Morphological Aspects and Viremia Analysis of BALB/c Murine Model Experimentally Infected with Dengue Virus Serotype 4
Abstract
:1. Introduction
2. Materials and Methods
2.1. Ethics Statement
2.2. Viral Strain
2.3. Viral Stock Production
2.4. Animals
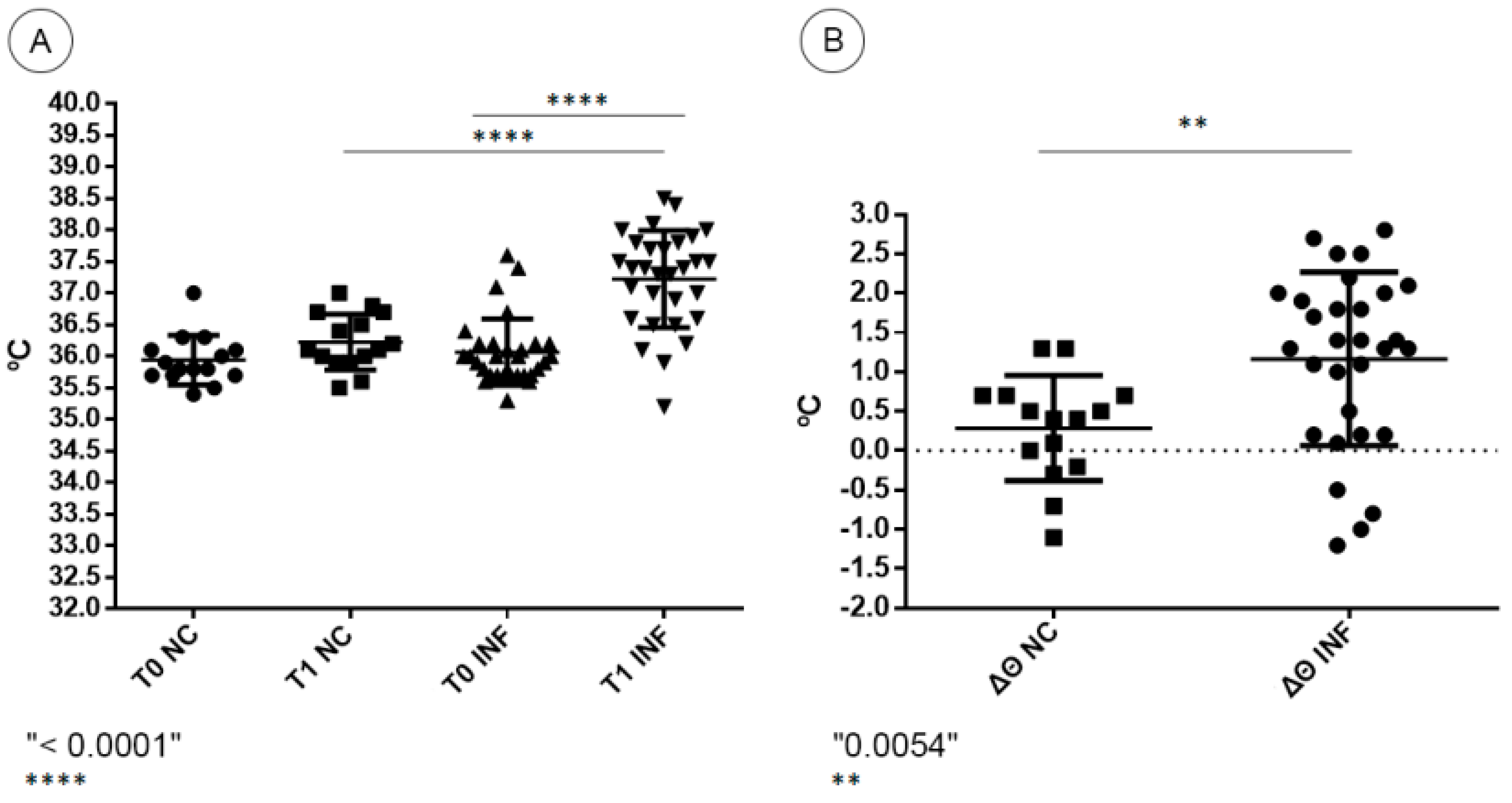
2.5. Temperature Gauging and Clinical Aspects
2.6. Statistical Analysis
2.7. Experimental Infection
2.8. Euthanasia and Organ Excision
2.9. Serum Sampling
2.10. Bright Field Microscopy
2.11. Fixation by Perfusion and Transmission Electron Microscopy
2.12. Immunohistochemistry
2.13. Molecular Biology
2.14. Real-Time Quantitative RT-PCR
3. Results
3.1. Clinical Aspects
3.2. Morphological Analysis
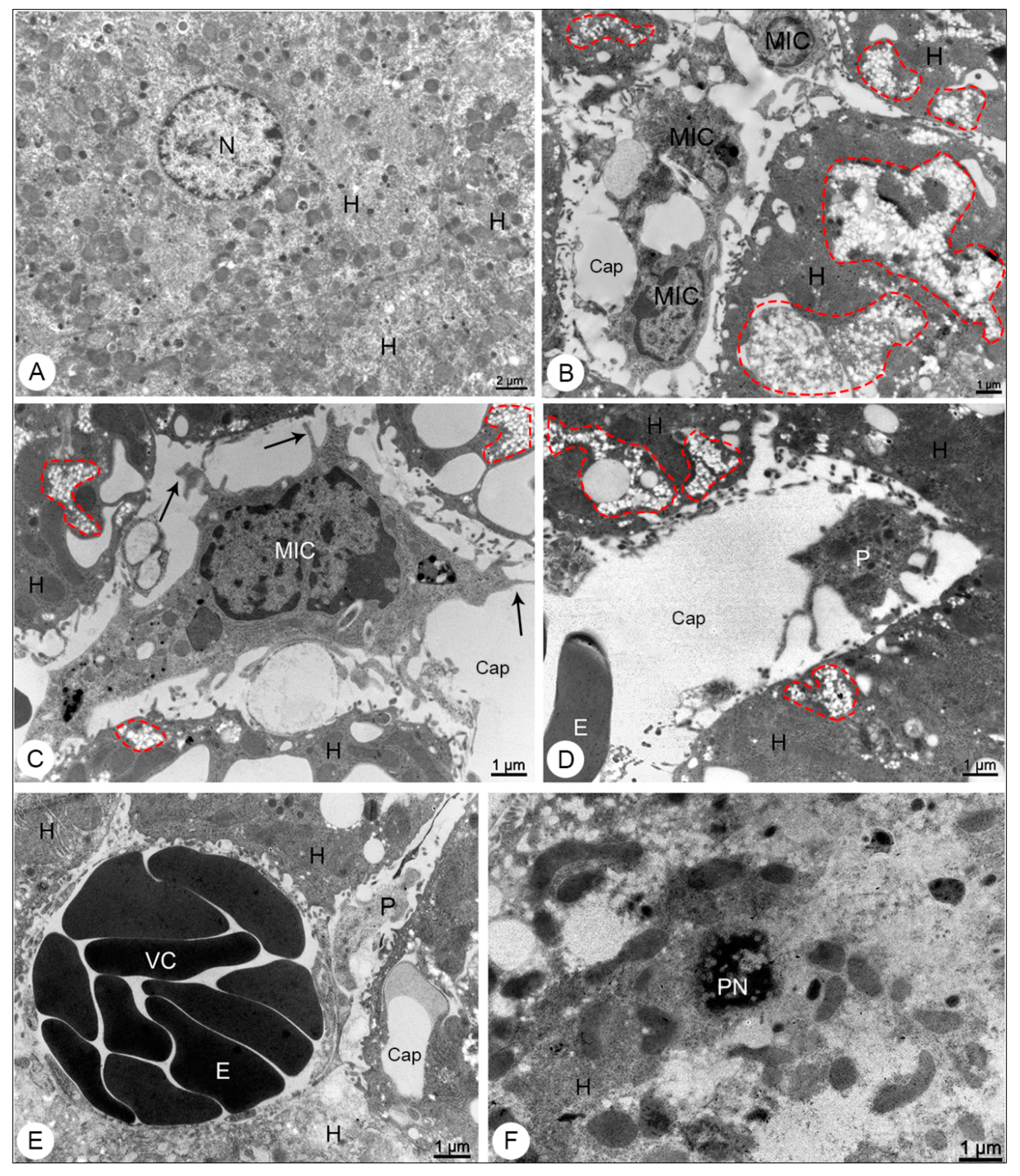
3.2.1. Liver Samples
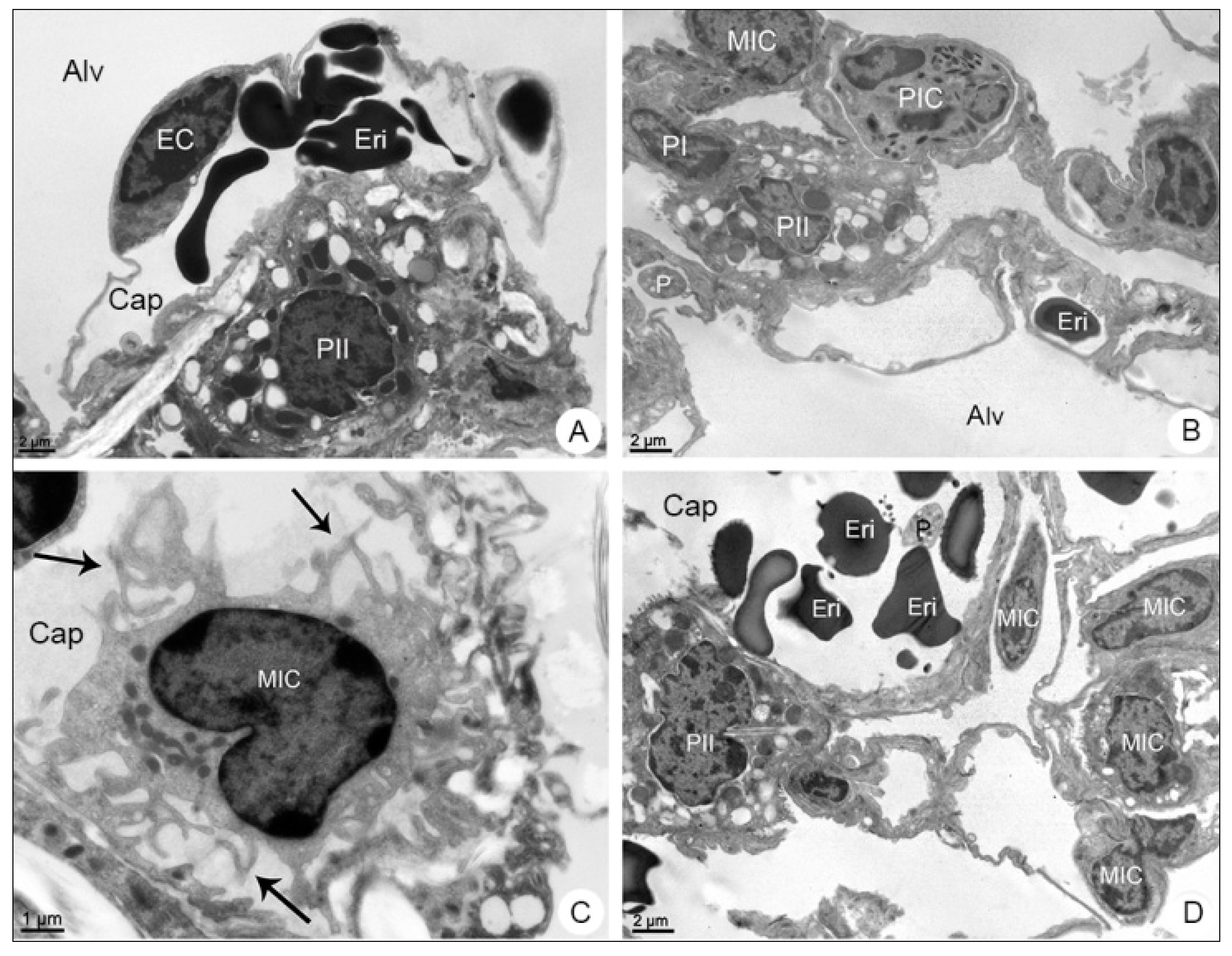
3.2.2. Lung Samples
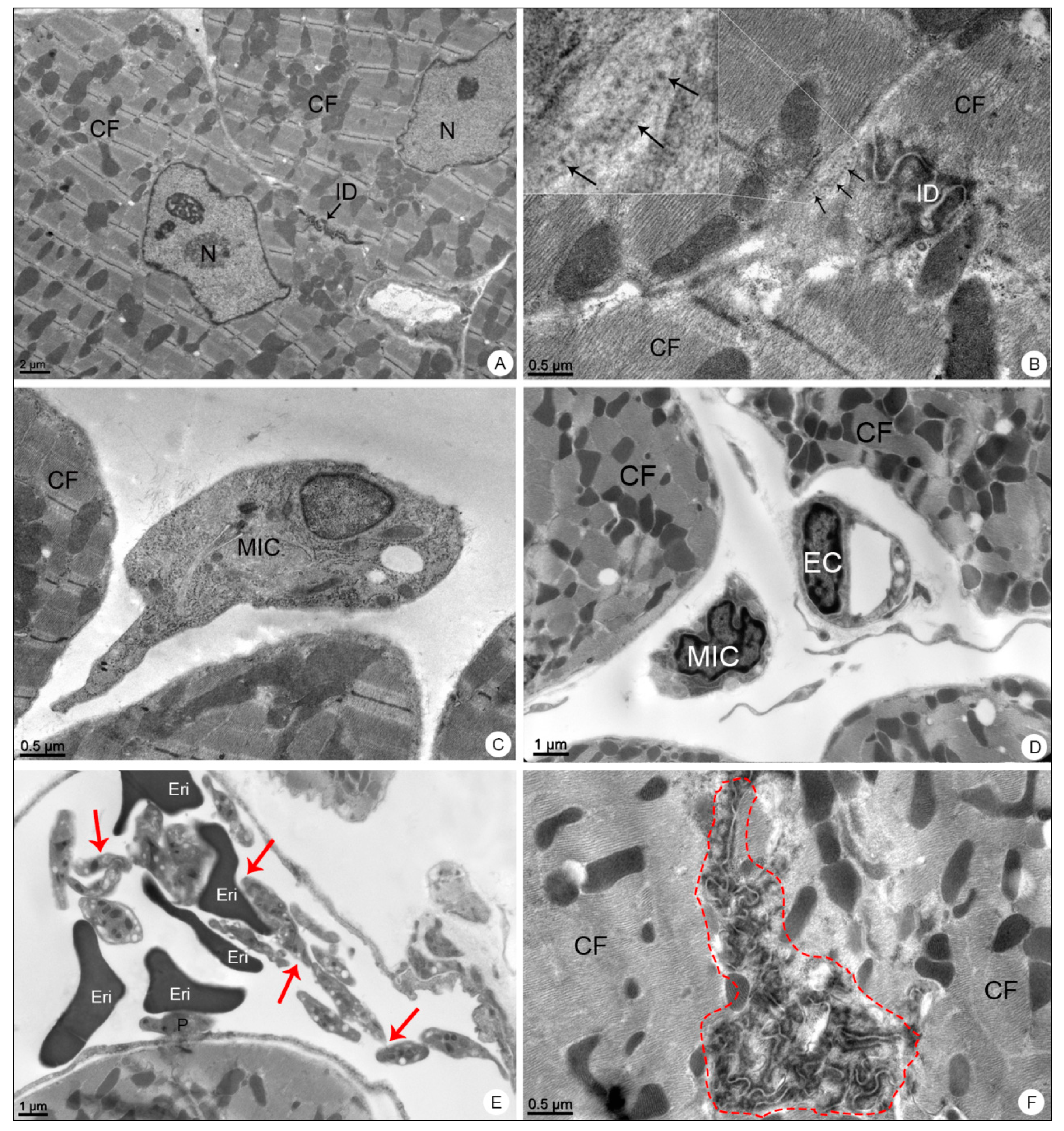
3.2.3. Heart Samples
3.3. Immunohistochemistry
3.4. Molecular Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gubler, D.J. Epidemic dengue/dengue hemorrhagic fever as a public health, social and economic problem in the 21st century. Trends Microbiol. 2002, 10, 100–103. [Google Scholar] [CrossRef]
- Stanaway, J.D.; Shepard, D.S.; Undurraga, E.A.; Halasa, Y.A.; Coffeng, L.E.; Brady, O.J.; Hay, S.I.; Bedi, N.; Bensenor, I.M.; Castañeda-Orjuela, C.A.; et al. The global burden of dengue: An analysis from the Global Burden of Disease Study 2013. Lancet Infect. Dis. 2016, 16, 712–723. [Google Scholar] [CrossRef] [Green Version]
- Nogueira, R.M.; Eppinghaus, A.L. Dengue virus type 4 arrives in the state of Rio de Janeiro: A challenge for epidemiological surveillance and control. Mem. Inst. Oswaldo Cruz 2011, 106, 255–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Secretaria de Vigilância em Saúde/Ministério da Saúde. Boletim Epidemiológico-Volume 43-nº 1–2012. Dengue: Situação Epidemiológica (de Janeiro a Abril de 2012). Available online: https://antigo.saude.gov.br/images/pdf/2014/julho/23/BE-2012-43--1--pag-11-a-15-Dengue.pdf (accessed on 17 June 2021).
- Burke, D.S.; Nisalak, A.; Johnson, D.E.; Scott, R.M. A prospective study of dengue infections in Bangkok. Am. J. Trop. Med. Hyg. 1988, 38, 172–180. [Google Scholar] [CrossRef]
- Cardosa, M. Dengue vaccine design: Issues and challenges. Br. Med. Bull. 1998, 54, 395–405. [Google Scholar] [CrossRef] [Green Version]
- Bäck, A.; Lundkvist, Å. Dengue viruses—An overview. Infect. Ecol. Epidemiol. 2013, 3, 19839. [Google Scholar]
- Fernando, S.; Wijewickrama, A.; Gomes, L.; Punchihewa, C.T.; Madusanka, S.D.P.; Dissanayake, H.; Jeewandara, C.; Peiris, H.; Ogg, G.S.; Malavige, G.N. Patterns and causes of liver involvement in acute dengue infection. BMC Infect. Dis. 2016, 16, 319. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kularatne, S.A.; Gawarammana, I.B.; Kumarasiri, P.R. Epidemiology, clinical features, laboratory investigations and early diagnosis of dengue fever in adults: A descriptive study in Sri Lanka. Southeast Asian J. Trop. Med. Public Health 2005, 36, 686–692. [Google Scholar]
- Gulati, S.; Maheshwari, A. Atypical manifestations of dengue. Trop. Med. Int. Health 2007, 12, 1087–1095. [Google Scholar] [CrossRef]
- Sam, S.S.; Omar, S.F.; Teoh, B.T.; Abd-Jamil, J.; AbuBakar, S. Review of dengue hemorrhagic fever fatal cases seen among adults: A retrospective study. PLoS Negl. Trop. Dis. 2013, 7, e2194. [Google Scholar] [CrossRef] [Green Version]
- Arora, S.; Nathaniel, S.D.; Paul, J.C.; Hansdak, S.G. Acute liver failure in dengue haemorrhagic fever. BMJ Case Rep. 2015, 2015, bcr2015209443. [Google Scholar] [CrossRef] [Green Version]
- Motimath, P.; Patil, R.S.; Morkar, D.N.; Krishna, P. Acute fulminant hepatic failure due to primary dengue: A case report. J. Assoc. Physicians India 2016, 64, 120–121. [Google Scholar]
- Basílio-de-Oliveira, C.A.; Aguiar, G.R.; Baldanza, M.S.; Barth, O.M.; Eyer-Silva, W.A.; Paes, M.V. Pathologic study of a fatal case of dengue-3 virus infection in Rio de Janeiro, Brazil. Braz. J. Infect. Dis. 2005, 9, 341–347. [Google Scholar] [CrossRef] [Green Version]
- Póvoa, T.F.; Alves, A.M.B.; Oliveira, C.A.B.; Nuovo, G.J.; Chagas, V.L.A.; Paes, M.V. The pathology of severe dengue in multiple organs of human fatal cases: Histopathology, ultrastructure and virus replication. PLoS ONE 2014, 9, e83386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paes, M.V.; Pinhão, A.T.; Barreto, D.F.; Costa, S.M.; Oliveira, M.P.; Nogueira, A.C.; Takiya, C.M.; Farias-Filho, J.C.; Schatzmayr, H.G.; Alves, A.M.B.; et al. Liver injury and viremia in mice infected with dengue-2 virus. Virology 2005, 338, 236–246. [Google Scholar] [CrossRef] [Green Version]
- Barth, O.M.; Barreto, D.F.; Paes, M.V.; Takiya, C.M.; Pinhão, A.T.; Schatzmayr, H.G. Morphological studies in a model for dengue-2 virus infection in mice. Mem. Inst. Oswaldo Cruz 2006, 101, 905–915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caldas, G.C. Estudos Morfológicos e Moleculares de Tecido de Modelo Murino Infectados Experimentalmente Com Vírus Dengue Sorotipo 3; Rio de Janeiro Federal University: Rio de Janeiro, Brazil, 2016. [Google Scholar]
- Jácome, F.C.; Caldas, G.C.; Rasinhas, A.C.; de Almeida, A.L.T.; de Souza, D.D.C.; Paulino, A.C.; Leonardo, R.; Barth, O.M.; dos Santos, F.B.; Barreto-Vieira, D.F. Comparative analysis of liver involvement caused by two DENV-2 lineages using an immunocompetent murine model. Sci. Rep. 2021, 11, 9723. [Google Scholar] [CrossRef] [PubMed]
- Marchiori, E.; Ferreira, J.L.N.; Bittencourt, C.N.; de Araújo Neto, C.A.; Zanetti, G.; Mano, C.M.; Santos, A.A.S.D.; Vianna, A.D. Pulmonary hemorrhage syndrome associated with dengue fever, high-resolution computed tomography findings: A case report. Orphanet J. Rare Dis. 2009, 4, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, I.K.; Lee, W.H.; Liu, J.W.; Yang, K.D. Acute myocarditis in dengue hemorrhagic fever: A case report and review of cardiac complications in dengue-affected patients. Int. J. Infect. Dis. 2010, 14, e919–e922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodrigues, R.S.; Brum, A.L.G.; Paes, M.V.; Póvoa, T.F.; Basilio-de-Oliveira, C.A.; Marchiori, E.; Borghi, D.P.; Ramos, G.V.; Bozza, F.A. Lung in dengue: Computed tomography findings. PLoS ONE 2014, 9, e96313. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barreto, D.F.; Paes, M.V.; Takiya, C.M.; Pinhão, A.T.; Côrtes, L.M.C.; Majerowicz, S.; Barth, O.M. Mice lung experimentally infected with dengue-2 virus: Ultrastructural aspects. Virus Rev. Res. 2002, 7, e96313. [Google Scholar] [CrossRef] [Green Version]
- Barreto, D.F.; Takiya, C.M.; Schatzmayr, H.G.; Nogueira, R.M.R.; Farias-Filho, J.C.; Barth, O.M. Histopathological and ultrastructural aspects of mice lungs experimentally infected with dengue virus serotype 2. Mem. Inst. Oswaldo Cruz 2007, 102, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Jácome, F.C.; Teixeira de Almeida, A.L.; Coutinho de Souza, D.D.; da Costa Rasinhas, A.; Caldas, G.C.; Nunes da Silva, M.A.; Barth, O.M.; Barreto-Vieira, D.F. Secondary dengue infection in immunocompetent murine model leads to heart tissue damage. Acta Virol. 2019, 63, 292–300. [Google Scholar] [CrossRef] [PubMed]
- Shivanthan, M.; Navinan, M.; Constantine, G.; Rajapakse, S. Cardiac involvement in dengue infection. J. Infect. Dev. Ctries. 2015, 9, 338–346. [Google Scholar] [CrossRef] [Green Version]
- Gupta, V.K.; Gadpayle, A.K. Subclinicical cardiac involvement in dengue haemorrahgic fever. J. Indian Acad. Clin. Med. 2010, 11, 107–111. [Google Scholar]
- Yacoub, S.; Griffiths, A.; Chau, T.T.H.; Simmons, C.P.; Wills, B.; Hien, T.T.; Henein, M.; Farrar, J. Cardiac function in Vietnamese patients with different dengue severity grades. J. Crit. Care Med. 2012, 40, 477–483. [Google Scholar] [CrossRef] [Green Version]
- Weerakoon, K.G.; Kularatne, S.A.; Edussuriya, D.H.; Kodikara, S.K.; Gunatilake, L.P.; Pinto, V.G.; Seneviratne, A.B.; Gunasena, S. Histopathological diagnosis of myocarditis in a dengue outbreak in Sri Lanka, 2009. BMC Res. Notes 2011, 4, 268. [Google Scholar] [CrossRef] [Green Version]
- Veloso, H.H.; Júnior, J.A.F.; de Paiva, J.M.B.; Honório, J.F.H.; Bellei, N.C.H.; de Paola, A.A.V. Acute atrial fibrillation during dengue hemorrhagic fever. Braz. J. Infect. Dis. 2003, 7, 418–422. [Google Scholar] [CrossRef] [Green Version]
- Salgado, D.M.; Eltit, J.M.; Mansfield, K.; Panqueba, C.; Castro, D.; Vega, M.R.; Xhaja, K.; Schmidt, D.; Martin, K.J.; Allen, P.D.; et al. Heart and skeletal muscle are targets of dengue virus infection. Pediatr. Infect. Dis. J. 2010, 29, 238–242. [Google Scholar] [CrossRef] [Green Version]
- Navinan, M.R.; Yudhishdran, J.; Herath, S.; Liyanage, I.; Kugadas, T.; Kumara, D.; Kulatunga, A. Complete heart block in dengue complicating management of shock due to both bleeding and leakage: A case report. BMC Res. Notes 2015, 8, 68. [Google Scholar] [CrossRef] [Green Version]
- Sheetal, S.; Jacob, E. A Study on the cardiac manifestations of dengue. J. Assoc. Physicians India 2016, 64, 30–34. [Google Scholar]
- Pereda, M.G.; López, M.; Mariluz, M. Myocarditis and complicated dengue: A case report. Rev. Chil. Infectol. 2015, 32, 238–239. [Google Scholar] [CrossRef] [Green Version]
- Miranda, C.H.; Borges, M.C.; Schmidt, A.; Pazin-Filho, A.; Rossi, M.A.; Ramos, S.G.; da Fonseca, B.A.L. A case presentation of a fatal dengue myocarditis showing evidence for dengue virus-induced lesion. Eur. Heart J. Acute Cardiovasc. Care 2013, 2, 127–130. [Google Scholar] [CrossRef] [Green Version]
- Thein, S.; Aung, M.M.; Shwe, T.N.; Aye, M.; Zaw, A.; Aye, K.; Aye, K.M.; Aaskov, J. Risk factors in dengue shock syndrome. Am. J. Trop. Med. Hyg. 1997, 56, 566–572. [Google Scholar] [CrossRef]
- Simmons, C.; Farrar, J.; van Vinh Chau, N.; Wills, B. Dengue. N. Engl. J. Med. 2012, 366, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- Mawson, A.R. Retinoids, race and the pathogenesis of dengue hemorrhagic fever. Med. Hypotheses 2013, 81, 1069–1074. [Google Scholar] [CrossRef] [PubMed]
- Zubair, M.; Ashraf, M.; Ahsan, A.; Nazir, N.; Hanif, H.; Khan, H.A. Dengue viral infections in Pakistan and other Asian countries: A comprehensive review. J. Pak. Med. Assoc. 2016, 66, 884–888. [Google Scholar] [PubMed]
- Rico-Hesse, R.; Harrison, L.M.; Salas, R.A.; Tovar, D.; Nisalak, A.; Ramos, C.; Boshell, J.; de Mesa, M.T.; Nogueira, R.M.; da Rosa, A.T.; et al. Origins of dengue type 2 viruses associated with increased pathogenicity in the Americas. Virology 1997, 230, 244–251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rothman, A.; Ennis, F. Immunopathogenesis of dengue hemorrhagic fever. Virology 1999, 257, 1–6. [Google Scholar] [CrossRef]
- Yauch, L.; Shresta, S. Mouse models of dengue virus infection and disease. Antivir. Res. 2008, 80, 87–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, K.; Zompi, S.; Beatty, P.; Harris, E. A Mouse model for studying dengue virus pathogenesis and immune response. Ann. N. Y. Acad. Sci. 2009, 1171, E12–E23. [Google Scholar] [CrossRef]
- del Angel, R.; Reyes-del Valle, J. Dengue vaccines: Strongly sought but not a reality just yet. PLoS Pathog. 2013, 9, e1003551. [Google Scholar] [CrossRef] [Green Version]
- Wan, S.; Lin, C.; Wang, S.; Chen, Y.; Yeh, T.; Liu, H.; Anderson, R.; Lin, Y. Current progress in dengue vaccines. J. Biomed. Sci. 2013, 20, 37. [Google Scholar] [CrossRef] [Green Version]
- Salazar, M.; del Angel, R.; Lanz-Mendoza, H.; Ludert, J.; Pando-Robles, V. The role of cell proteins in dengue virus infection. J. Proteom. 2014, 111, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Zellweger, R.; Shresta, S. Mouse models to study dengue virus immunology and pathogenesis. Front. Immunol. 2014, 5, 151. [Google Scholar] [CrossRef] [Green Version]
- Sarathy, V.V.; Infante, E.; Li, L.; Campbell, G.A.; Wang, T.; Paessler, S.; Beatty, R.; Harris, E.; Milligan, G.N.; Bourne, N.; et al. Characterization of lethal dengue virus type 4 (DENV-4) TVP-376 infection in mice lacking both IFN-α/β and IFN-γ receptors (AG129) and comparison with the DENV-2 AG129 mouse model. J. Gen. Virol. 2015, 96, 3035–3048. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, R.A.S.; da Silva, M.M.C.; Calzavara-Silva, C.E.; Silva, A.M.; Cordeiro, M.T.; de Moura, P.M.M.F.; Baptista Filho, P.N.; Marques Júnior, E.T.A.M.; Gil, L.H.V.G. Primary dengue haemorrhagic fever in patients from northeast of Brazil is associated with high levels of interferon-β; during acute phase. Mem. Inst. Oswaldo Cruz 2016, 111, 378–384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costa, S.M.; Yorio, A.P.; Gonçalves, A.J.S.; Vidale, M.M.; Costa, E.C.B.; Mohana-Borges, R.; Motta, M.A.; Freire, M.S.; Alves, A.M.B. Induction of a protective response in mice by the dengue virus NS3 protein using DNA vaccines. PLoS ONE 2011, 6, e25685. [Google Scholar] [CrossRef] [Green Version]
- Lazo, L.; Izquierdo, A.; Suzarte, E.; Gil, L.; Valdés, I.; Marcos, E.; Álvarez, M.; Romero, Y.; Guzmán, M.G.; Guillén, G.; et al. Evaluation in mice of the immunogenicity and protective efficacy of a tetravalent subunit vaccine candidate against dengue virus. Microbiol. Immunol. 2014, 58, 219–226. [Google Scholar] [CrossRef]
- Zompi, S.; Harris, E. Animal models of dengue virus infection. Viruses 2012, 4, 62–82. [Google Scholar] [CrossRef] [Green Version]
- Runtuwene, L.R.; Konishi, E.; Yamanaka, A.; Makino, Y.; Suzuki, Y.; Takasaki, T.; Kurane, I.; Kobayashi, T.; Eshita, Y. Dengue transmission model by means of viremic adult immuno-competent mouse. Parasites Vectors 2014, 7, 143. [Google Scholar] [CrossRef] [Green Version]
- Rasinhas, A.C. Análises Morfológicas de Tecido Cardíaco de Camundongo BALB/c Com Quadro de Infecção Primária e Secundária Pelos Vírus Dengue Sorotipo 1, 2 e 3; Fluminese Federal University: Rio de Janeiro, Brazil, 2013. [Google Scholar]
- Barreto-Vieira, D.F.; Barth, O.M. Negative and Positive Staining in Transmission Electron Microscopy for Virus Diagnosis. In Microbiology in Agriculture and Human Health; Shah, M.M., Ed.; InTech: London, UK, 2015; pp. 45–56. [Google Scholar]
- Christofferson, R.; McCracken, M.; Johnson, A.; Chisenhall, D.; Mores, C. Development of a transmission model for dengue virus. Virol. J. 2013, 10, 127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, K.; Li, S.J.; Chen, S.; Liu, H.; Lin, Y.; Yeh, T.; Liu, C.; Lei, H. Manifestation of thrombocytopenia in dengue-2-virus-infected mice. J. Gen. Virol. 2000, 81, 2177–2182. [Google Scholar] [CrossRef]
- Shresta, S.; Kyle, J.L.; Beatty, P.R.; Harris, E. Early activation of natural killer and B cells in response to primary dengue virus infection in A/J mice. Virology 2004, 319, 262–273. [Google Scholar] [CrossRef] [Green Version]
- Hotta, H.; Hotta, S. Dengue virus multiplication in cultures of mouse peritoneal macrophages: Effects of macrophage activators. Microbiol. Immunol. 1982, 26, 665–676. [Google Scholar] [CrossRef] [Green Version]
- Atrasheuskaya, A.; Petzelbauer, P.; Fredeking, T.; Ignatyev, G. Anti-TNF antibody treatment reduces mortality in exper-imental dengue virus infection. FEMS Immunol. Med. Microbiol. 2003, 35, 33–42. [Google Scholar] [CrossRef]
- França, R.F.; Zucoloto, S.; da Fonseca, B.A.A. BALB/c mouse model shows that liver involvement in dengue disease is immune-mediated. Exp. Mol. Pathol. 2010, 89, 321–326. [Google Scholar] [CrossRef]
- Velandia-Romero, M.; Calderón-Peláez, M.; Castellanos, J. In vitro infection with dengue virus induces changes in the structure and function of the mouse brain endothelium. PLoS ONE 2016, 11, e0157786. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Balsitis, S.J.; Williams, K.L.; Lachica, R.; Flores, D.; Kyle, J.L.; Mehlhop, E.; Johnson, S.; Diamond, M.S.; Beatty, P.R.; Harris, E. Lethal antibody enhancement of dengue disease in mice is prevented by Fc modification. PLoS Pathog. 2010, 6, e1000790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Clark, K.; Onlamoon, N.; Hsiao, H.; Perng, G.; Villinger, F. Can non-human primates serve as models for investigating dengue disease pathogenesis? Front. Microbiol. 2013, 4, 305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Alwis, R.; Williams, K.L.; Schmid, M.A.; Lai, C.; Patel, B.; Smith, S.A.; Crowe, J.E.; Wang, W.; Harris, E.; de Silva, A.M. Dengue viruses are enhanced by distinct populations of serotype cross-reactive antibodies in human immune sera. PLoS Pathog. 2014, 10, e1004386. [Google Scholar] [CrossRef] [Green Version]
- Johnson, B.W.; Russell, B.J.; Lanciotti, R.S. Serotype-specific detection of dengue viruses in a fourplex real-time reverse transcriptase PCR assay. J. Clin. Microbiol. 2005, 43, 4977–4983. [Google Scholar] [CrossRef] [Green Version]
- Igarashi, A. Isolation of a Singh’s Aedes albopictus cell clone sensitive to Dengue and Chikungunya viruses. J. Gen. Virol. 1978, 40, 531–544. [Google Scholar] [CrossRef]
- Gubler, D.J.; Kuno, G.; Sather, G.E.; Velez, M.; Oliver, A. Mosquito cell cultures and specific monoclonal antibodies in surveillance for dengue viruses. Am. J. Trop. Med. Hyg. 1984, 33, 158–165. [Google Scholar] [CrossRef]
- Reed, L.J.; Muench, H. A simple method of estimating fifty per cent endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Barreto-Vieira, D.F.; Barth-Schatzmayr, O.M.; Schatzmayr, H.G. Modelo Animal Experimental Para o Estudo da Patogênese dos Vírus Dengue Sorotipos 1 e 2. Manual de Técnicas; Interciência: Rio de Janeiro, Brazil, 2010. [Google Scholar]
- Reynolds, E. The use of lead citrate at high pH as an electron-opaque stain in electron. J. Cell Biol. 1963, 17, 208–212. [Google Scholar] [CrossRef] [Green Version]
- Balmaseda, A.; Hammond, S.N.; Pérez, L.; Tellez, Y.; Saborío, S.I.; Mercado, J.C.; Cuadra, R.; Rocha, J.; Pérez, M.A.; Silva, S.; et al. Serotype-specific Differences in Clinical Manifestations of Dengue. Am. J. Trop. Med. Hyg. 2006, 74, 449–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Halstead, S. Pathogenesis of Dengue: Dawn of a new era. F1000Research 2015, 4, F1000. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, S.E. Physiological Characteristics in Biology of the Laboratory Mouse; Green, E.L., Ed.; McGraw-Hill: New York, NY, USA, 1968. [Google Scholar]
- Mouse Genome Informatics-Informatics.jax.org. Mouse Facts. 2021. Available online: http://www.informatics.jax.org/mgihome/other/mouse_facts1.shtml#physiology (accessed on 9 March 2021).
- Zhang, J.; Lan, Y.; Li, M.Y.; Lamers, M.M.; Fusade-Boyer, M.; Klemm, E.; Thiele, C.; Ashour, J.; Sanyal, S. Flaviviruses Exploit the Lipid Droplet Protein AUP1 to Trigger Lipophagy and Drive Virus Production. Cell Host Microbe 2018, 23, 819–831.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakinah, S.; Priya, S.P.; Kumari, S.; Amira, F.K.P.; Alsaeedy, H.; Ling, M.P.; Chee, H.; Higuchi, A.; Alarfaj, A.A.; Munusamy, M.A.; et al. Impact of dengue virus (serotype DENV-2) infection on liver of BALB/c mice: A histopathological analysis. Tissue Cell 2017, 49, 86–94. [Google Scholar] [CrossRef] [PubMed]
- Shresta, S.; Sharar, K.L.; Prigozhin, D.M.; Beatty, P.R.; Harris, E. Murine model for dengue virus-induced lethal disease with increased vascular permeability. J. Virol. 2006, 80, 10208–10217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nascimento, E.J.M.; Hottz, E.D.; Garcia-Bates, T.M.; Bozza, F.; Marques, E.T.A., Jr.; Barratt-Boyes, S.M. Emerging concepts in dengue pathogenesis: Interplay between plasmablasts, platelets, and complement in triggering vasculopathy. Crit. Rev. Immunol. 2014, 34, 227–240. [Google Scholar] [CrossRef] [Green Version]
- Chao, C.; Wu, W.; Lai, Y.; Tsai, P.; Perng, G.; Lin, Y.; Yeh, T. Dengue virus nonstructural protein 1 activates platelets via Toll-like receptor 4, leading to thrombocytopenia and hemorrhage. PLoS Pathog. 2019, 15, e1007625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malavige, G.N.; Ogg, G.S. Pathogenesis of Vascular Leak in Dengue Virus Infection. Immunology 2017, 151, 261–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puerta-Guardo, H.; Glasner, D.R.; Espinosa, D.A.; Biering, S.B.; Patana, M.; Ratnasiri, K.; Wang, C.; Beatty, P.R.; Harris, E. Flavivirus NS1 Triggers Tissue-Specific Vascular Endothelial Dysfunction Reflecting Disease Tropism. Cell Rep. 2019, 26, 1598–1613.e8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Borges, E.R.; Ab’Saber, A.M.; Barbas, C.S.V. Pulmonary hemorrhage syndromes. J. Bras. Pneumol. 2005, 31 (Suppl. S1), S36–S43. [Google Scholar] [CrossRef]
- King, C.A.; Marshall, J.S.; Alshurafa, H.; Anderson, R. Release of vasoactive cytokines by antibody-enhanced dengue virus infection of a human mast cell/basophil line. J. Virol. 2000, 74, 7146–7150. [Google Scholar] [CrossRef] [Green Version]
- Tribulova, N.; Egan Benova, T.; Szeiffova Bacova, B.; Viczenczova, C.; Barancik, M. New aspects of pathogenesis of atrial fibrillation: Remodelling of intercalated discs. J. Physiol. Pharmacol. 2015, 66, 625–634. [Google Scholar]
- Ehler, E. Cardiac cytoarchitecture—Why the “hardware” is important for heart function! Biochim. Biophys. Acta. 2016, 1863 Pt B, 1857–1863. [Google Scholar] [CrossRef] [Green Version]
- Paes, M.V.; Lenzi, H.L.; Nogueira, A.C.M.; Nuovo, G.J.; Pinhão, A.T.; Mota, E.M.; Basílio-de-Oliveira, C.A.; Schatzmayr, H.; Barth, O.M.; Alves, A.M.B. Hepatic damage associated with dengue-2 virus replication in liver cells of BALB/c mice. Lab. Invest. 2009, 89, 1140–1151. [Google Scholar] [CrossRef] [Green Version]
- Rivera, J.; Neira, M.; Parra, E.; Méndez, J.; Sarmiento, L.; Caldas, M.L. Detección de antígenos del virus dengue en tejidos post mortem. Biomedica 2014, 34, 514–520. [Google Scholar] [CrossRef]
- Lardo, S.; Utami, Y.; Yohan, B.; Tarigan, S.M.; Santoso, W.D.; Nainggolan, L.; Sasmono, R.T. Concurrent infections of dengue viruses serotype 2 and 3 in patient with severe dengue from Jakarta, Indonesia. Asian Pac. J. Trop. Med. 2016, 9, 134–140. [Google Scholar] [CrossRef] [PubMed]
- Sariol, C.A.; Pelegrino, J.L.; Martinez, A.; Arteaga, E.; Kouri, G.; Guzman, M.G. Detection and genetic relationship of dengue virus sequences in seventeen-year-old paraffin-embedded samples from Cuba. Am. J. Trop. Med. Hyg. 1999, 61, 994–1000. [Google Scholar] [CrossRef]
- Rosen, L.; Drouet, M.T.; Deubel, V. Detection of dengue virus RNA by reverse transcription-polymerase chain reaction in the liver and lymphoid organs but not in the brain in fatal human infection. Am. J. Trop. Med. Hyg. 1999, 61, 720–724. [Google Scholar] [CrossRef] [Green Version]
- Lima, M.R.Q.; Nogueira, R.M.R.; Schatzmayr, H.G.; de Filippis, A.M.B.; Limonta, D.; dos Santos, F.B. A new approach to dengue fatal cases diagnosis: NS1 antigen capture in tissues. PLoS Negl. Trop. Dis. 2011, 5, e1147. [Google Scholar] [CrossRef] [PubMed]
- Bhatnagar, J.; Blau, D.M.; Shieh, W.; Paddock, C.D.; Drew, C.; Liu, L.; Jones, T.; Patel, M.; Zaki, S.R. Molecular detection and typing of dengue viruses from archived tissues of fatal cases by rt-PCR and sequencing: Diagnostic and epidemiologic implications. Am. J. Trop. Med. Hyg. 2012, 86, 335–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marques, N.; Gan, V.C.; Leo, Y.S. Dengue myocarditis in Singapore: Two case reports. Infection 2013, 41, 709–714. [Google Scholar] [CrossRef] [PubMed]
- Limonta, D.; Falcón, V.; Torres, G.; Capó, V.; Menéndez, I.; Rosario, D.; Castellanos, Y.; Alvarez, M.; Rodríguez-Roche, R.; de la Rosa, M.C.; et al. Dengue virus identification by transmission electron microscopy and molecular methods in fatal dengue hemorrhagic fever. Infection 2012, 40, 689–694. [Google Scholar] [CrossRef]






| N = 75 | Histopathology/IHQ/qRT-PCR | TEM | Viremia |
|---|---|---|---|
| DENV-4 72 h.p.i. | 15 | 15 | 30 |
| Negative Control | 10 | 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Costa Rasinhas, A.; Cunha Jácome, F.; Cardoso Caldas, G.; Teixeira de Almeida, A.L.; Nunes da Silva, M.A.; Dias Coutinho de Souza, D.; Carlos Paulino, A.; Mendes Bandeira, D.; Leonardo, R.; Conrado Guerra Nunes, P.; et al. Morphological Aspects and Viremia Analysis of BALB/c Murine Model Experimentally Infected with Dengue Virus Serotype 4. Viruses 2021, 13, 1954. https://doi.org/10.3390/v13101954
da Costa Rasinhas A, Cunha Jácome F, Cardoso Caldas G, Teixeira de Almeida AL, Nunes da Silva MA, Dias Coutinho de Souza D, Carlos Paulino A, Mendes Bandeira D, Leonardo R, Conrado Guerra Nunes P, et al. Morphological Aspects and Viremia Analysis of BALB/c Murine Model Experimentally Infected with Dengue Virus Serotype 4. Viruses. 2021; 13(10):1954. https://doi.org/10.3390/v13101954
Chicago/Turabian Styleda Costa Rasinhas, Arthur, Fernanda Cunha Jácome, Gabriela Cardoso Caldas, Ana Luisa Teixeira de Almeida, Marcos Alexandre Nunes da Silva, Daniel Dias Coutinho de Souza, Amanda Carlos Paulino, Derick Mendes Bandeira, Raphael Leonardo, Priscila Conrado Guerra Nunes, and et al. 2021. "Morphological Aspects and Viremia Analysis of BALB/c Murine Model Experimentally Infected with Dengue Virus Serotype 4" Viruses 13, no. 10: 1954. https://doi.org/10.3390/v13101954
APA Styleda Costa Rasinhas, A., Cunha Jácome, F., Cardoso Caldas, G., Teixeira de Almeida, A. L., Nunes da Silva, M. A., Dias Coutinho de Souza, D., Carlos Paulino, A., Mendes Bandeira, D., Leonardo, R., Conrado Guerra Nunes, P., Mohana-Borges, R., Monika Barth, O., Barreto dos Santos, F., & Ferreira Barreto Vieira, D. (2021). Morphological Aspects and Viremia Analysis of BALB/c Murine Model Experimentally Infected with Dengue Virus Serotype 4. Viruses, 13(10), 1954. https://doi.org/10.3390/v13101954






