Cell Fusion and Syncytium Formation in Betaherpesvirus Infection
Abstract
:1. Introduction
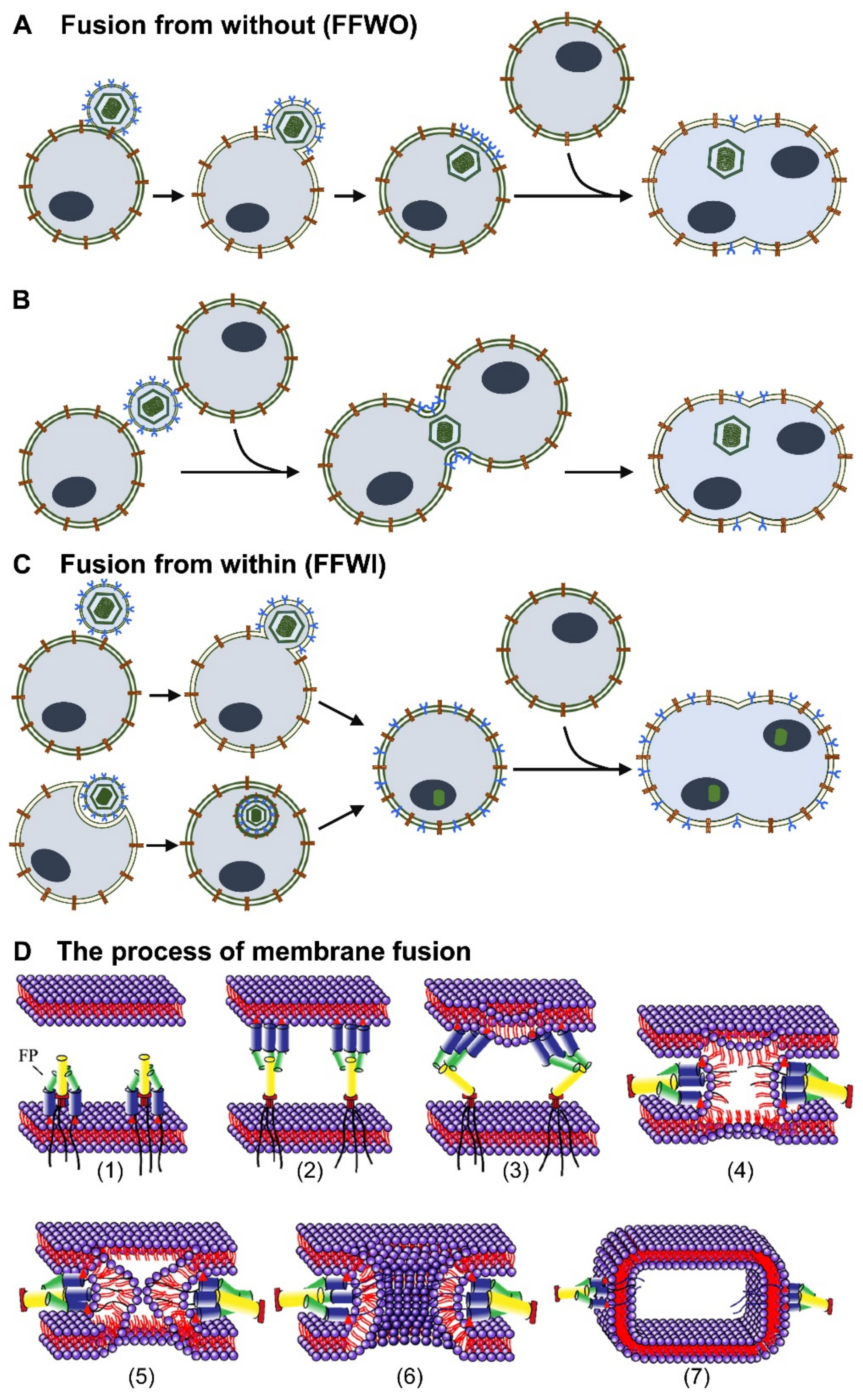
2. Syncytium Formation by Herpesviruses
3. Determinants and Mechanism of Syncytium Formation
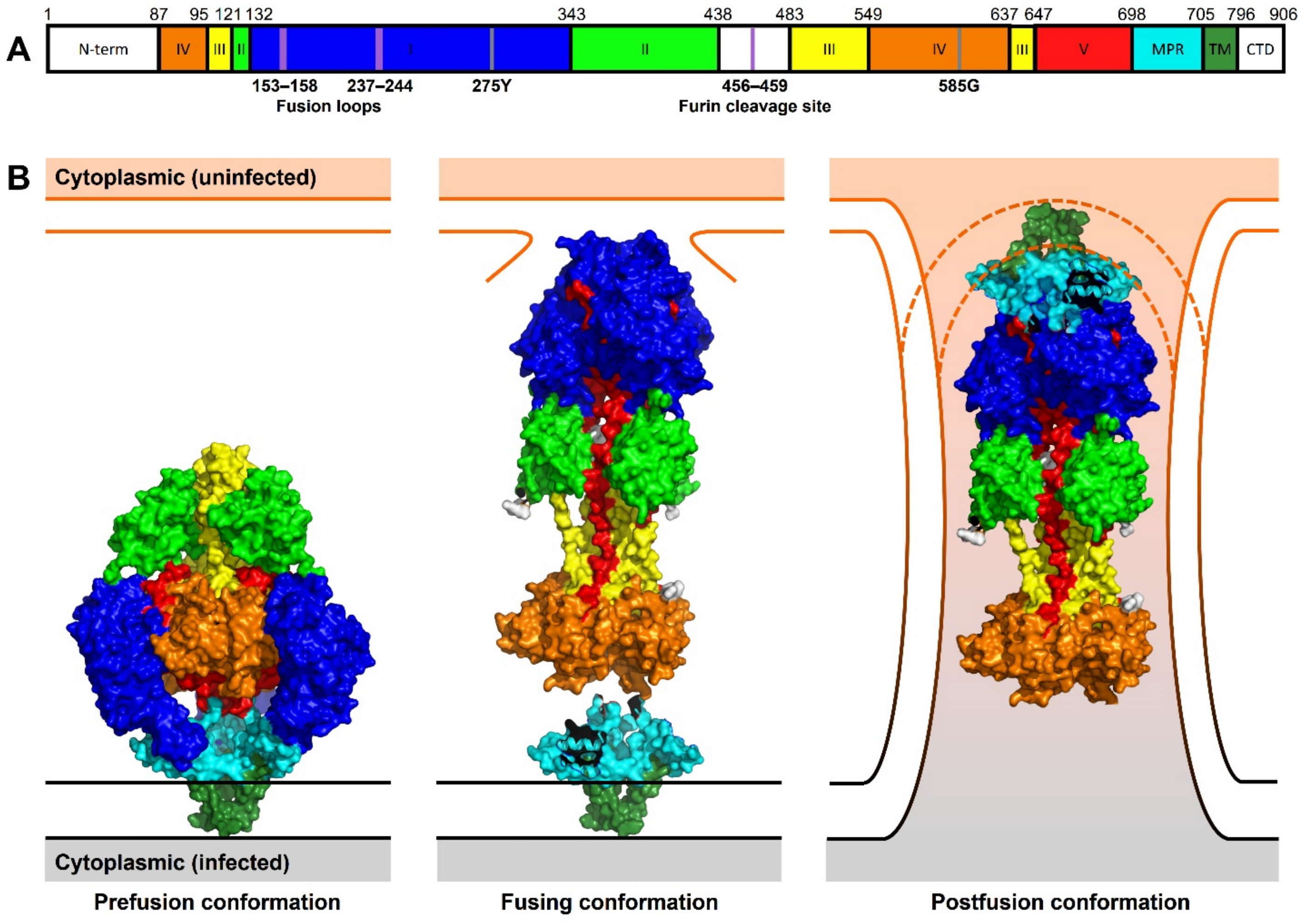
3.1. The HCMV Core Fusion Machinery: Glycoprotein gB, the HCMV Fusogen
3.2. The HCMV Core Fusion Machinery: gH and gL, the HCMV Fusion Trigger
3.3. The HCMV Accessory Proteins Involved in Membrane Fusion
4. Cellular Determinants and Mechanism of Syncytium Formation
5. Role of Syncytia in Viral Pathogenesis
6. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hernandez, J.M.; Podbilewicz, B. The hallmarks of cell-cell fusion. Development 2017, 144, 4481–4495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brukman, N.G.; Uygur, B.; Podbilewicz, B.; Chernomordik, L.V. How cells fuse. J. Cell Biol. 2019, 218, 1436–1451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, X.; Kang, Y. Cell fusion as a hidden force in tumor progression. Cancer Res. 2009, 69, 8536–8539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Platt, J.L.; Cascalho, M. Cell Fusion in Malignancy: A Cause or Consequence? A Provocateur or Cure? Cells 2019, 8, 587. [Google Scholar] [CrossRef] [Green Version]
- Ku, J.W.K.; Chen, Y.; Lim, B.J.W.; Gasser, S.; Crasta, K.C.; Gan, Y.H. Bacterial-induced cell fusion is a danger signal triggering cGAS-STING pathway via micronuclei formation. Proc. Natl. Acad. Sci. USA 2020, 117, 15923–15934. [Google Scholar] [CrossRef]
- Stockton, J.L.; Torres, A.G. Multinucleated Giant Cell Formation as a Portal to Chronic Bacterial Infections. Microorganisms 2020, 8, 1637. [Google Scholar] [CrossRef]
- Diaz, A.; Sagasti, C.; Casaravilla, C. Granulomatous responses in larval taeniid infections. Parasite Immunol. 2018, 40, e12523. [Google Scholar] [CrossRef]
- McNally, A.K.; Anderson, J.M. Macrophage fusion and multinucleated giant cells of inflammation. Adv. Exp. Med. Biol. 2011, 713, 97–111. [Google Scholar] [CrossRef]
- Higuchi, H.; Bronk, S.F.; Bateman, A.; Harrington, K.; Vile, R.G.; Gores, G.J. Viral fusogenic membrane glycoprotein expression causes syncytia formation with bioenergetic cell death: Implications for gene therapy. Cancer Res. 2000, 60, 6396–6402. [Google Scholar]
- Sodroski, J.; Goh, W.C.; Rosen, C.; Campbell, K.; Haseltine, W.A. Role of the HTLV-III/LAV envelope in syncytium formation and cytopathicity. Nature 1986, 322, 470–474. [Google Scholar] [CrossRef]
- Cathomen, T.; Naim, H.Y.; Cattaneo, R. Measles viruses with altered envelope protein cytoplasmic tails gain cell fusion competence. J. Virol. 1998, 72, 1224–1234. [Google Scholar] [CrossRef] [Green Version]
- Bateman, A.; Bullough, F.; Murphy, S.; Emiliusen, L.; Lavillette, D.; Cosset, F.L.; Cattaneo, R.; Russell, S.J.; Vile, R.G. Fusogenic membrane glycoproteins as a novel class of genes for the local and immune-mediated control of tumor growth. Cancer Res. 2000, 60, 1492–1497. [Google Scholar]
- Leroy, H.; Han, M.; Woottum, M.; Bracq, L.; Bouchet, J.; Xie, M.; Benichou, S. Virus-Mediated Cell-Cell Fusion. Int. J. Mol. Sci. 2020, 21, 9644. [Google Scholar] [CrossRef]
- Cifuentes-Munoz, N.; Dutch, R.E.; Cattaneo, R. Direct cell-to-cell transmission of respiratory viruses: The fast lanes. PLoS Pathog. 2018, 14, e1007015. [Google Scholar] [CrossRef] [Green Version]
- Compton, A.A.; Schwartz, O. They Might Be Giants: Does Syncytium Formation Sink or Spread HIV Infection? PLoS Pathog. 2017, 13, e1006099. [Google Scholar] [CrossRef]
- Huerta, L.; Lopez-Balderas, N.; Rivera-Toledo, E.; Sandoval, G.; Gomez-Icazbalceta, G.; Villarreal, C.; Lamoyi, E.; Larralde, C. HIV-envelope-dependent cell-cell fusion: Quantitative studies. Sci. World J. 2009, 9, 746–763. [Google Scholar] [CrossRef] [Green Version]
- Spijkerman, I.; de Wolf, F.; Langendam, M.; Schuitemaker, H.; Coutinho, R. Emergence of syncytium-inducing human immunodeficiency virus type 1 variants coincides with a transient increase in viral RNA level and is an independent predictor for progression to AIDS. J. Infect. Dis. 1998, 178, 397–403. [Google Scholar] [CrossRef] [Green Version]
- Buchrieser, J.; Dufloo, J.; Hubert, M.; Monel, B.; Planas, D.; Rajah, M.M.; Planchais, C.; Porrot, F.; Guivel-Benhassine, F.; Van der Werf, S.; et al. Syncytia formation by SARS-CoV-2-infected cells. EMBO J. 2020, 39, e106267. [Google Scholar] [CrossRef]
- Bayliss, G.J.; Wolf, H. An Epstein--Barr virus early protein induces cell fusion. Proc. Natl. Acad. Sci. USA 1981, 78, 7162–7165. [Google Scholar] [CrossRef] [Green Version]
- Bracq, L.; Xie, M.; Benichou, S.; Bouchet, J. Mechanisms for Cell-to-Cell Transmission of HIV-1. Front. Immunol. 2018, 9, 260. [Google Scholar] [CrossRef]
- Ciechonska, M.; Key, T.; Duncan, R. Efficient reovirus- and measles virus-mediated pore expansion during syncytium formation is dependent on annexin A1 and intracellular calcium. J. Virol. 2014, 88, 6137–6147. [Google Scholar] [CrossRef] [Green Version]
- Vitale, I.; Galluzzi, L.; Castedo, M.; Kroemer, G. Mitotic catastrophe: A mechanism for avoiding genomic instability. Nat. Rev. Mol. Cell Biol. 2011, 12, 385–392. [Google Scholar] [CrossRef]
- Braga, L.; Ali, H.; Secco, I.; Chiavacci, E.; Neves, G.; Goldhill, D.; Penn, R.; Jimenez-Guardeno, J.M.; Ortega-Prieto, A.M.; Bussani, R.; et al. Drugs that inhibit TMEM16 proteins block SARS-CoV-2 spike-induced syncytia. Nature 2021, 594, 88–93. [Google Scholar] [CrossRef]
- Burton, C.; Bartee, E. Syncytia Formation in Oncolytic Virotherapy. Mol. Ther. Oncolytics 2019, 15, 131–139. [Google Scholar] [CrossRef] [Green Version]
- Eisenberg, R.J.; Atanasiu, D.; Cairns, T.M.; Gallagher, J.R.; Krummenacher, C.; Cohen, G.H. Herpes virus fusion and entry: A story with many characters. Viruses 2012, 4, 800–832. [Google Scholar] [CrossRef]
- Pellet, P.E.; Roizman, B. Herpesviridae . In Fields Virology, 6th ed.; Knipe, D.M., Howley, P.M., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013; pp. 1802–18022. [Google Scholar]
- Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis; Arvin, A.; Campadelli-Fiume, G.; Mocarski, E.; Moore, P.S.; Roizman, B.; Whitley, R.; Yamanishi, K. (Eds.) Cambridge University Press: Cambridge, UK, 2007; ISBN 978-0-521-82714-0. [Google Scholar]
- Griffiths, P.; Baraniak, I.; Reeves, M. The pathogenesis of human cytomegalovirus. J. Pathol. 2015, 235, 288–297. [Google Scholar] [CrossRef]
- Rawlinson, W.D.; Boppana, S.B.; Fowler, K.B.; Kimberlin, D.W.; Lazzarotto, T.; Alain, S.; Daly, K.; Doutre, S.; Gibson, L.; Giles, M.L.; et al. Congenital cytomegalovirus infection in pregnancy and the neonate: Consensus recommendations for prevention, diagnosis, and therapy. Lancet Infect. Dis. 2017, 17, e177–e188. [Google Scholar] [CrossRef]
- Agut, H.; Bonnafous, P.; Gautheret-Dejean, A. Human Herpesviruses 6A, 6B, and 7. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ayee, R.; Ofori, M.E.O.; Wright, E.; Quaye, O. Epstein Barr Virus Associated Lymphomas and Epithelia Cancers in Humans. J. Cancer 2020, 11, 1737–1750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katano, H. Pathological Features of Kaposi’s Sarcoma-Associated Herpesvirus Infection. Adv. Exp. Med. Biol. 2018, 1045, 357–376. [Google Scholar] [CrossRef] [PubMed]
- Booth, J.C.; Beesley, J.E.; Stern, H. Syncytium formation caused by human cytomegalovirus in human embryonic lung fibroblasts. Arch. Virol. 1978, 57, 143–152. [Google Scholar] [CrossRef]
- Bayliss, G.J.; Wolf, H. Epstein--Barr virus-induced cell fusion. Nature 1980, 287, 164–165. [Google Scholar] [CrossRef]
- Takimoto, T.; Sato, H.; Ogura, H.; Miyazaki, T. Cell fusion by nasopharyngeal carcinoma-derived Epstein-Barr virus. Arch. Otolaryngol. Head Neck Surg. 1987, 113, 510–513. [Google Scholar] [CrossRef]
- Kaleeba, J.A.; Berger, E.A. Broad target cell selectivity of Kaposi’s sarcoma-associated herpesvirus glycoprotein-mediated cell fusion and virion entry. Virology 2006, 354, 7–14. [Google Scholar] [CrossRef] [Green Version]
- Oliver, S.L.; Brady, J.J.; Sommer, M.H.; Reichelt, M.; Sung, P.; Blau, H.M.; Arvin, A.M. An immunoreceptor tyrosine-based inhibition motif in varicella-zoster virus glycoprotein B regulates cell fusion and skin pathogenesis. Proc. Natl. Acad. Sci. USA 2013, 110, 1911–1916. [Google Scholar] [CrossRef] [Green Version]
- Oliver, S.L.; Yang, E.; Arvin, A.M. Dysregulated Glycoprotein B-Mediated Cell-Cell Fusion Disrupts Varicella-Zoster Virus and Host Gene Transcription during Infection. J. Virol. 2017, 91, e01613-16. [Google Scholar] [CrossRef] [Green Version]
- Weed, D.J.; Nicola, A.V. Herpes simplex virus Membrane Fusion. Adv. Anat. Embryol. Cell Biol. 2017, 223, 29–47. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, Y.; Yamamoto, T.; Aoyama, Y.; Fujimoto, W. Cell-to-cell transmission of HSV-1 in differentiated keratinocytes promotes multinucleated giant cell formation. J. Dermatol. Sci. 2019, 93, 14–23. [Google Scholar] [CrossRef] [Green Version]
- Cifuentes-Munoz, N.; El Najjar, F.; Dutch, R.E. Viral cell-to-cell spread: Conventional and non-conventional ways. Adv. Virus Res. 2020, 108, 85–125. [Google Scholar] [CrossRef]
- Ambrosini, E.; Enquist, L. Cell-fusion events induced by a-herpesviruses. Future Virol. 2015, 10, 185–200. [Google Scholar] [CrossRef]
- Mohl, B.S.; Chen, J.; Sathiyamoorthy, K.; Jardetzky, T.S.; Longnecker, R. Structural and Mechanistic Insights into the Tropism of Epstein-Barr Virus. Mol. Cells 2016, 39, 286–291. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cole, N.L.; Grose, C. Membrane fusion mediated by herpesvirus glycoproteins: The paradigm of varicella-zoster virus. Rev. Med. Virol. 2003, 13, 207–222. [Google Scholar] [CrossRef] [PubMed]
- Sweeney, S.A.; Sulit, D.J.; Adams, E.G.; Shvartsman, K.R.; Rapini, R.P. Grape Cells (Multinucleated Keratinocytes) in Noninfectious Dermatoses: Case Series and Review of the Literature. Am. J. Dermatopathol. 2015, 37, e143–e146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, E.; Arvin, A.M.; Oliver, S.L. The Glycoprotein B Cytoplasmic Domain Lysine Cluster Is Critical for Varicella-Zoster Virus Cell-Cell Fusion Regulation and Infection. J. Virol. 2017, 91, e01707-16. [Google Scholar] [CrossRef] [Green Version]
- Blank, H.; Burgoon, C.F.; Baldridge, G.D.; Mc, C.P.; Urbach, F. Cytologic smears in diagnosis of herpes simplex, herpes zoster, and varicella. J. Am. Med. Assoc. 1951, 146, 1410–1412. [Google Scholar] [CrossRef]
- Esiri, M.M.; Tomlinson, A.H. Herpes Zoster. Demonstration of virus in trigeminal nerve and ganglion by immunofluorescence and electron microscopy. J. Neurol. Sci. 1972, 15, 35–48. [Google Scholar] [CrossRef]
- Mohl, B.S.; Chen, J.; Longnecker, R. Gammaherpesvirus entry and fusion: A tale how two human pathogenic viruses enter their host cells. Adv. Virus Res. 2019, 104, 313–343. [Google Scholar] [CrossRef] [Green Version]
- Sinzger, C.; Bissinger, A.L.; Viebahn, R.; Oettle, H.; Radke, C.; Schmidt, C.A.; Jahn, G. Hepatocytes are permissive for human cytomegalovirus infection in human liver cell culture and In vivo. J. Infect. Dis. 1999, 180, 976–986. [Google Scholar] [CrossRef]
- Diosi, P.; Babusceac, L.; Gherman, D. Cytophagia in cell cultures infected with cytomegalovirus. J. Infect. Dis. 1972, 125, 669–671. [Google Scholar] [CrossRef]
- Takeuchi, T.; Fujii, A.; Okumiya, T.; Watabe, S.; Ishikawa, T.; Umeda, A.; Masuda, M.; Takeuchi, H. The study of cytopathological aspects induced by human cytomegalovirus infection. Diagn. Cytopathol. 2004, 31, 289–293. [Google Scholar] [CrossRef]
- Gerna, G.; Sarasini, A.; Patrone, M.; Percivalle, E.; Fiorina, L.; Campanini, G.; Gallina, A.; Baldanti, F.; Revello, M.G. Human cytomegalovirus serum neutralizing antibodies block virus infection of endothelial/epithelial cells, but not fibroblasts, early during primary infection. J. Gen. Virol. 2008, 89, 853–865. [Google Scholar] [CrossRef]
- Galitska, G.; Biolatti, M.; De Andrea, M.; Leone, A.; Coscia, A.; Bertolotti, L.; Ala, U.; Bertino, E.; Dell’Oste, V.; Landolfo, S. Biological relevance of Cytomegalovirus genetic variability in congenitally and postnatally infected children. J. Clin. Virol. 2018, 108, 132–140. [Google Scholar] [CrossRef]
- Vanarsdall, A.L.; Ryckman, B.J.; Chase, M.C.; Johnson, D.C. Human cytomegalovirus glycoproteins gB and gH/gL mediate epithelial cell-cell fusion when expressed either in cis or in trans. J. Virol. 2008, 82, 11837–11850. [Google Scholar] [CrossRef] [Green Version]
- Itell, H.L.; Kaur, A.; Deere, J.D.; Barry, P.A.; Permar, S.R. Rhesus monkeys for a nonhuman primate model of cytomegalovirus infections. Curr. Opin. Virol. 2017, 25, 126–133. [Google Scholar] [CrossRef] [Green Version]
- Bowman, J.J.; Lacayo, J.C.; Burbelo, P.; Fischer, E.R.; Cohen, J.I. Rhesus and human cytomegalovirus glycoprotein L are required for infection and cell-to-cell spread of virus but cannot complement each other. J. Virol. 2011, 85, 2089–2099. [Google Scholar] [CrossRef] [Green Version]
- Beisser, P.S.; Grauls, G.; Bruggeman, C.A.; Vink, C. Deletion of the R78 G protein-coupled receptor gene from rat cytomegalovirus results in an attenuated, syncytium-inducing mutant strain. J. Virol. 1999, 73, 7218–7230. [Google Scholar] [CrossRef] [Green Version]
- Van Den Pol, A.N.; Mocarski, E.; Saederup, N.; Vieira, J.; Meier, T.J. Cytomegalovirus cell tropism, replication, and gene transfer in brain. J. Neurosci. 1999, 19, 10948–10965. [Google Scholar] [CrossRef] [Green Version]
- Camalxaman, S.N.; Zeenathul, N.A.; Quah, Y.W.; Loh, H.S.; Zuridah, H.; Hani, H.; Sheikh-Omar, A.R.; Mohd-Azmi, M.L. Establishment of rat brain endothelial cells susceptible to rat cytomegalovirus ALL-03 infection. In Vitro Cell. Dev. Biol. Anim. 2013, 49, 238–244. [Google Scholar] [CrossRef]
- Kilham, L.; Margolis, G. Encephalitis in suckling rats induced with rat cytomegalovirus. Lab. Investig. 1975, 33, 200–206. [Google Scholar]
- Margolis, G.; Kilham, L. Neuronal parasitism and cell fusion in mouse cytomegalovirus encephalitis. Exp. Mol. Pathol. 1976, 25, 20–30. [Google Scholar] [CrossRef]
- Mori, Y.; Seya, T.; Huang, H.L.; Akkapaiboon, P.; Dhepakson, P.; Yamanishi, K. Human herpesvirus 6 variant A but not variant B induces fusion from without in a variety of human cells through a human herpesvirus 6 entry receptor, CD46. J. Virol. 2002, 76, 6750–6761. [Google Scholar] [CrossRef] [Green Version]
- Secchiero, P.; Berneman, Z.N.; Gallo, R.C.; Lusso, P. Biological and molecular characteristics of human herpesvirus 7: In vitro growth optimization and development of a syncytia inhibition test. Virology 1994, 202, 506–512. [Google Scholar] [CrossRef] [Green Version]
- Cui, X.; Freed, D.C.; Wang, D.; Qiu, P.; Li, F.; Fu, T.M.; Kauvar, L.M.; McVoy, M.A. Impact of Antibodies and Strain Polymorphisms on Cytomegalovirus Entry and Spread in Fibroblasts and Epithelial Cells. J. Virol. 2017, 91. [Google Scholar] [CrossRef] [Green Version]
- Roizman, B. Polykaryocytosis. Cold. Spring Harb. Symp. Quant. Biol. 1962, 27, 327–342. [Google Scholar] [CrossRef]
- Kielian, M. Mechanisms of Virus Membrane Fusion Proteins. Annu. Rev. Virol. 2014, 1, 171–189. [Google Scholar] [CrossRef] [Green Version]
- Lorizate, M.; Krausslich, H.G. Role of lipids in virus replication. Cold. Spring Harb. Perspect. Biol. 2011, 3, a004820. [Google Scholar] [CrossRef] [Green Version]
- Leikin, S.L.; Kozlov, M.M.; Chernomordik, L.V.; Markin, V.S.; Chizmadzhev, Y.A. Membrane fusion: Overcoming of the hydration barrier and local restructuring. J. Theor. Biol. 1987, 129, 411–425. [Google Scholar] [CrossRef]
- Chernomordik, L.V.; Kozlov, M.M. Mechanics of membrane fusion. Nat. Struct. Mol. Biol. 2008, 15, 675–683. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sapir, A.; Avinoam, O.; Podbilewicz, B.; Chernomordik, L.V. Viral and developmental cell fusion mechanisms: Conservation and divergence. Dev. Cell 2008, 14, 11–21. [Google Scholar] [CrossRef] [Green Version]
- Segev, N.; Avinoam, O.; Podbilewicz, B. Fusogens. Curr. Biol. 2018, 28, R378–R380. [Google Scholar] [CrossRef] [Green Version]
- Cooper, R.S.; Heldwein, E.E. Herpesvirus gB: A Finely Tuned Fusion Machine. Viruses 2015, 7, 6552–6569. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Falke, D.; Knoblich, A.; Muller, S. Fusion from without induced by herpes simplex virus type 1. Intervirology 1985, 24, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Connolly, S.A.; Jackson, J.O.; Jardetzky, T.S.; Longnecker, R. Fusing structure and function: A structural view of the herpesvirus entry machinery. Nat. Rev. Microbiol. 2011, 9, 369–381. [Google Scholar] [CrossRef] [PubMed]
- Heldwein, E.E.; Krummenacher, C. Entry of herpesviruses into mammalian cells. Cell. Mol. Life Sci. 2008, 65, 1653–1668. [Google Scholar] [CrossRef]
- Nguyen, C.C.; Kamil, J.P. Pathogen at the Gates: Human Cytomegalovirus Entry and Cell Tropism. Viruses 2018, 10, 704. [Google Scholar] [CrossRef] [Green Version]
- Sathiyamoorthy, K.; Chen, J.; Longnecker, R.; Jardetzky, T.S. The COMPLEXity in herpesvirus entry. Curr. Opin. Virol. 2017, 24, 97–104. [Google Scholar] [CrossRef]
- Vanarsdall, A.L.; Johnson, D.C. Human cytomegalovirus entry into cells. Curr. Opin. Virol. 2012, 2, 37–42. [Google Scholar] [CrossRef] [Green Version]
- Foglierini, M.; Marcandalli, J.; Perez, L. HCMV Envelope Glycoprotein Diversity Demystified. Front. Microbiol. 2019, 10, 1005. [Google Scholar] [CrossRef]
- Weiler, N.; Paal, C.; Adams, K.; Calcaterra, C.; Fischer, D.; Stanton, R.J.; Stohr, D.; Laib Sampaio, K.; Sinzger, C. Role of Envelope Glycoprotein Complexes in Cell-Associated Spread of Human Cytomegalovirus. Viruses 2021, 13, 614. [Google Scholar] [CrossRef]
- Muggeridge, M.I. Characterization of cell-cell fusion mediated by herpes simplex virus 2 glycoproteins gB, gD, gH and gL in transfected cells. J. Gen. Virol. 2000, 81, 2017–2027. [Google Scholar] [CrossRef]
- Kinzler, E.R.; Compton, T. Characterization of human cytomegalovirus glycoprotein-induced cell-cell fusion. J. Virol. 2005, 79, 7827–7837. [Google Scholar] [CrossRef] [Green Version]
- Tugizov, S.; Navarro, D.; Paz, P.; Wang, Y.; Qadri, I.; Pereira, L. Function of human cytomegalovirus glycoprotein B: Syncytium formation in cells constitutively expressing gB is blocked by virus-neutralizing antibodies. Virology 1994, 201, 263–276. [Google Scholar] [CrossRef]
- Chowdary, T.K.; Cairns, T.M.; Atanasiu, D.; Cohen, G.H.; Eisenberg, R.J.; Heldwein, E.E. Crystal structure of the conserved herpesvirus fusion regulator complex gH-gL. Nat. Struct. Mol. Biol. 2010, 17, 882–888. [Google Scholar] [CrossRef] [Green Version]
- Buscher, N.; Paulus, C.; Nevels, M.; Tenzer, S.; Plachter, B. The proteome of human cytomegalovirus virions and dense bodies is conserved across different strains. Med. Microbiol. Immunol. 2015, 204, 285–293. [Google Scholar] [CrossRef]
- Isaacson, M.K.; Compton, T. Human cytomegalovirus glycoprotein B is required for virus entry and cell-to-cell spread but not for virion attachment, assembly, or egress. J. Virol. 2009, 83, 3891–3903. [Google Scholar] [CrossRef] [Green Version]
- Wille, P.T.; Wisner, T.W.; Ryckman, B.; Johnson, D.C. Human cytomegalovirus (HCMV) glycoprotein gB promotes virus entry in trans acting as the viral fusion protein rather than as a receptor-binding protein. mBio 2013, 4, e00332-13. [Google Scholar] [CrossRef] [Green Version]
- Potzsch, S.; Spindler, N.; Wiegers, A.K.; Fisch, T.; Rucker, P.; Sticht, H.; Grieb, N.; Baroti, T.; Weisel, F.; Stamminger, T.; et al. B cell repertoire analysis identifies new antigenic domains on glycoprotein B of human cytomegalovirus which are target of neutralizing antibodies. PLoS Pathog. 2011, 7, e1002172. [Google Scholar] [CrossRef] [Green Version]
- Burke, H.G.; Heldwein, E.E. Crystal Structure of the Human Cytomegalovirus Glycoprotein B. PLoS Pathog. 2015, 11, e1005227. [Google Scholar] [CrossRef] [Green Version]
- Si, Z.; Zhang, J.; Shivakoti, S.; Atanasov, I.; Tao, C.L.; Hui, W.H.; Zhou, K.; Yu, X.; Li, W.; Luo, M.; et al. Different functional states of fusion protein gB revealed on human cytomegalovirus by cryo electron tomography with Volta phase plate. PLoS Pathog. 2018, 14, e1007452. [Google Scholar] [CrossRef]
- Liu, Y.; Heim, K.P.; Che, Y.; Chi, X.; Qiu, X.; Han, S.; Dormitzer, P.R.; Yang, X. Prefusion structure of human cytomegalovirus glycoprotein B and structural basis for membrane fusion. Sci. Adv. 2021, 7, eabf3178. [Google Scholar] [CrossRef]
- Adams, M.J.; Carstens, E.B. Ratification vote on taxonomic proposals to the International Committee on Taxonomy of Viruses (2012). Arch. Virol. 2012, 157, 1411–1422. [Google Scholar] [CrossRef] [Green Version]
- Ruel, N.; Zago, A.; Spear, P.G. Alanine substitution of conserved residues in the cytoplasmic tail of herpes simplex virus gB can enhance or abolish cell fusion activity and viral entry. Virology 2006, 346, 229–237. [Google Scholar] [CrossRef] [Green Version]
- Fan, Z.; Grantham, M.L.; Smith, M.S.; Anderson, E.S.; Cardelli, J.A.; Muggeridge, M.I. Truncation of herpes simplex virus type 2 glycoprotein B increases its cell surface expression and activity in cell-cell fusion, but these properties are unrelated. J. Virol. 2002, 76, 9271–9283. [Google Scholar] [CrossRef] [Green Version]
- Gage, P.J.; Levine, M.; Glorioso, J.C. Syncytium-inducing mutations localize to two discrete regions within the cytoplasmic domain of herpes simplex virus type 1 glycoprotein B. J. Virol. 1993, 67, 2191–2201. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rogalin, H.B.; Heldwein, E.E. Interplay between the Herpes Simplex Virus 1 gB Cytodomain and the gH Cytotail during Cell-Cell Fusion. J. Virol. 2015, 89, 12262–12272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engel, J.P.; Boyer, E.P.; Goodman, J.L. Two novel single amino acid syncytial mutations in the carboxy terminus of glycoprotein B of herpes simplex virus type 1 confer a unique pathogenic phenotype. Virology 1993, 192, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Diakidi-Kosta, A.; Michailidou, G.; Kontogounis, G.; Sivropoulou, A.; Arsenakis, M. A single amino acid substitution in the cytoplasmic tail of the glycoprotein B of herpes simplex virus 1 affects both syncytium formation and binding to intracellular heparan sulfate. Virus Res. 2003, 93, 99–108. [Google Scholar] [CrossRef]
- Reuter, N.; Kropff, B.; Schneiderbanger, J.K.; Alt, M.; Krawczyk, A.; Sinzger, C.; Winkler, T.H.; Britt, W.J.; Mach, M.; Thomas, M. Cell Fusion Induced by a Fusion-Active Form of Human Cytomegalovirus Glycoprotein B (gB) Is Inhibited by Antibodies Directed at Antigenic Domain 5 in the Ectodomain of gB. J. Virol. 2020, 94, e01276-20. [Google Scholar] [CrossRef]
- Kirchmeier, M.; Fluckiger, A.C.; Soare, C.; Bozic, J.; Ontsouka, B.; Ahmed, T.; Diress, A.; Pereira, L.; Schodel, F.; Plotkin, S.; et al. Enveloped virus-like particle expression of human cytomegalovirus glycoprotein B antigen induces antibodies with potent and broad neutralizing activity. Clin. Vaccine Immunol. 2014, 21, 174–180. [Google Scholar] [CrossRef] [Green Version]
- Heldwein, E.E.; Lou, H.; Bender, F.C.; Cohen, G.H.; Eisenberg, R.J.; Harrison, S.C. Crystal structure of glycoprotein B from herpes simplex virus 1. Science 2006, 313, 217–220. [Google Scholar] [CrossRef] [Green Version]
- Backovic, M.; Longnecker, R.; Jardetzky, T.S. Structure of a trimeric variant of the Epstein-Barr virus glycoprotein B. Proc. Natl. Acad. Sci. USA 2009, 106, 2880–2885. [Google Scholar] [CrossRef] [Green Version]
- Campelo, F.; McMahon, H.T.; Kozlov, M.M. The hydrophobic insertion mechanism of membrane curvature generation by proteins. Biophys. J. 2008, 95, 2325–2339. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Wisner, T.W.; Johnson, D.C.; Heldwein, E.E. HCMV gB shares structural and functional properties with gB proteins from other herpesviruses. Virology 2013, 435, 239–249. [Google Scholar] [CrossRef] [Green Version]
- Chandramouli, S.; Ciferri, C.; Nikitin, P.A.; Calo, S.; Gerrein, R.; Balabanis, K.; Monroe, J.; Hebner, C.; Lilja, A.E.; Settembre, E.C.; et al. Structure of HCMV glycoprotein B in the postfusion conformation bound to a neutralizing human antibody. Nat. Commun. 2015, 6, 8176. [Google Scholar] [CrossRef] [Green Version]
- Luo, S.; Hu, K.; He, S.; Wang, P.; Zhang, M.; Huang, X.; Du, T.; Zheng, C.; Liu, Y.; Hu, Q. Contribution of N-linked glycans on HSV-2 gB to cell-cell fusion and viral entry. Virology 2015, 483, 72–82. [Google Scholar] [CrossRef] [Green Version]
- Tang, J.; Frascaroli, G.; Lebbink, R.J.; Ostermann, E.; Brune, W. Human cytomegalovirus glycoprotein B variants affect viral entry, cell fusion, and genome stability. Proc. Natl. Acad. Sci. USA 2019, 116, 18021–18030. [Google Scholar] [CrossRef] [Green Version]
- Chou, S. Molecular epidemiology of envelope glycoprotein H of human cytomegalovirus. J. Infect. Dis. 1992, 166, 604–607. [Google Scholar] [CrossRef]
- Heldwein, E.E. gH/gL supercomplexes at early stages of herpesvirus entry. Curr. Opin. Virol. 2016, 18, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Matsuura, H.; Kirschner, A.N.; Longnecker, R.; Jardetzky, T.S. Crystal structure of the Epstein-Barr virus (EBV) glycoprotein H/glycoprotein L (gH/gL) complex. Proc. Natl. Acad. Sci. USA 2010, 107, 22641–22646. [Google Scholar] [CrossRef] [Green Version]
- Ciferri, C.; Chandramouli, S.; Donnarumma, D.; Nikitin, P.A.; Cianfrocco, M.A.; Gerrein, R.; Feire, A.L.; Barnett, S.W.; Lilja, A.E.; Rappuoli, R.; et al. Structural and biochemical studies of HCMV gH/gL/gO and Pentamer reveal mutually exclusive cell entry complexes. Proc. Natl. Acad. Sci. USA 2015, 112, 1767–1772. [Google Scholar] [CrossRef] [Green Version]
- Atanasiu, D.; Whitbeck, J.C.; Cairns, T.M.; Reilly, B.; Cohen, G.H.; Eisenberg, R.J. Bimolecular complementation reveals that glycoproteins gB and gH/gL of herpes simplex virus interact with each other during cell fusion. Proc. Natl. Acad. Sci. USA 2007, 104, 18718–18723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanarsdall, A.L.; Howard, P.W.; Wisner, T.W.; Johnson, D.C. Human Cytomegalovirus gH/gL Forms a Stable Complex with the Fusion Protein gB in Virions. PLoS Pathog. 2016, 12, e1005564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Varnum, S.M.; Streblow, D.N.; Monroe, M.E.; Smith, P.; Auberry, K.J.; Pasa-Tolic, L.; Wang, D.; Camp, D.G., 2nd; Rodland, K.; Wiley, S.; et al. Identification of proteins in human cytomegalovirus (HCMV) particles: The HCMV proteome. J. Virol. 2004, 78, 10960–10966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryckman, B.J.; Rainish, B.L.; Chase, M.C.; Borton, J.A.; Nelson, J.A.; Jarvis, M.A.; Johnson, D.C. Characterization of the human cytomegalovirus gH/gL/UL128-131 complex that mediates entry into epithelial and endothelial cells. J. Virol. 2008, 82, 60–70. [Google Scholar] [CrossRef] [Green Version]
- Malito, E.; Chandramouli, S.; Carfi, A. From recognition to execution-the HCMV Pentamer from receptor binding to fusion triggering. Curr. Opin. Virol. 2018, 31, 43–51. [Google Scholar] [CrossRef]
- Roubalova, K.; Strunecky, O.; Vitek, A.; Zufanova, S.; Prochazka, B. Genetic variability of cytomegalovirus glycoprotein O in hematopoietic stem cell transplant recipients. Transpl. Infect. Dis. 2011, 13, 237–243. [Google Scholar] [CrossRef]
- Wille, P.T.; Knoche, A.J.; Nelson, J.A.; Jarvis, M.A.; Johnson, D.C. An HCMV gO-null mutant fails to incorporate gH/gL into the virion envelope and is unable to enter fibroblasts, epithelial, and endothelial cells. J. Virol. 2009, 84, 2585–2596. [Google Scholar] [CrossRef] [Green Version]
- Hobom, U.; Brune, W.; Messerle, M.; Hahn, G.; Koszinowski, U.H. Fast screening procedures for random transposon libraries of cloned herpesvirus genomes: Mutational analysis of human cytomegalovirus envelope glycoprotein genes. J. Virol. 2000, 74, 7720–7729. [Google Scholar] [CrossRef] [Green Version]
- Huber, M.T.; Compton, T. The human cytomegalovirus UL74 gene encodes the third component of the glycoprotein H-glycoprotein L-containing envelope complex. J. Virol. 1998, 72, 8191–8197. [Google Scholar] [CrossRef] [Green Version]
- Hahn, G.; Revello, M.G.; Patrone, M.; Percivalle, E.; Campanini, G.; Sarasini, A.; Wagner, M.; Gallina, A.; Milanesi, G.; Koszinowski, U.; et al. Human cytomegalovirus UL131-128 genes are indispensable for virus growth in endothelial cells and virus transfer to leukocytes. J. Virol. 2004, 78, 10023–10033. [Google Scholar] [CrossRef] [Green Version]
- Wang, D.; Shenk, T. Human cytomegalovirus UL131 open reading frame is required for epithelial cell tropism. J. Virol. 2005, 79, 10330–10338. [Google Scholar] [CrossRef] [Green Version]
- Baldanti, F.; Paolucci, S.; Campanini, G.; Sarasini, A.; Percivalle, E.; Revello, M.G.; Gerna, G. Human cytomegalovirus UL131A, UL130 and UL128 genes are highly conserved among field isolates. Arch. Virol. 2006, 151, 1225–1233. [Google Scholar] [CrossRef]
- Adler, B.; Scrivano, L.; Ruzcics, Z.; Rupp, B.; Sinzger, C.; Koszinowski, U. Role of human cytomegalovirus UL131A in cell type-specific virus entry and release. J. Gen. Virol. 2006, 87, 2451–2460. [Google Scholar] [CrossRef]
- Huber, M.T.; Compton, T. Characterization of a novel third member of the human cytomegalovirus glycoprotein H-glycoprotein L complex. J. Virol. 1997, 71, 5391–5398. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Nelson, J.A.; Britt, W.J. Glycoprotein H-related complexes of human cytomegalovirus: Identification of a third protein in the gCIII complex. J. Virol. 1997, 71, 3090–3097. [Google Scholar] [CrossRef] [Green Version]
- Gerna, G.; Percivalle, E.; Perez, L.; Lanzavecchia, A.; Lilleri, D. Monoclonal Antibodies to Different Components of the Human Cytomegalovirus (HCMV) Pentamer gH/gL/pUL128L and Trimer gH/gL/gO as well as Antibodies Elicited during Primary HCMV Infection Prevent Epithelial Cell Syncytium Formation. J. Virol. 2016, 90, 6216–6223. [Google Scholar] [CrossRef] [Green Version]
- Zhou, M.; Lanchy, J.M.; Ryckman, B.J. Human Cytomegalovirus gH/gL/gO Promotes the Fusion Step of Entry into All Cell Types, whereas gH/gL/UL128-131 Broadens Virus Tropism through a Distinct Mechanism. J. Virol. 2015, 89, 8999–9009. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Zhou, M.; Stanton, R.; Kamil, J.; Ryckman, B.J. Expression Levels of Glycoprotein O (gO) Vary between Strains of Human Cytomegalovirus, Influencing the Assembly of gH/gL Complexes and Virion Infectivity. J. Virol. 2018, 92, e00606-18. [Google Scholar] [CrossRef] [Green Version]
- Vo, M.; Aguiar, A.; McVoy, M.A.; Hertel, L. Cytomegalovirus Strain TB40/E Restrictions and Adaptations to Growth in ARPE-19 Epithelial Cells. Microorganisms 2020, 8, 615. [Google Scholar] [CrossRef]
- Murrell, I.; Bedford, C.; Ladell, K.; Miners, K.L.; Price, D.A.; Tomasec, P.; Wilkinson, G.W.G.; Stanton, R.J. The pentameric complex drives immunologically covert cell-cell transmission of wild-type human cytomegalovirus. Proc. Natl. Acad. Sci. USA 2017, 114, 6104–6109. [Google Scholar] [CrossRef] [Green Version]
- Calo, S.; Cortese, M.; Ciferri, C.; Bruno, L.; Gerrein, R.; Benucci, B.; Monda, G.; Gentile, M.; Kessler, T.; Uematsu, Y.; et al. The Human Cytomegalovirus UL116 Gene Encodes an Envelope Glycoprotein Forming a Complex with gH Independently from gL. J. Virol. 2016, 90, 4926–4938. [Google Scholar] [CrossRef] [Green Version]
- Vezzani, G.; Amendola, D.; Yu, D.; Chandramouli, S.; Frigimelica, E.; Maione, D.; Merola, M. The Human Cytomegalovirus UL116 Glycoprotein Is a Chaperone to Control gH-Based Complexes Levels on Virions. Front. Microbiol. 2021, 12, 630121. [Google Scholar] [CrossRef]
- Siddiquey, M.N.A.; Schultz, E.P.; Yu, Q.; Amendola, D.; Vezzani, G.; Yu, D.; Maione, D.; Lanchy, J.M.; Ryckman, B.J.; Merola, M.; et al. The human cytomegalovirus protein UL116 interacts with the viral ER resident glycoprotein UL148 and promotes the incorporation of gH/gL complexes into virions. J. Virol. 2021. [Google Scholar] [CrossRef]
- Gatault, P.; Jones, I.K.A.; Meyer, C.; Kreklywich, C.; Alexander, T.; Smith, P.P.; Denton, M.; Powell, J.; Orloff, S.L.; Streblow, D.N. Rat and human cytomegalovirus ORF116 encodes a virion envelope glycoprotein required for infectivity. Virology 2021, 557, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Lehner, R.; Meyer, H.; Mach, M. Identification and characterization of a human cytomegalovirus gene coding for a membrane protein that is conserved among human herpesviruses. J. Virol. 1989, 63, 3792–3800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mach, M.; Kropff, B.; Dal Monte, P.; Britt, W. Complex formation by human cytomegalovirus glycoproteins M (gpUL100) and N (gpUL73). J. Virol. 2000, 74, 11881–11892. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimamura, M.; Mach, M.; Britt, W.J. Human cytomegalovirus infection elicits a glycoprotein M (gM)/gN-specific virus-neutralizing antibody response. J. Virol. 2006, 80, 4591–4600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beisser, P.S.; Goh, C.S.; Cohen, F.E.; Michelson, S. Viral chemokine receptors and chemokines in human cytomegalovirus trafficking and interaction with the immune system. CMV chemokine receptors. Curr. Top. Microbiol. Immunol. 2002, 269, 203–234. [Google Scholar] [CrossRef]
- Chee, M.S.; Satchwell, S.C.; Preddie, E.; Weston, K.M.; Barrell, B.G. Human cytomegalovirus encodes three G protein-coupled receptor homologues. Nature 1990, 344, 774–777. [Google Scholar] [CrossRef]
- Frank, T.; Niemann, I.; Reichel, A.; Stamminger, T. Emerging roles of cytomegalovirus-encoded G protein-coupled receptors during lytic and latent infection. Med. Microbiol. Immunol. 2019, 208, 447–456. [Google Scholar] [CrossRef]
- Krishna, B.A.; Miller, W.E.; O’Connor, C.M. US28: HCMV’s Swiss Army Knife. Viruses 2018, 10, 445. [Google Scholar] [CrossRef] [Green Version]
- Noriega, V.M.; Gardner, T.J.; Redmann, V.; Bongers, G.; Lira, S.A.; Tortorella, D. Human cytomegalovirus US28 facilitates cell-to-cell viral dissemination. Viruses 2014, 6, 1202–1218. [Google Scholar] [CrossRef]
- Pleskoff, O.; Treboute, C.; Alizon, M. The cytomegalovirus-encoded chemokine receptor US28 can enhance cell-cell fusion mediated by different viral proteins. J. Virol. 1998, 72, 6389–6397. [Google Scholar] [CrossRef] [Green Version]
- Dhyani, V.; Gare, S.; Gupta, R.K.; Swain, S.; Venkatesh, K.V.; Giri, L. GPCR mediated control of calcium dynamics: A systems perspective. Cell Signal. 2020, 74, 109717. [Google Scholar] [CrossRef]
- Okubo, Y.; Uchida, H.; Wakata, A.; Suzuki, T.; Shibata, T.; Ikeda, H.; Yamaguchi, M.; Cohen, J.B.; Glorioso, J.C.; Tagaya, M.; et al. Syncytial Mutations Do Not Impair the Specificity of Entry and Spread of a Glycoprotein D Receptor-Retargeted Herpes Simplex Virus. J. Virol. 2016, 90, 11096–11105. [Google Scholar] [CrossRef] [Green Version]
- Isaacson, M.K.; Feire, A.L.; Compton, T. Epidermal growth factor receptor is not required for human cytomegalovirus entry or signaling. J. Virol. 2007, 81, 6241–6247. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Huong, S.M.; Chiu, M.L.; Raab-Traub, N.; Huang, E.S. Epidermal growth factor receptor is a cellular receptor for human cytomegalovirus. Nature 2003, 424, 456–461. [Google Scholar] [CrossRef]
- Lee, B.J.; Min, C.K.; Hancock, M.; Streblow, D.N.; Caposio, P.; Goodrum, F.D.; Yurochko, A.D. Human Cytomegalovirus Host Interactions: EGFR and Host Cell Signaling Is a Point of Convergence Between Viral Infection and Functional Changes in Infected Cells. Front. Microbiol. 2021, 12, 660901. [Google Scholar] [CrossRef]
- Feire, A.L.; Koss, H.; Compton, T. Cellular integrins function as entry receptors for human cytomegalovirus via a highly conserved disintegrin-like domain. Proc. Natl. Acad. Sci. USA 2004, 101, 15470–15475. [Google Scholar] [CrossRef] [Green Version]
- Soroceanu, L.; Akhavan, A.; Cobbs, C.S. Platelet-derived growth factor-alpha receptor activation is required for human cytomegalovirus infection. Nature 2008, 455, 391–395. [Google Scholar] [CrossRef]
- Martinez-Martin, N.; Viejo-Borbolla, A.; Martin, R.; Blanco, S.; Benovic, J.L.; Thelen, M.; Alcami, A. Herpes simplex virus enhances chemokine function through modulation of receptor trafficking and oligomerization. Nat. Commun. 2015, 6, 6163. [Google Scholar] [CrossRef] [Green Version]
- Brooks, P.J.; Glogauer, M.; McCulloch, C.A. An Overview of the Derivation and Function of Multinucleated Giant Cells and Their Role in Pathologic Processes. Am. J. Pathol. 2019, 189, 1145–1158. [Google Scholar] [CrossRef] [Green Version]
- Scheller, C.; Jassoy, C. Syncytium formation amplifies apoptotic signals: A new view on apoptosis in HIV infection in vitro. Virology 2001, 282, 48–55. [Google Scholar] [CrossRef] [Green Version]
- Chen, E.H.; Grote, E.; Mohler, W.; Vignery, A. Cell-cell fusion. FEBS Lett. 2007, 581, 2181–2193. [Google Scholar] [CrossRef] [Green Version]
- Martens, S.; McMahon, H.T. Mechanisms of membrane fusion: Disparate players and common principles. Nat. Rev. Mol. Cell Biol. 2008, 9, 543–556. [Google Scholar] [CrossRef]
- Vanarsdall, A.L.; Chase, M.C.; Johnson, D.C. Human cytomegalovirus glycoprotein gO complexes with gH/gL, promoting interference with viral entry into human fibroblasts but not entry into epithelial cells. J. Virol. 2011, 85, 11638–11645. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muggeridge, M.I.; Grantham, M.L.; Johnson, F.B. Identification of syncytial mutations in a clinical isolate of herpes simplex virus 2. Virology 2004, 328, 244–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McCarthy, K.M.; Tank, D.W.; Enquist, L.W. Pseudorabies virus infection alters neuronal activity and connectivity in vitro. PLoS Pathog. 2009, 5, e1000640. [Google Scholar] [CrossRef] [PubMed]
- Paradowska, E.; Jablonska, A.; Studzinska, M.; Kasztelewicz, B.; Wisniewska-Ligier, M.; Dzierzanowska-Fangrat, K.; Wozniakowska-Gesicka, T.; Czech-Kowalska, J. Distribution of the CMV glycoprotein gH/gL/gO and gH/gL/pUL128/pUL130/pUL131A complex variants and associated clinical manifestations in infants infected congenitally or postnatally. Sci. Rep. 2019, 9, 16352. [Google Scholar] [CrossRef] [PubMed]
- Schleiss, M.R. Congenital cytomegalovirus infection: Molecular mechanisms mediating viral pathogenesis. Infect. Disord. Drug Targets 2011, 11, 449–465. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tang, J.; Frascaroli, G.; Zhou, X.; Knickmann, J.; Brune, W. Cell Fusion and Syncytium Formation in Betaherpesvirus Infection. Viruses 2021, 13, 1973. https://doi.org/10.3390/v13101973
Tang J, Frascaroli G, Zhou X, Knickmann J, Brune W. Cell Fusion and Syncytium Formation in Betaherpesvirus Infection. Viruses. 2021; 13(10):1973. https://doi.org/10.3390/v13101973
Chicago/Turabian StyleTang, Jiajia, Giada Frascaroli, Xuan Zhou, Jan Knickmann, and Wolfram Brune. 2021. "Cell Fusion and Syncytium Formation in Betaherpesvirus Infection" Viruses 13, no. 10: 1973. https://doi.org/10.3390/v13101973
APA StyleTang, J., Frascaroli, G., Zhou, X., Knickmann, J., & Brune, W. (2021). Cell Fusion and Syncytium Formation in Betaherpesvirus Infection. Viruses, 13(10), 1973. https://doi.org/10.3390/v13101973






