, M. A Critical Role for Induced IgM in the Protection against West Nile Virus Infection. J. Exp. Med. 2003, 198, 1853–1862. [Google Scholar] [CrossRef]
- Halevy, M.; Akov, Y.; Ben-Nathan, D.; Kobiler, D.; Lachmi, B.; Lustig, S. Loss of active neuroinvasiveness in attenuated strains of West Nile virus: Pathogenicity in immunocompetent and SCID mice. Arch. Virol. 1994, 137, 355–370. [Google Scholar] [CrossRef]
- Ochsenbein, A.F.; Zinkernagel, R.M. Natural antibodies and complement link innate and acquired immunity. Immunol. Today 2000, 21, 624–630. [Google Scholar] [CrossRef]
- Ahmed, M.U.; Hanif, M.; Ali, M.J.; Haider, M.A.; Kherani, D.; Memon, G.M.; Karim, A.H.; Sattar, A. Neurological Manifestations of COVID-19 (SARS-CoV-2): A Review. Front. Neurol. 2020, 11, 518. [Google Scholar] [CrossRef]
- Andriuta, D.; Roger, P.-A.; Thibault, W.; Toublanc, B.; Sauzay, C.; Castelain, S.; Godefroy, O.; Brochot, E. COVID-19 encephalopathy: Detection of antibodies against SARS-CoV-2 in CSF. J. Neurol. 2020, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Marten, N.W.; Stohlman, S.A.; Bergmann, C.C. MHV Infection of the CNS: Mechanisms of Immune-Mediated Control. Viral Immunol. 2001, 14, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Matthews, A.E.; Weiss, S.R.; Shlomchik, M.J.; Hannum, L.G.; Gombold, J.L.; Paterson, Y.Y. Antibody is required for clearance of infectious murine hepatitis virus A59 from the central nervous system, but not the liver. J. Immunol. 2001, 167, 5254–5263. [Google Scholar] [CrossRef] [PubMed]
- Phares, T.W.; Disano, K.D.; Stohlman, S.A.; Bergmann, C.C. Progression from IgD+ IgM+ to Isotype-Switched B Cells Is Site Specific during Coronavirus-Induced Encephalomyelitis. J. Virol. 2014, 88, 8853–8867. [Google Scholar] [CrossRef] [Green Version]
- Tarantola, A. Four Thousand Years of Concepts Relating to Rabies in Animals and Humans, Its Prevention and Its Cure. Trop. Med. Infect. Dis. 2017, 2, 5. [Google Scholar] [CrossRef] [Green Version]
- Hooper, D.; Phares, T.W.; Fabis, M.J.; Roy, A. The Production of Antibody by Invading B Cells Is Required for the Clearance of Rabies Virus from the Central Nervous System. PLoS Negl. Trop. Dis. 2009, 3, e535. [Google Scholar] [CrossRef]
- Gnanadurai, C.W.; Zhou, M.; He, W.; Leyson, C.M.; Huang, C.-T.; Salyards, G.; Harvey, S.B.; Chen, Z.; He, B.; Yang, Y.; et al. Presence of Virus Neutralizing Antibodies in Cerebral Spinal Fluid Correlates with Non-Lethal Rabies in Dogs. PLoS Negl. Trop. Dis. 2013, 7, e2375. [Google Scholar] [CrossRef] [Green Version]
- Roy, A.; Hooper, D. Lethal Silver-Haired Bat Rabies Virus Infection Can Be Prevented by Opening the Blood-Brain Barrier. J. Virol. 2007, 81, 7993–7998. [Google Scholar] [CrossRef] [Green Version]
- Hayashida, E.; Ling, Z.L.; Ashhurst, T.M.; Viengkhou, B.; Jung, S.R.; Songkhunawej, P.; West, P.K.; King, N.J.; Hofer, M.J. Zika virus encephalitis in immunocompetent mice is dominated by innate immune cells and does not require T or B cells. J. Neuroinflam. 2019, 16, 177. [Google Scholar] [CrossRef] [Green Version]
- Brooke, C.B.; Deming, D.J.; Whitmore, A.C.; White, L.J.; Johnston, R.E. T Cells Facilitate Recovery from Venezuelan Equine Encephalitis Virus-Induced Encephalomyelitis in the Absence of Antibody. J. Virol. 2010, 84, 4556–4568. [Google Scholar] [CrossRef] [Green Version]
- Mascola, J.R.; Louder, M.K.; VanCott, T.C.; Sapan, C.V.; Lambert, J.S.; Muenz, L.R.; Bunow, B.; Birx, D.L.; Robb, M.L. Potent and synergistic neutralization of human immunodeficiency virus (HIV) type 1 primary isolates by hyperimmune anti-HIV immunoglobulin combined with monoclonal antibodies 2F5 and 2G12. J. Virol. 1997, 71, 7198–7206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ter Meulen, J.; Brink, E.N.V.D.; Poon, L.L.M.; Marissen, W.E.; Leung, C.S.W.; Cox, F.; Cheung, C.Y.; Bakker, A.Q.; Bogaards, J.A.; Van Deventer, E.; et al. Human Monoclonal Antibody Combination against SARS Coronavirus: Synergy and Coverage of Escape Mutants. PLoS Med. 2006, 3, e237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlsen, T.H.; Pedersen, J.; Prentoe, J.; Giang, E.; Keck, Z.-Y.; Mikkelsen, L.S.; Law, M.; Foung, S.K.H.; Bukh, J. Breadth of neutralization and synergy of clinically relevant human monoclonal antibodies against HCV genotypes 1a, 1b, 2a, 2b, 2c, and 3a. Hepatology 2014, 60, 1551–1562. [Google Scholar] [CrossRef] [PubMed]
- Pollara, J.; Bonsignori, M.; Moody, M.A.; Liu, P.; Alam, S.M.; Hwang, K.-K.; Gurley, T.C.; Kozink, D.M.; Armand, L.C.; Marshall, D.J.; et al. HIV-1 Vaccine-Induced C1 and V2 Env-Specific Antibodies Synergize for Increased Antiviral Activities. J. Virol. 2014, 88, 7715–7726. [Google Scholar] [CrossRef] [Green Version]
- Mankowski, M.; Kinchen, V.; Wasilewski, L.N.; Flyak, A.I.; Ray, S.C.; Crowe, J.E.; Bailey, J.R. Synergistic anti-HCV broadly neutralizing human monoclonal antibodies with independent mechanisms. Proc. Natl. Acad. Sci. USA 2017, 115, E82–E91. [Google Scholar] [CrossRef] [Green Version]
- Howell, K.A.; Brannan, J.M.; Bryan, C.; McNeal, A.; Davidson, E.; Turner, H.L.; Vu, H.; Shulenin, S.; He, S.; Kuehne, A.; et al. Cooperativity Enables Non-neutralizing Antibodies to Neutralize Ebolavirus. Cell Rep. 2017, 19, 413–424. [Google Scholar] [CrossRef]
- LaZear, E.; Whitbeck, J.C.; Ponce-De-Leon, M.; Cairns, T.M.; Willis, S.H.; Zuo, Y.; Krummenacher, C.; Cohen, G.H.; Eisenberg, R.J. Antibody-Induced Conformational Changes in Herpes Simplex Virus Glycoprotein gD Reveal New Targets for Virus Neutralization. J. Virol. 2011, 86, 1563–1576. [Google Scholar] [CrossRef] [Green Version]
- Heinz, F.X.; Mandl, C.; Berger, R.; Tuma, W.; Kunz, C. Antibody-induced conformational changes result in enhanced avidity of antibodies to different antigenic sites on the tick-borne encephalitis virus glycoprotein. Virology 1984, 133, 25–34. [Google Scholar] [CrossRef]
- Kaneko, Y.; Nimmerjahn, F.; Ravetch, J.V. Anti-Inflammatory Activity of Immunoglobulin G Resulting from Fc Sialylation. Science 2006, 313, 670–673. [Google Scholar] [CrossRef] [Green Version]
- Bozza, S.; Käsermann, F.; Kaveri, S.V.; Romani, L.; Bayry, J. Intravenous immunoglobulin protects from experimental allergic bronchopulmonary aspergillosis via a sialylation-dependent mechanism. Eur. J. Immunol. 2018, 49, 195–198. [Google Scholar] [CrossRef] [Green Version]
- Anthony, R.M.; Nimmerjahn, F.; Ashline, D.J.; Reinhold, V.N.; Paulson, J.C.; Ravetch, J.V. Recapitulation of IVIG Anti-Inflammatory Activity with a Recombinant IgG Fc. Science 2008, 320, 373–376. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwab, I.; Seeling, M.; Biburger, M.; Aschermann, S.; Nitschke, L.; Nimmerjahn, F. B cells and CD22 are dispensable for the immediate antiinflammatory activity of intravenous immunoglobulins in vivo. Eur. J. Immunol. 2012, 42, 3302–3309. [Google Scholar] [CrossRef] [PubMed]
- Washburn, N.; Schwab, I.; Ortiz, D.; Bhatnagar, N.; Lansing, J.C.; Medeiros, A.; Tyler, S.; Mekala, D.; Cochran, E.; Sarvaiya, H.; et al. Controlled tetra-Fc sialylation of IVIg results in a drug candidate with consistent enhanced anti-inflammatory activity. Proc. Natl. Acad. Sci. USA 2015, 112, E1297–E1306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohmi, Y.; Ise, W.; Harazono, A.; Takakura, D.; Fukuyama, H.; Baba, Y.; Narazaki, M.; Shoda, H.; Takahashi, N.; Ohkawa, Y.; et al. Sialylation converts arthritogenic IgG into inhibitors of collagen-induced arthritis. Nat. Commun. 2016, 7, 11205. [Google Scholar] [CrossRef] [Green Version]
- Bartsch, Y.C.; Rahmöller, J.; Mertes, M.M.M.; Eiglmeier, S.; Lorenz, F.K.M.; Stoehr, A.D.; Braumann, D.; Lorenz, A.K.; Winkler, A.; Lilienthal, G.-M.; et al. Sialylated Autoantigen-Reactive IgG Antibodies Attenuate Disease Development in Autoimmune Mouse Models of Lupus Nephritis and Rheumatoid Arthritis. Front. Immunol. 2018, 9, 1183. [Google Scholar] [CrossRef]
- LaRocca, T.J.; Holthausen, D.J.; Hsieh, C.; Renken, C.; Mannella, C.A.; Benach, J.L. The bactericidal effect of a complement-independent antibody is osmolytic and specific to Borrelia. Proc. Natl. Acad. Sci. USA 2009, 106, 10752–10757. [Google Scholar] [CrossRef] [Green Version]
- Irvine, E.B.; Alter, G. Understanding the role of antibody glycosylation through the lens of severe viral and bacterial diseases. Glycobiology 2020, 30, 241–253. [Google Scholar] [CrossRef] [Green Version]
- Tian, H.; Weber, S.; Thorkildson, P.; Kozel, T.R.; Pirofski, L. Efficacy of Opsonic and Nonopsonic Serotype 3 Pneumococcal Capsular Polysaccharide-Specific Monoclonal Antibodies against Intranasal Challenge with Streptococcus pneumoniae in Mice. Infect. Immun. 2009, 77, 1502–1513. [Google Scholar] [CrossRef] [Green Version]
- LaRocca, T.J.; Katona, L.I.; Thanassi, D.G.; Benach, J.L. Bactericidal action of a complement-independent antibody against relapsing fever Borrelia resides in its variable region. J. Immunol. 2008, 180, 6222–6228. [Google Scholar] [CrossRef] [Green Version]
- Connolly, S.E.; Thanassi, D.G.; Benach, J.L. Generation of a Complement-Independent Bactericidal IgM against a Relapsing Fever Borrelia. J. Immunol. 2004, 172, 1191–1197. [Google Scholar] [CrossRef] [Green Version]
- Mahendra, A.; Sharma, M.; Rao, D.N.; Peyron, I.; Planchais, C.; Dimitrov, J.D.; Kaveri, S.V.; Lacroix-Desmazes, S. Antibody-mediated catalysis: Induction and therapeutic relevance. Autoimmun. Rev. 2013, 12, 648–652. [Google Scholar] [CrossRef] [PubMed]
- Planque, S.; Mitsuda, Y.; Taguchi, H.; Salas, M.; Morris, M.-K.; Nishiyama, Y.; Kyle, R.; Okhuysen, P.C.; Escobar, M.; Hunter, R.; et al. Characterization of gp120 Hydrolysis by IgA Antibodies from Humans without HIV Infection. AIDS Res. Hum. Retrovir. 2007, 23, 1541–1554. [Google Scholar] [CrossRef] [PubMed]
- Bowen, A.; Wear, M.; Casadevall, A. Antibody-Mediated Catalysis in Infection and Immunity. Infect. Immun. 2017, 85, e00202-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Després, P.; Griffin, J.W.; Griffin, D.E. Antiviral activity of alpha interferon in Sindbis virus-infected cells is restored by anti-E2 monoclonal antibody treatment. J. Virol. 1995, 69, 7345–7348. [Google Scholar] [CrossRef] [Green Version]
- Ubol, S.; Levine, B.; Lee, S.H.; Greenspan, N.S.; Griffin, D.E. Roles of immunoglobulin valency and the heavy-chain constant domain in antibody-mediated downregulation of Sindbis virus replication in persistently infected neurons. J. Virol. 1995, 69, 1990–1993. [Google Scholar] [CrossRef] [Green Version]
- Nilaratanakul, V.; Hauer, D.A.; Griffin, D.E. Visualization of cell-type dependent effects of anti-E2 antibody and interferon-gamma treatments on localization and expression of Broccoli aptamer-tagged alphavirus RNAs. Sci. Rep. 2020, 10, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Mallery, D.L.; McEwan, W.A.; Bidgood, S.R.; Towers, G.J.; Johnson, C.M.; James, L.C. Antibodies mediate intracellular immunity through tripartite motif-containing 21 (TRIM21). Proc. Natl. Acad. Sci. USA 2010, 107, 19985–19990. [Google Scholar] [CrossRef] [Green Version]
| Virus | Viral Family | Target Location | Target Cells | Animal Species | Additional Human CNS Presentation | Reference |
|---|---|---|---|---|---|---|
| Eastern equine encephalitis | Togaviridae | Olfactory bulb, widespread | Neurons | Mouse | None associated | [8,9] |
| Western equine encephalitis | Togaviridae | Olfactory bulb, substantia nigra widespread | Neurons | Mouse | Similar to Parkinson’s, cogwheel rigidity | [10] |
| Venezuelan Equine Encephalitis Virus | Togaviridae | Olfactory bulb, widespread | Neurons | Mouse | None associated | [11,12] |
| Sindbis virus | Togaviridae | Hippocampus and brainstem | Immature and mature neurons | Mouse | None associated | [13,14] |
| Semliki Forest Virus | Togaviridae | Corpus callosum | Neurons, oligodendrocytes | Mouse | None associated | [15,16] |
| Chikungunya virus | Togaviridae | Not determined | Astrocytes | Mouse, human cell culture | Guillain–Barre syndrome | [17] |
| Japanese encephalitis virus | Flaviviridae | Basal ganglia | Neurons | Rat | Similar to Parkinson’s | [18] |
| Zika virus | Flaviviridae | Frontal cortex, hippocampus, striatum | Mature neurons | Mouse, human cell culture | Memory impairment | [19] |
| Severe acute respiratory syndrome coronavirus 2 | Coronaviridae | Not determined | Presumed olfactory neurons | Human | Guillain–Barre syndrome, smell/taste dysfunction | [20,21] |
| JHM mouse hepatitis virus | Coronaviridae | Not determined | Neurons, Glia cells | Mouse | None associated | [22,23] |
| Poliovirus | Picornaviridae | Brainstem/spinal cord | Motor neurons | Mouse | Paralysis | [24] |
| Theiler’s murine encephalomyelitis virus | Picornaviridae | hippocampus periventricular thalamic nuclei; septal nuclei; and piriform, parietal, and entorhinal cortices | Glia, macrophages | Mouse | spontaneous recurrent epileptic seizures | [25] |
| Nipah virus | Paramyxoviridae | Cribriform plate, olfactory bulb | Neurons | Hamster | None associated | [26] |
| Rabies virus | Rhabdoviridae | Not determined | Neurons | Mouse | Agitation, cognitive dysfunction | [27] |
| Virus | Abbr. | Chemokines | Cytokines | Surface Receptors | Reference |
|---|---|---|---|---|---|
| Sindbis virus | SINV | CXCL9, CXCL10, CCL1, CCL2, CCL5 | BAFF, IL−10, and IL−21 | CXCR3, CXCR5, CCR7 | [2] |
| West Nile virus | WNV | CXCL9, CXCL10, CCL2, CCL5, CCL7 | TNF-α, IFN-γ | CXCR3, CCR1, CCR2, CCR5 | [34] |
| JHM mouse hepatitis virus | JHMV | CXCL9, CXCL10 | APRIL, BAFF, IL−6, IL−10, IL−21 | CXCR3, B220, sIg, CD19 | [7,33] |
| Rabies virus | RABV | CXCL10, CX3CL1, CCL4, CCL5, CCL7, CCL21 | IL−6, IL−1, IL−12, TNF-α, IFN-γ | Unknown | [35,36] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bartlett, M.L.; Griffin, D.E. Acute RNA Viral Encephalomyelitis and the Role of Antibodies in the Central Nervous System. Viruses 2020, 12, 988. https://doi.org/10.3390/v12090988
Bartlett ML, Griffin DE. Acute RNA Viral Encephalomyelitis and the Role of Antibodies in the Central Nervous System. Viruses. 2020; 12(9):988. https://doi.org/10.3390/v12090988
Chicago/Turabian StyleBartlett, Maggie L., and Diane E. Griffin. 2020. "Acute RNA Viral Encephalomyelitis and the Role of Antibodies in the Central Nervous System" Viruses 12, no. 9: 988. https://doi.org/10.3390/v12090988
APA StyleBartlett, M. L., & Griffin, D. E. (2020). Acute RNA Viral Encephalomyelitis and the Role of Antibodies in the Central Nervous System. Viruses, 12(9), 988. https://doi.org/10.3390/v12090988





