The Cross-Talk between Thrombosis and Inflammatory Storm in Acute and Long-COVID-19: Therapeutic Targets and Clinical Cases
Abstract
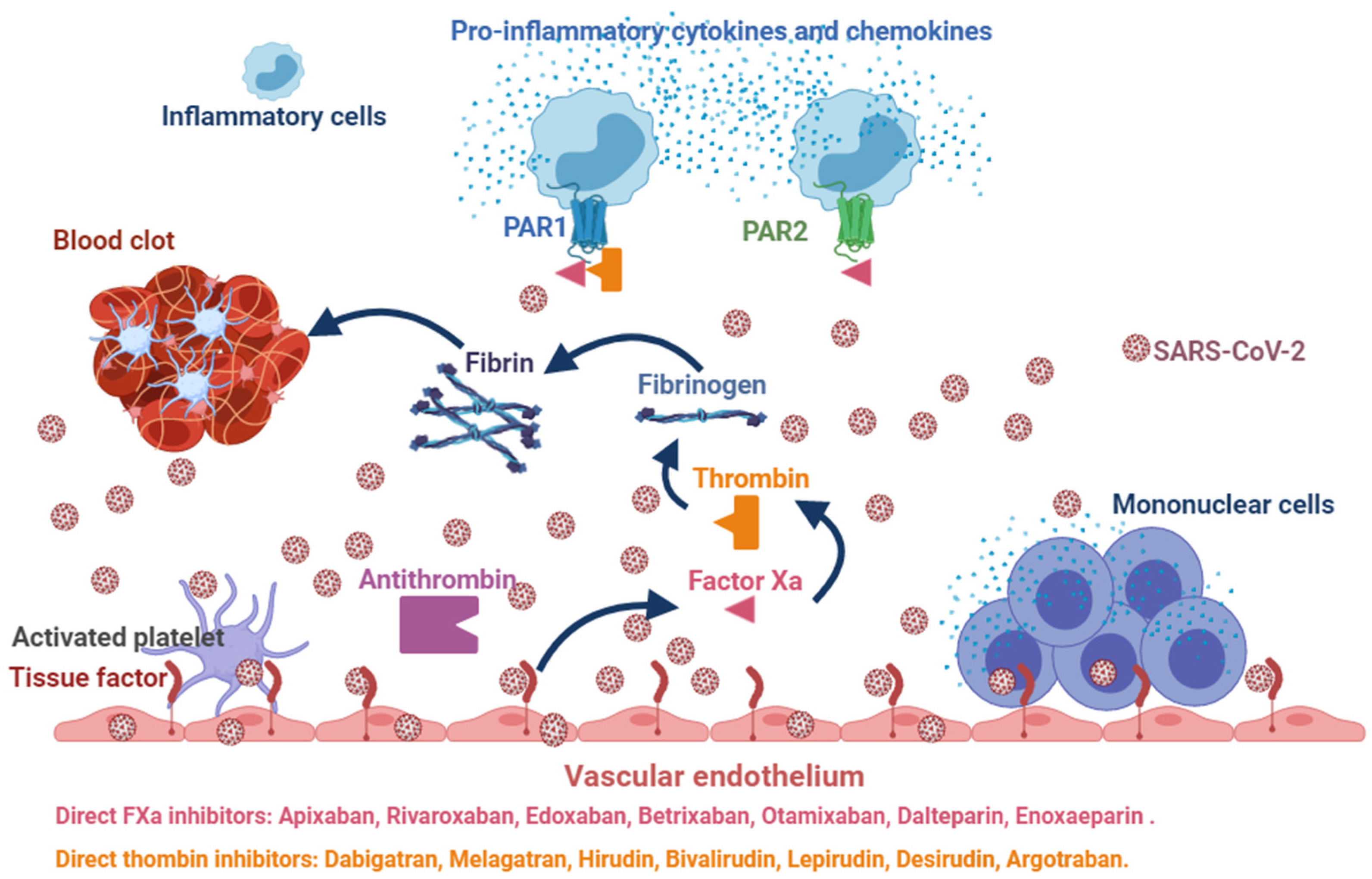
:1. Introduction
2. COVID-19 and Older Adults with Comorbidities
3. Uncertain Effects of Anticoagulants in COVID-19
4. Potential Benefits of DOACs in COVID-19
5. Clinical Case
6. Long-COVID-19
7. Materials and Methods and Preliminary Results
8. Clinical Case
9. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, Y.; Xiao, M.; Zhang, S.; Xia, P.; Cao, W.; Jiang, W.; Chen, H.; Ding, X.; Zhao, H.; Zhang, H.; et al. Coagulopathy and Antiphospholipid Antibodies in Patients with COVID-19. N. Engl. J. Med. 2020, 382, e38. [Google Scholar] [CrossRef]
- Iba, T.; Watanabe, E.; Umemura, Y.; Wada, T.; Hayashida, K.; Kushimoto, S.; Wada, H. Sepsis-associated disseminated intravascular coagulation and its differential diag-noses. J. Intensive Care 2019, 32, 7–13. [Google Scholar]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Wichmann, D.; Sperhake, J.-P.; Lütgehetmann, M.; Steurer, S.; Edler, C.; Heinemann, A.; Heinrich, F.; Mushumba, H.; Kniep, I.; Schröder, A.S.; et al. Autopsy Findings and Venous Thromboembolism in Patients With COVID-19. Ann. Intern. Med. 2020, 173, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.; Li, D.; Wang, X.; Sun, Z. Abnormal coagulation parameters are associated with poor prognosis in patients with novel coronavirus pneumonia. J. Thromb. Haemost. 2020, 18, 844–847. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lillicrap, D. Disseminated intravascular coagulation in patients with 2019-nCoV pneumonia. J. Thromb. Haemost. 2020, 18, 786–787. [Google Scholar] [CrossRef] [Green Version]
- Bernard, G.R.; Vincent, J.-L.; Laterre, P.-F.; LaRosa, S.P.; Dhainaut, J.-F.; Lopez-Rodriguez, A.; Steingrub, J.S.; Garber, G.E.; Helterbrand, J.D.; Ely, E.W.; et al. Efficacy and Safety of Recombinant Human Activated Protein C for Severe Sepsis. N. Engl. J. Med. 2001, 344, 699–709. [Google Scholar] [CrossRef] [Green Version]
- Alaniz, C. An Update on Activated Protein C (Xigris) In the Management of Sepsis. Pharm. Ther. 2010, 35, 504–508. [Google Scholar]
- Paranjpe, I.; Fuster, V.; Lala, A.; Russak, A.J.; Glicksberg, B.; Levin, M.A.; Charney, A.W.; Narula, J.; Fayad, Z.A.; Bagiella, E.; et al. Association of Treatment Dose Anticoagulation With In-Hospital Survival Among Hospitalized Patients With COVID-19. J. Am. Coll. Cardiol. 2020, 76, 122–124. [Google Scholar] [CrossRef]
- Jose, R.J.P.; Manuel, A. COVID-19 cytokine storm: The interplay between inflammation and coagulation. Lancet Respir. Med. 2020, 8, e46–e47. [Google Scholar] [CrossRef]
- Iba, T.; Levy, J.H.; Warkentin, T.E.; Thachil, J.; van der Poll, T.; Levi, M. Diagnosis and management of sepsis-induced coagulopathy and disseminated in-travascular coagulation. J. Thromb. Haemost. 2019, 11, 1989–1994. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iba, T.; Di Nisio, M.; Levy, J.H.; Kitamura, N.; Thachil, J. New criteria for sepsis-induced coagulopathy (SIC) following the revised sepsis definition: A retrospective analysis of a nationwide survey. BMJ Open 2017, 7, e017046. [Google Scholar] [CrossRef] [Green Version]
- Du, R.-H.; Liang, L.-R.; Yang, C.-Q.; Wang, W.; Cao, T.-Z.; Li, M.; Guo, G.-Y.; Du, J.; Zheng, C.-L.; Zhu, Q.; et al. Predictors of mortality for patients with COVID-19 pneumonia caused by SARS-CoV-2: A prospective cohort study. Eur. Respir. J. 2020, 55, 2000524. [Google Scholar] [CrossRef] [Green Version]
- Liang, W.-H.; Guan, W.-J.; Li, C.; Li, Y.-M.; Liang, H.-R.; Zhao, Y.; Liu, X.-Q.; Sang, L.; Chen, R.-C.; Tang, C.-L.; et al. Clinical characteristics and outcomes of hospitalised patients with COVID-19 treated in Hubei (epicentre) and outside Hubei (non-epicentre): A nationwide analysis of China. Eur. Respir. J. 2020, 55, 2000562. [Google Scholar] [CrossRef] [Green Version]
- Istituto Superiore di Sanità Caratteristiche dei Pazienti Deceduti Positivi a COVID-19 in Italia. Available online: www.epicentro.iss.it/coronavirus/bollettino/Report-COVID-2019_2_aprile_eng.pdf. (accessed on 1 July 2021).
- Casucci, G.; Acanfora, D.; Incalzi, R.A. The Cross-Talk between Age, Hypertension and Inflammation in COVID-19 Patients: Therapeutic Targets. Drugs Aging. 2020, 37, 779–785. [Google Scholar] [CrossRef]
- Li, J.-Y.; You, Z.; Wang, Q.; Zhou, Z.-J.; Qiu, Y.; Luo, R.; Ge, X.-Y. The epidemic of 2019-novel-coronavirus (2019-nCoV) pneumonia and insights for emerging infectious diseases in the future. Microbes Infect. 2020, 22, 80–85. [Google Scholar] [CrossRef]
- Guan, W.-J.; Ni, Z.-Y.; Hu, Y.; Liang, W.-H.; Ou, C.-Q.; He, J.-X.; Liu, L.; Shan, H.; Lei, C.-L.; Hui, D.S.; et al. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Yu, Y.; Xu, J.; Shu, H.; Xia, J.; Liu, H.; Wu, Y.; Zang, L.; Yu, Z.; Fang, M.; et al. Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: A single-centered, retrospective, observational study. Lancet Respir. Med. 2020, 8, 475–481. [Google Scholar] [CrossRef] [Green Version]
- Franceschi, C.; Garagnani, P.; Parini, P.; Giuliani, C.; Santoro, A. Inflammaging: A new immune–metabolic viewpoint for age-related diseases. Nat. Rev. Endocrinol. 2018, 14, 576–590. [Google Scholar] [CrossRef]
- Brook, C.E.; Boots, M.; Chandran, K.; Dobson, A.P.; Drosten, C.; Graham, A.L.; Grenfell, B.T.; Müller, M.A.; Ng, M.; Wang, L.-F.; et al. Accelerated viral dynamics in bat cell lines, with implications for zoonotic emergence. eLife 2020, 9, e48401. [Google Scholar] [CrossRef]
- He, X.; Li, Z.; Tang, X.; Zhang, L.; Wang, L.; He, Y.; Jin, T.; Yuan, D. Age- and sex-related differences in body composition in healthy subjects aged 18 to 82 years. Medicine 2018, 97, e11152. [Google Scholar] [CrossRef] [PubMed]
- Bourgonje, A.R.; Abdulle, A.E.; Timens, W.; Hillebrands, J.; Navis, G.J.; Gordijn, S.J.; Bolling, M.C.; Dijkstra, G.; Voors, A.A.; Osterhaus, A.D.M.E.; et al. Angiotensin-converting enzyme 2 (ACE2), SARS-CoV-2 and the pathophysiology of coronavirus disease 2019 (COVID-19). J. Pathol. 2020, 251, 228–248. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Zheng, Y.; Gou, X.; Pu, K.; Chen, Z.; Guo, Q.; Ji, R.; Wang, H.; Wang, Y.; Zhou, Y. Prevalence of comorbidities in the novel Wuhan coronavirus (COVID-19) infection: A sys-tematic review and meta-analysis. Int. J. Infect. Dis. 2020, 9712, 91–95. [Google Scholar] [CrossRef]
- Varga, Z.; Flammer, A.J.; Steiger, P.; Haberecker, M.; Andermatt, R.; Zinkernagel, A.S.; Mehra, M.R.; Schuepbach, R.; Ruschitzka, F.; Moch, H. Endothelial cell infection and endotheliitis in COVID-19. Lancet 2020, 395, 1417–1418. [Google Scholar] [CrossRef]
- Bikdeli, B.; Madhavan, M.V.; Jimenez, D.; Chuich, T.; Dreyfus, I.; Driggin, E.; Der Nigoghossian, C.; Ageno, W.; Madjid, M.; Guo, Y.; et al. COVID-19 and thrombotic or thromboembolic disease: Implication for pre-vention, antithrombotic therapy, and follow-up. J. Am. Coll. Cardiol. 2020, 75, 2950–2973. [Google Scholar] [CrossRef]
- Poterucha, T.J.; Libby, P.; Goldhaber, S.Z. More than an anticoagulant: Do heparins have direct anti-inflammatory effects? Thromb. Haemost. 2017, 3, 437–444. [Google Scholar] [CrossRef]
- Tang, N.; Bai, H.; Chen, X.; Gong, J.; Li, D.; Sun, Z. Anticoagulant treatment is associated with decreased mortality in severe coronavirus disease 2019 patients with coagulopathy. J. Thromb. Haemost. 2020, 18, 1094–1099. [Google Scholar] [CrossRef]
- Shi, C.; Wang, C.; Wang, H.; Yang, C.; Cai, F.; Zeng, F.; Cheng, F.; Liu, Y.; Zhou, T.; Deng, B.; et al. Clinical observation of low molecular weight heparin in relieving inflammation in COVID-19 patients: A retrospective cohort study. Clin. Transl. Sci. 2020, 13, 1087–1095. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Huang, M.; Li, D.; Tang, N. Difference of coagulation features between severe pneumonia induced by SARS-CoV2 and non-SARS-CoV2. J. Thromb. Thrombolysis 2020, 51, 1107–1110. [Google Scholar] [CrossRef] [Green Version]
- Spronk, H.M.; Cate, H.T. The blood coagulation system as a molecular machine. BioEssays 2003, 25, 1220–1228. [Google Scholar] [CrossRef]
- Mackman, N.; Tilley, R.E.; Key, N.S. Role of the Extrinsic Pathway of Blood Coagulation in Hemostasis and Thrombosis. Arter. Thromb. Vasc. Biol. 2007, 27, 1687–1693. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monroe, D.M.; Hoffman, M. What does it take to make the perfect clot? Arter. Thromb. Vasc. Biol. 2006, 26, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Heuberger, D.M.; Schuepbach, R.A. Protease-activated receptors (PARs): Mechanisms of action and potential therapeutic modulators in PAR-driven inflammatory diseases. Thromb. J. 2019, 17, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Posma, J.J.; Grover, S.; Hisada, Y.; Owens, A.P.; Antoniak, S.; Spronk, H.M.; Mackman, N. Roles of Coagulation Proteases and PARs (Protease-Activated Receptors) in Mouse Models of Inflammatory Diseases. Arter. Thromb. Vasc. Biol. 2019, 39, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Geisbert, T.W.; Hensley, L.E.; Jahrling, P.B.; Larsen, T.; Geisbert, J.B.; Paragas, J.; Young, H.A.; Fredeking, T.; Rote, W.E.; Vlasuk, G.P. Treatment of Ebola virus infection with a recombinant inhibitor of factor VIIa/tissue factor: A study in rhesus monkeys. Lancet 2003, 362, 1953–1958. [Google Scholar] [CrossRef] [Green Version]
- Antoniak, S.; Mackman, N. Multiple roles of the coagulation protease cascade during virus infection. Blood 2014, 123, 2605–2613. [Google Scholar] [CrossRef] [Green Version]
- Lê, V.B.; Riteau, B.; Alessi, M.-C.; Couture, C.; Jandrot-Perrus, M.; Rhéaume, C.; Hamelin, M.; Boivin, G. Protease-activated receptor 1 inhibition protects mice against thrombin-dependent respiratory syncytial virus and human metapneumovirus infections. Br. J. Pharmacol. 2017, 175, 388–403. [Google Scholar] [CrossRef] [Green Version]
- Ellinghaus, P.; Perzborn, E.; Hauenschild, P.; Gerdes, C.; Heitmeier, S.; Visser, M.; Summer, H.; Laux, V. Expression of pro-inflammatory genes in human endothelial cell: Compar-ison of rivaroxaban and dabigatran. Thromb. Res. 2016, 142, 44–51. [Google Scholar] [CrossRef] [Green Version]
- Subbe, C.; Kruger, M.; Rutherford, P.; Gemmel, L. Validation of a modified Early Warning Score in medical admissions. Qjm: Int. J. Med. 2001, 94, 521–526. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Zhang, Q.; Huang, C.; Shi, C.; Wang, L.; Shi, N.; Fang, C.; Shan, F.; Mei, X.; Shi, J.; et al. CT quantification of pneumonia lesions in early days predicts progression to severe illness in a cohort of COVID-19 patients. Theranostics 2020, 10, 5613–5622. [Google Scholar] [CrossRef]
- Taylor, F.B., Jr.; Toh, C.H.; Hoots, W.K.; Wada, H.; Levi, M. Scientific Subcommittee on Disseminated Intravascular Coagulation (DIC) of the International Society on Thrombosis and Haemostasis (ISTH). Towards definition, clinical and laboratory criteria, and a scoring system for disseminated intravascular coagulation. Thromb. Haemost. 2001, 86, 1327–1330. [Google Scholar] [CrossRef] [Green Version]
- Spyropoulos, A.C.; Ageno, W.; Albers, G.W.; Elliott, C.G.; Halperin, J.L.; Hiatt, W.R.; Maynard, G.A.; Steg, P.G.; Weitz, J.I.; Lu, W.; et al. Post-Discharge Prophylaxis with Rivaroxaban Reduces Fatal and Major Thromboembolic Events in Medically Ill Patients. J. Am. Coll. Cardiol. 2020, 75, 3140–3147. [Google Scholar] [CrossRef]
- Ayoubkhani, D.; Khunti, K.; Nafilyan, V.; Maddox, T.; Humberstone, B.; Diamond, I.; Banerjee, A. Post-COVID syndrome in individuals admitted to hospital with COVID-19: Retrospective cohort study. BMJ 2021, 372, n693. [Google Scholar] [CrossRef]
- Shah, W.; Hillman, T.; Playford, E.D.; Hishmeh, L. Managing the long term effects of COVID-19: Summary of NICE, SIGN, and RCGP rapid guideline. BMJ 2021, 372, n136. [Google Scholar] [CrossRef]
- Mandal, S.; Barnett, J.; Brill, S.E.; Brow n, J.S.; Denneny, E.K.; Hare, S.S.; Heightman, M.; Hillman, T.E.; Jacob, J.; Jarvis, H.C.; et al. ‘Long-COVID’: A cross-sectional study of persisting symptoms, biomarker and imaging abnormalities following hospitalisation for COVID-19. Thorax 2020, 76, 396–398. [Google Scholar] [CrossRef]
- Townsend, L.; Fogarty, H.; Dyer, A.; Martin-Loeches, I.; Bannan, C.; Nadarajan, P.; Bergin, C.; Farrelly, C.O.; Conlon, N.; Bourke, N.M.; et al. Prolonged elevation of D-dimer levels in convalescent COVID-19 patients is independent of the acute phase response. J. Thromb. Haemost. 2021, 19, 1064–1070. [Google Scholar] [CrossRef]
- De Terwangne, C.; Laouni, J.; Jouffe, L.; Lechien, J.; Bouillon, V.; Place, S.; Capulzini, L.; Machayekhi, S.; Ceccarelli, A.; Saussez, S.; et al. Predictive Accuracy of COVID-19 World Health Organization (WHO) Severity Classification and Comparison with a Bayesian-Method-Based Severity Score (EPI-SCORE). Pathogens 2020, 9, 880. [Google Scholar] [CrossRef] [PubMed]
- Klok, F.A.; Boon, G.J.; Barco, S.; Endres, M.; Geelhoed, J.M.; Knauss, S.; Rezek, S.A.; Spruit, M.A.; Vehreschild, J.; Siegerink, B. The Post-COVID-19 Functional Status scale: A tool to measure functional status over time after COVID-19. Eur. Respir. J. 2020, 56, 2001494. [Google Scholar] [CrossRef] [PubMed]
- Andrade, B.S.; Siqueira, S.; Soares, W.D.A.; Rangel, F.D.S.; Santos, N.; Freitas, A.D.S.; da Silveira, P.R.; Tiwari, S.; Alzahrani, K.; Góes-Neto, A.; et al. Long-COVID and Post-COVID Health Complications: An Up-to-Date Review on Clinical Conditions and Their Possible Molecular Mechanisms. Viruses 2021, 13, 700. [Google Scholar] [CrossRef]
- Mcmurray, J.; Packer, M.; Desai, A.S.; Gong, J.; Lefkowitz, M.P.; Rizkala, A.R.; Rouleau, J.L.; Shi, V.C.; Solomon, S.D.; Swedberg, K.; et al. Angiotensin–Neprilysin Inhibition versus Enalapril in Heart Failure. N. Engl. J. Med. 2014, 371, 993–1004. [Google Scholar] [CrossRef] [Green Version]
- Acanfora, D.; Ciccone, M.M.; Scicchitano, P.; Acanfora, C.; Casucci, G. Neprilysin inhibitor–angiotensin II receptor blocker combination (sacubitril/valsartan): Rationale for adoption in SARS-CoV-2 patients. Eur. Hear. J.—Cardiovasc. Pharmacother. 2020, 6, 135–136. [Google Scholar] [CrossRef] [Green Version]
- Eikelboom, J.W.; Connolly, S.J.; Bosch, J.; Dagenais, G.R.; Hart, R.G.; Shestakovska, O.; Diaz, R.; Alings, M.; Lonn, E.M.; Anand, S.S.; et al. Rivaroxaban with or without Aspirin in Stable Cardiovascular Disease. N. Engl. J. Med. 2017, 377, 1319–1330. [Google Scholar] [CrossRef] [PubMed]
- Shi, S.; Qin, M.; Shen, B.; Cai, Y.; Liu, T.; Yang, F.; Gong, W.; Liu, X.; Liang, J.; Zhao, Q.; et al. Association of Cardiac Injury with Mortality in Hospitalized Patients with COVID-19 in Wuhan, China. JAMA Cardiol. 2020, 5, 802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cate, H.T. Surviving COVID-19 with Heparin? N. Engl. J. Med. 2021, 385, 845–846. [Google Scholar] [CrossRef] [PubMed]
- ATTACC Investigators; ACTIV-4a Investigators; REMAP-CAP Investigators. Therapeutic Anticoagulation with Heparin in Noncritically Ill Patients with COVID-19. N. Engl. J. Med. 2021, 385, 790–802. [Google Scholar] [CrossRef] [PubMed]




| Laboratory Values (Reference Range) | 24 November 2020 | 25 November 2020 | 26 November 2020 | 27 November 2020 | 28 November 2020 | 29 November 2020 | 30 November 2020 |
|---|---|---|---|---|---|---|---|
| White Blood Cells count (3.7–10.3), ×109/L | 13.52 | 14.6 | 14.3 | 13.7 | 12.5 | 13.9 | 13.58 |
| Neutrophils (40–75), % | 87.6 | 88.0 | 87.2 | 86.8 | 81.0 | 82.1 | 77.2 |
| Lymphocytes (19–48), % | 6.6 | 6.0 | 6.5 | 9.2 | 10.2 | 10.6 | 11 |
| Eosinophils (0–7), % | 0 | 1 | 2 | 2 | 1 | 2 | 0.3 |
| Red Blood Cells count (4.2–6.0), ×106/L | 5.32 | 5.42 | 5.12 | 4.92 | 4.91 | 5.2 | 5.31 |
| Haemoglobin (13.7–17.5), g/dL | 15.4 | 14.9 | 14.6 | 13.2 | 13.6 | 14.2 | 15.1 |
| Platelet count (155–369), ×109/L | 311 | 70 | 90 | 180 | 220 | 310 | 346 |
| Prothrombin time (9.6–12.5), second | 13.4 | 18.2 | 16.2 | 13.2 | 10.2 | 10.2 | 10.4 |
| International normalized ratio (INR) (0.9–1.2) | 0.99 | 1.1 | 1.2 | 1.0 | 0.9 | 1.0 | 1.1 |
| Activated partial thromboplastin time (19–30), s | 29.3 | 33.2 | 34.6 | 35.1 | 29.1 | 28.5 | 27.6 |
| Fibrinogen (150–450), mg/dL | 570 | 220 | 300 | 420 | 510 | 480 | 366 |
| Lactate dehydrogenase (140–280), U/L | 1149 | 1520 | 1480 | 921 | 843 | 601 | 570 |
| Creatinine (0.8–1.30), mg/dL | 0.8 | 1.0 | 1.1 | 1.0 | 0.9 | 0.9 | 0.9 |
| Erytrocite Sedimentation Rate (0–15), mm | 62 | 121 | 144 | 80 | 73 | 52 | 31 |
| High Sensitivity C Reactive Proteine (0–45), mg/L | 104.9 | 158.8 | 161.2 | 82.1 | 40.1 | 18.2 | 2.23 |
| IL-6 (0–6.4) pg/mL | 36.74 | 84.2 | 96.8 | 72.3 | 42.1 | 16.3 | 5.56 |
| D-dimer (250–500), ng/mL | 1044 | 13,298 | 18,481 | 4280 | 3187 | 2128 | 347 |
| Disseminated Intravascular Coagulation Score | 0 | 6 | 6 | 4 | 1 | 0 | 0 |
| Demographic, Medical History and Vital Signs | Long-COVID-19 | No COVID-19 |
|---|---|---|
| Number of patients, n | 30 | 20 |
| Sex, M/F, n | 17/13 | 8/12 |
| Age, years a | 58.6 ± 17.6 | 56.3 ± 14.7 |
| Weight, kg a | 77.1 ± 14.5 | 73.8 ± 12 |
| Height, cm a | 164.6 ± 11.4 | 169.1 ± 8.7 |
| Body mass index, kg/m2 a | 28.4 ± 4.2 | 25.7 ± 2.4 |
| Pre-existing conditions in the last year, n (%) | ||
| Cancer | 2 (6.7%) | 1 (5.0%) |
| Chronic heart disease | 13 (43.3%) | 6 (30.0%) |
| Chronic kidney disease | 5 (16.6%) | 2 (10.0%) |
| Chronic liver disease | 3 (10.0%) | 1 (5.0%) |
| Chronic lung disease | 7 (23.3%) | 7 (35.0%) |
| Chronic neurological disease | 9 (30.0%) | 5 (25.0%) |
| Diabetes | 7 (23.7%) | 3 (15.0%) |
| Hypertension | 19 (63.3%) | 11 (55.0%) |
| Mental health conditions | 2 (6.66%) | 1 (5.0%) |
| Obesity (Body Mass Index > 30) | 11 (36.6%) | 3 (15.0%) |
| Heart rate, bpm a | 73 ± 15 | 70 ± 13 |
| Systolic arterial pressure, mmHg a | 121 ± 15 | 121 ± 17 |
| Diastolic arterial pressure, mmHg a | 78 ± 12 | 76 ± 10 |
| Therapies, n (%) | ||
| ACE-I/ARB/ARNIs | 19 (63%) | 12 (60%) |
| Beta-blocker | 11 (37%) | 8 (40%) |
| ASA | 13 (43%) | 9 (45%) |
| Diuretics | 11 (37%) | 6 (30%) |
| Anticoagulants | 12 (40%) | 6 (30%) |
| Echocardiography Measurements | ||
| LV end diastolic dimension, cm a | 4.8 ± 1 | 4.5 ± 0.6 |
| LV end diastolic volume, mL a | 114.6 ± 52.5 | 94.1 ± 27.9 |
| LV end systolic dimension, cm a | 3.2 ± 1.04 | 2.6 ± 0.5 * |
| LV end systolic volume, mL a | 48.7 ± 38.5 | 28 ± 10.5 † |
| LV ejection fraction, % a | 61.9 ± 13.7 | 70.4 ± 5.7 • |
| Left atrial anteroposterior dimension, cm a | 3.7 ± 1.3 | 3.5 ± 0.5 |
| E/A ratio a | 1.02 ± 0.4 | 1.1 ± 0.3 |
| SPAP, mmHg a | 13.8 ± 10.5 | 14.6 ± 8.6 |
| Laboratory Values (Reference Range) | Long-COVID-19 | No COVID-19 |
|---|---|---|
| White Blood Cells count (3.7–10.3), ×109/L a | 6.84 ± 2.6 | 7.14 ± 2.3 |
| Red Blood Cells count (4.0–10.0), ×106/L a | 4.53 ± 0.6 | 4.8 ± 0.58 |
| Haemoglobin (13.7–17.5), g/dL a | 14.9 ± 6.4 | 14.2 ± 1.8 |
| Platelet count (155–369), ×109/L a | 221 ± 92 | 244 ± 50 |
| Prothrombin time (9.6–12.5), s a | 14.2 ± 2.5 | 13.5 ± 1.2 |
| International normalized ratio (0.9–1.2) a | 1.07 ± 0.2 | 1.00 ± 0.09 |
| Activated Partial Thromboplastin Time (19–30), s a | 30.6 ± 5.1 | 28.8 ± 2.6 |
| Fibrinogen (150–450), mg/dL a | 364.8 ± 154.4 | 326.9 ± 86.1 |
| Lactate dehydrogenase (140–280), U/L a | 448.1 ± 133 | 342.45 ± 90.5 * |
| Creatinine (0.8–1.30), mg/dL a | 0.92 ± 0.25 | 0.86 ± 0.23 |
| Aspartate Aminotrasferase (0–31), U/L a | 25.04 ± 12.2 | 21.6 ± 12.2 |
| Alanine Aminotrasferase (0–34), U/L a | 25.2 ± 14.5 | 20.9 ± 14.6 |
| High Sensitivity C Reactive Protein (0–45), mg/L a | 16.3 ± 50.1 | 3.95 ± 8.8 |
| Sodium (135–155), mEq/L a | 139 ± 2.7 | 139 ± 2.02 |
| Potassium (3.5–5.5), mEq/L a | 4.1 ± 0.27 | 4.3 ± 0.4 |
| D-dimer (250–500), ng/mL a | 1044.4 ± 1022 | 273.7 ± 106 † |
| Erythrocyte Sedimentation Rate (0–15), mm a | 25.7 ± 33.2 | 15.5 ± 17.2 |
| Albuminuria (0–2.5), mg/dL a | 120.7 ± 134.7 | 64.6 ± 17.7 |
| Interleukin-6 (0–6.4), pg/mL a | 13.2 ± 3 | 3 ± 2.7 • |
| High-sensitivity Cardiac Troponin (<19), ng/mL a | 9 ± 26.3 | 1.6 ± 0.3 |
| NT-ProBNP (<450), pg/mL a | 587.4 ± 273 | 273.5 ± 147.9 ◊ |
| SARS-CoV-2 Anti-Spike IgM (<1), EU/mL a | 12.2 ± 35.5 | 1.04 ± 2.4 |
| SARS-CoV-2 Anti-Spike IgG (<10), EU/mL a | 91.5 ± 130.1 | 35.9 ± 61.5 |
| Serum Ferritin (20–300), ng/mL a | 144.6 ± 158.6 | 113 ± 85.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Acanfora, D.; Acanfora, C.; Ciccone, M.M.; Scicchitano, P.; Bortone, A.S.; Uguccioni, M.; Casucci, G. The Cross-Talk between Thrombosis and Inflammatory Storm in Acute and Long-COVID-19: Therapeutic Targets and Clinical Cases. Viruses 2021, 13, 1904. https://doi.org/10.3390/v13101904
Acanfora D, Acanfora C, Ciccone MM, Scicchitano P, Bortone AS, Uguccioni M, Casucci G. The Cross-Talk between Thrombosis and Inflammatory Storm in Acute and Long-COVID-19: Therapeutic Targets and Clinical Cases. Viruses. 2021; 13(10):1904. https://doi.org/10.3390/v13101904
Chicago/Turabian StyleAcanfora, Domenico, Chiara Acanfora, Marco Matteo Ciccone, Pietro Scicchitano, Alessandro Santo Bortone, Massimo Uguccioni, and Gerardo Casucci. 2021. "The Cross-Talk between Thrombosis and Inflammatory Storm in Acute and Long-COVID-19: Therapeutic Targets and Clinical Cases" Viruses 13, no. 10: 1904. https://doi.org/10.3390/v13101904
APA StyleAcanfora, D., Acanfora, C., Ciccone, M. M., Scicchitano, P., Bortone, A. S., Uguccioni, M., & Casucci, G. (2021). The Cross-Talk between Thrombosis and Inflammatory Storm in Acute and Long-COVID-19: Therapeutic Targets and Clinical Cases. Viruses, 13(10), 1904. https://doi.org/10.3390/v13101904







