Newcastle Disease Virus Vectored Chicken Infectious Anaemia Vaccine Induces Robust Immune Response in Chickens
Abstract
:1. Introduction
2. Materials and Methods
2.1. Viruses, Cells and Specific Pathogen-Free (SPF) Eggs
2.2. Generation of Recombinant NDV Containing the VP2 and VP1 Genes of CIAV
2.3. Characterisation of Recombinant Viruses
2.4. Fluorescence Assay
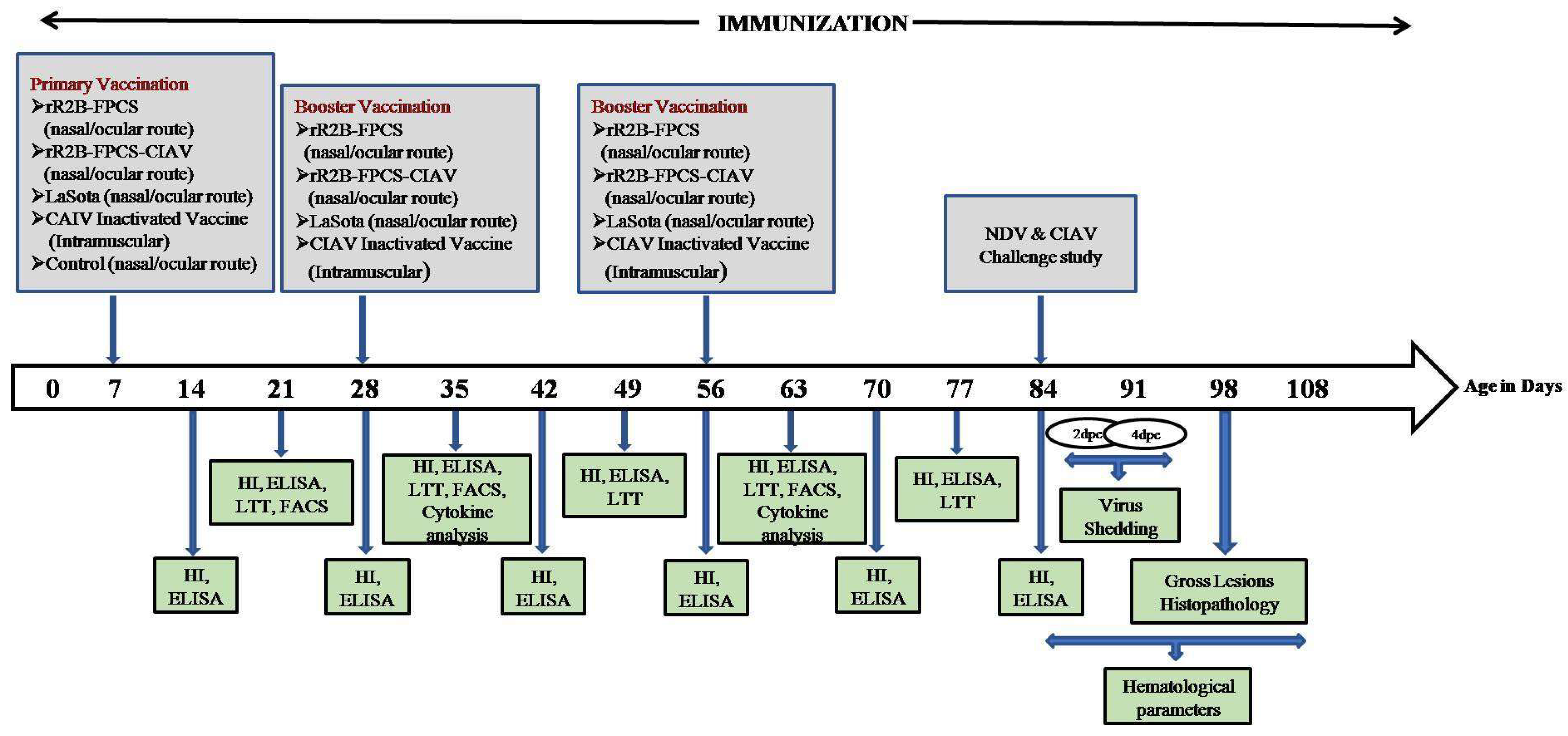
2.5. In Vivo Animal Experimentation Studies
2.6. Haematology
2.7. Histopathology and Lesion Scoring
2.8. Statistical Analysis
3. Results
3.1. Generation of Recombinant NDV Containing the Immunogenic Genes of CIAV
3.2. Molecular and Biological Characterisation of rR2B-FPCS-CIAV Virus
3.3. Humoral Immune Response
3.4. Cell-Mediated Immune (CMI) Response
3.5. Challenge Studies
3.5.1. Virus Shedding Studies
3.5.2. Haematology
3.5.3. Gross and Histopathological Findings
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gelderblom, H.; Kling, S.; Lurz, R.; Tischer, I.; Bülow, V.V. Morphological characterization of chicken anaemia agent (CAA). Arch. Virol. 1989, 109, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Noteborn, M.H.; De Boer, G.F.; Van Roozelaar, D.J.; Karreman, C.; Kranenburg, O.; Vos, J.G.; Jeurissen, S.H.; Hoeben, R.C.; Zantema, A.; Koch, G. Characterization of cloned chicken anemia virus DNA that contains all elements for the infectious replication cycle. J. Virol. 1991, 6, 3131–3139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noteborn, M.H.; Koch, G. Chicken anaemia virus infection: Molecular basis of pathogenicity. Avian Pathol. 1995, 24, 11–31. [Google Scholar] [CrossRef] [PubMed]
- Noteborn, M.H.; Verschueren, C.A.; Koch, G.; Van der Eb, A.J. Simultaneous expression of recombinant baculovirus-encoded chicken anaemia virus (CAV) proteins VP1 and VP2 is required for formation of the CAV-specific neutralizing epitope. J. Gen.Virol. 1998, 79, 3073–3077. [Google Scholar] [CrossRef] [Green Version]
- Chellappa, M.M.; Dey, S.; Gaikwad, S.; Kataria, J.M.; Vakharia, V.N. Complete genome sequence of Newcastle disease virus mesogenic vaccine strain R2B from India. J. Virol. 2012, 86, 13814–13815. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chellappa, M.M.; Dey, S.; Gaikwad, S.; Pathak, D.C.; Vakharia, V.N. Rescue of a recombinant Newcastle disease virus strain R2B expressing green fluorescent protein. Virus Genes 2017, 3, 410–417. [Google Scholar] [CrossRef]
- Dey, S.; Chellappa, M.M.; Gaikwad, S.; Kataria, J.M.; Vakharia, V.N. Genotype characterization of commonly used Newcastle disease virus vaccine strains of India. PLoS ONE 2014, 9, e98869. [Google Scholar]
- Yadav, K.; Pathak, D.C.; Saikia, D.P.; Debnath, A.; Ramakrishnan, S.; Dey, S.; Chellappa, M.M. Generation and evaluation of a recombinant Newcastle disease virus strain R2B with an altered fusion protein cleavage site as a vaccine candidate. Microb. Pathog. 2018, 118, 230–237. [Google Scholar] [CrossRef]
- Saikia, D.P.; Yadav, K.; Pathak, D.C.; Ramamurthy, N.; D’Silva, A.L.; Marriappan, A.K.; Ramakrishnan, S.; Vakharia, V.N.; Chellappa, M.M.; Dey, S. Recombinant Newcastle disease virus (NDV) expressing sigma C protein of avian reovirus (ARV) protects against both ARV and NDV in chickens. Pathogens 2019, 8, 145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Debnath, A.; Pathak, D.C.; D’silva, A.L.; Batheja, R.; Ramamurthy, N.; Vakharia, V.N.; Chellappa, M.M.; Dey, S. Newcastle disease virus vectored rabies vaccine induces strong humoral and cell mediated immune responses in mice. Vet. Microbiol. 2020, 251, 108890. [Google Scholar] [CrossRef]
- Todd, D. Circoviruses: Immunosuppressive threats to avian species: A review. Avian Pathol. 2000, 29, 373–394. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Wu, B.; Liu, Y.; Chen, W.; Dai, Z.; Bi, Y.; Xie, Q. Assessing the efficacy of an inactivated chicken anemia virus vaccine. Vaccine 2015, 33, 1916–1922. [Google Scholar] [CrossRef]
- Moeini, H.; Omar, A.R.; Rahim, R.A.; Yuso, K. Development of a DNA vaccine against chicken anemia virus by using a bicistronic vector expressing VP1 and VP2 proteins of CAV. Comp. Immunol. Microbiol. Infect. Dis. 2011, 34, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Moeini, H.; Omar, A.R.; Rahim, R.A.; Yuso, K. Improving the potency of DNA vaccine against Chicken Anemia Virus (CAV) by fusing VP1 protein of CAV to Marek’s Disease Virus (MDV)Type-1 VP22 protein. Virol. J. 2011, 8, 119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sawant, P.M.; Dhama, K.; Rawool, D.B.; Wani, M.Y.; Tiwari, R.; Singh, S.D.; Singh, R.K. Development of a DNA vaccine for chicken infectious anemia and its immunogenicity studies using high mobility group box 1 protein as a novel immunoadjuvant indicated induction of promising protective immune responses. Vaccine 2015, 33, 333–340. [Google Scholar] [CrossRef]
- Shen, S.Y.; Chang, W.C.; Yi, H.H.; Tsai, S.S.; Liu, H.J.; Liao, P.C.; Chuang, K.P. Development of a subunit vaccine containing recombinant chicken anemia virus VP1 and pigeon IFN-γ. Vet. Immunol. Immunopathol. 2015, 167, 200–204. [Google Scholar] [CrossRef]
- Fang, L.; Li, Y.; Wang, Y.; Fu, J.; Cui, S.; Li, X.; Chang, S.; Zhao, P. Genetic analysis of two chicken infectious anemia virus variants-related Gyrovirus in stray mice and dogs: The First Report in China, 2015. Biomed. Res. Int. 2017, 2017, 6707868. [Google Scholar] [CrossRef] [Green Version]
- Koch, G.; van Roozelaar, D.J.; Verschueren, C.A.; van der Eb, A.J.; Noteborn, M.H. Immunogenic and protective properties of chicken anaemia virus proteins expressed by baculovirus. Vaccine 1995, 13, 763–770. [Google Scholar] [CrossRef]
- Tseng, T.Y.; Liu, Y.C.; Hsu, Y.C.; Chang, P.C.; Hsieh, M.K.; Shien, J.H.; Ou, S.C. Preparation of chicken anemiavirus (CAV) virus-Like particles and chicken interleukin-12 for vaccine development using a baculovirus expression system. Pathogens 2019, 8, 262. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Q.L.; Zhang, Z.F.; He, J.L. Simultaneous expression of chicken anaemia virus proteins VP1 and VP2 in silkworms. Sheng Wu Gong Cheng Xue Bao 2001, 17, 283–287. [Google Scholar]
- Panda, A.; Huang, Z.; Elankumaran, S.; Rockemann, D.D.; Samal, S.K. Role of fusion protein cleavage site in the virulence of Newcastle disease virus. Microb. Pathog. 2004, 36, 1–10. [Google Scholar] [CrossRef]
- Reed, L.J.; Muench, H. A simple method of estimation of fifty per cent end-points. Am. J. Hyg. 1938, 27, 493–497. [Google Scholar]
- Alexander, D.J.; Senne, D. Newcastle disease other avian paramyxovirus and pneumovirus infection. In Diseases of Poultry, 12th ed.; Saif, Y.M., Ed.; Blackwell Publishing: Ames, IA, USA, 2008; pp. 75–115. [Google Scholar]
- Dey, S.; Chellappa, M.M.; Pathak, D.C.; Gaikwad, S.; Yadav, K.; Ramakrishnan, S.; Vakharia, V.N. Newcastle disease virus vectored bivalent vaccine against virulent infectious bursal disease and Newcastle disease of chickens. Vaccines 2017, 5, 31. [Google Scholar] [CrossRef] [Green Version]
- Campbell, T.W. Avian Haematology and Cytology, 2nd ed.; Iowa State University Press: Ames, IA, USA, 1995. [Google Scholar]
- Wani, M.Y.; Dhama, K.; Malik, Y.S. Impact of virus load on immunocytological and histopathological parameters during clinical chicken anemia virus (CAV) infection in poultry. Microb. Pathog. 2016, 96, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Bancroft, J.D.; Cook, H.C. Manual of Histological Techniques and Their Diagnostic Applications; Churchill Livingstone: Edinburgh, UK, 1999. [Google Scholar]
- Hussein, E.A.; Hair-Bejo, M.; Adamu, L.; Omar, A.R.; Arshad, S.S.; Awad, E.A.; Aini, I. Scoring system for lesions induced by different strains of Newcastle disease virus in chicken. Vet. Med. Int. 2018, 9, 9296520. [Google Scholar] [CrossRef] [PubMed]
- Todd, D.; Creelan, J.L.; Mackie, D.P.; Rixon, F.; McNulty, M.S. Purification and biochemical characterization of chicken anaemia agent. J. Gen. Virol. 1990, 71, 819–823. [Google Scholar] [CrossRef] [PubMed]
- Wen, G.; Chen, C.; Guo, J.; Zhang, Z.; Shang, Y.; Shao, H.; Luo, Q.; Yang, J.; Wang, H.; Wang, H.; et al. Development of a novel thermostable Newcastle disease virus vaccine vector for expression of a heterologous gene. J. Gen. Virol. 2015, 96, 1219–1228. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Sun, Q.; Mei, Y.; Liu, Y.; Zhao, L. Newcastle disease virus co-expressing interleukin 7 and interleukin 15 modified tumor cells as a vaccine for cancer immunotherapy. Cancer Sci. 2018, 109, 279–288. [Google Scholar] [CrossRef] [Green Version]
- Veits, J.; Wiesner, D.; Fuchs, W.; Homann, B.; Granzow, H.; Starick, E.; Mundt, E.; Schirrmeier, H.; Mebatsion, T.; Mettenleiter, T.C.; et al. Newcastle disease virus expressing H5 hemagglutinin gene protects chickens against Newcastle disease and avian influenza. Proc. Natl. Acad. Sci. USA 2006, 103, 8197–8202. [Google Scholar] [CrossRef] [Green Version]
- Yu, Q.; Spatz, S.; Li, Y.; Yang, J.; Zhao, W.; Zhang, Z.; Wen, G.; Garcia, M.; Zsak, L. Newcastle disease virus vectored infectious laryngotracheitis vaccines protect commercial broiler chickens in the presence of maternally derived antibodies. Vaccine 2017, 35, 789–795. [Google Scholar] [CrossRef] [Green Version]
- Shirvani, E.; Paldurai, A.; Manoharan, V.K.; Varghese, B.P.; Samal, S.K. A recombinant Newcastle Disease Virus (NDV) expressing S protein of infectious bronchitis virus (IBV) protects chickens against IBV and NDV. Sci. Rep. 2018, 8, 11951. [Google Scholar] [CrossRef]
- Wigley, P.; Kaiser, P. Avian cytokines in health and disease. Braz. J. Poultry Sci. 2003, 5, 1–14. [Google Scholar] [CrossRef]
- Xie, P.; Li, Y.; Li, Y.; Liang, J.; Xiang, B.; Lin, Q.; Jin, J.; Ding, C.; Xu, C.; Ren, T. Immune effect of a Newcastle disease virus DNA vaccine with IL-12 as a molecular adjuvant delivered by electroporation. Arch. Virol. 2020, 165, 1959–1968. [Google Scholar] [CrossRef]
- Su, B.S.; Yin, H.S.; Chiu, H.H.; Hung, L.H.; Huang, J.P.; Shien, J.H.; Lee, L.H. Immunoadjuvant activities of a recombinant chicken IL-12 in chickens vaccinated with Newcastle disease virus recombinant HN protein. Vet. Microbiol. 2011, 151, 220–228. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Arslan, M.; Liu, X.; Song, H.; Du, M.; Li, Y.; Zhang, Z. IFN-γ establishes interferon-stimulated gene-mediated antiviral state against Newcastle disease virus in chicken fibroblasts. Acta Biochim. Biophys. Sin. 2020, 52, 268–280. [Google Scholar] [CrossRef] [Green Version]
- Orakpoghenor, O. Chicken infectious anemia: Emerging viral disease of poultry-an overview. Comp. Clin. Path. 2019, 28, 651–654. [Google Scholar] [CrossRef]
- McNulty, M.S. Chicken anemia agent: A review. Avian Pathol. 1991, 20, 187–203. [Google Scholar] [CrossRef] [PubMed]
- Adair, B.M. Immunopathogenesis of chicken anemia virus infection. Dev. Comp. Immunol. 2000, 24, 247–255. [Google Scholar] [CrossRef]
- Mariappan, A.K.; Munusamy, P.; Kumar, D.; Latheef, S.K.; Singh, S.D.; Singh, R.; Dhama, K. Pathological and molecular investigation of velogenic viscerotropic Newcastle disease outbreak in a vaccinated chicken flocks. Virus Dis. 2018, 29, 180–191. [Google Scholar] [CrossRef]
- Anis, Z.; Morita, T.; Azuma, K.; Ito, H.; Ito, T.; Shimada, A. Histopathological alterations in immune organs of chickens and ducks after experimental infection with virulent 9a5b Newcastle disease virus. J. Comp. Pathol. 2013, 149, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Karel, A.S.; Bernd, K.; Pete, K. Avian Immunology, 2nd ed.; Chapter 16-Avian Immunosuppressive Diseases and Immunoevasion; Elsevier Academic Press: Amsterdam, The Netherlands, 2014; pp. 275–297. [Google Scholar]











| Parameters | Group | CIAV Post Inoculation Days | ||||
|---|---|---|---|---|---|---|
| 5 | 10 | 15 | 20 | 25 | ||
| PCV % | Control unchallenged | 29.66 ± 1.52 a | 31.33 ± 1.52 a | 30.20 ± 0.72 a | 30.23 ± 0.68 a | 29.83 ± 0.76 a |
| Control challenged | 29.33 ± 0.57 a | 26.33 ± 1.52 b | 21.83 ± 1.75 b | 24.30 ± 0.60 b | 25.73 ± 0.64 b | |
| rR2B-FPCS-CIAV | 29.50 ± 0.50 a | 29.66 ± 2.08 a | 30.10 ± 0.85 a | 30.06 ± 0.90 a | 30.30 ± 0.60 a | |
| CIAV-Inactivated | 31.67 ± 0.57 a | 29.66 ± 1.52 a | 29.13 ± 0.80 a | 29.16 ± 1.25 a | 30.16 ± 0.76 a | |
| TEC (Millions/mm3) | Control unchallenged | 3.2 0 ± 0.10 a | 3.76 ± 0.10 a | 3.46 ± 0.10 a | 3.21 ± 0.10 a | 3.11 ± 0.09 a |
| Control challenged | 1.90 ± 0.05 b | 1.96 ± 0.01 b | 1.94 ± 0.14 b | 1.95 ± 0.02 b | 1.91 ± 0.07 b | |
| rR2B-FPCS-CIAV | 2.95 ± 0.03 b | 2.94 ± 0.04 c | 2.84 ± 0.10 c | 2.84 ± 0.06 c | 2.90 ± 0.09 c | |
| CIAV-Inactivated | 2.11 ± 0.07 b | 1.94 ± 0.04 b | 1.97 ± 0.12 b | 1.92 ± 0.07 b | 1.94 ± 0.05 b | |
| TLC (×103/mm 3) | Control unchallenged | 23,452 ± 97.51 a | 24,066 ± 57.30 a | 22,818 ± 76.37 a | 29,350 ± 50.00 a | 29,517 ± 76.37 a |
| Control challenged | 11,399 ± 99.00 b | 10,283 ± 76.37 b | 9790 ± 85.44 b | 10,157 ± 98.14 b | 10,817 ± 76.37 b | |
| rR2B-FPCS-CIAV | 20,317 ± 76.36 c | 19,733 ± 76.37 c | 18,853 ± 95.04 c | 19,543 ± 92.91 c | 19,787 ± 80.82 c | |
| CIAV-Inactivated | 18,483 ± 28.87 d | 16,066 ± 57.73 d | 16,383 ± 76.37 d | 17,217 ± 76.37 d | 17,717 ± 57.73 d | |
| PLC % | Control unchallenged | 45.67 ± 0.57 a | 46.83 ± 0.76 a | 45.23 ± 0.68 a | 46.16 ± 1.25 a | 47.20 ± 1.31 a |
| Control challenged | 21.67 ± 1.52 b | 22.16 ± 1.25 b | 21.16 ± 1.25 b | 23.20 ± 0.72 b | 26.23 ± 1.36 b | |
| rR2B-FPCS-CIAV | 42.33 ± 1.15 c | 39.16 ± 0.76 c | 40.06 ± 0.90 c | 40.30 ± 0.60 c | 42.30 ± 1.47 c | |
| CIAV-Inactivated | 43.67 ± 0.57 c | 39.73 ± 0.64 c | 39.10 ± 0.85 c | 39.10 ± 1.15 c | 40.20 ± 0.72 c | |
| PHC % | Control unchallenged | 53.67 ± 1.52 a | 53.10 ± 0.85 a | 54.16 ± 0.76 a | 54.20 ± 0.72 a | 53.20 ± 0.72 a |
| Control challenged | 74.33 ± 2.0 b | 75.13 ± 1.20 b | 76.20 ± 1.31 b | 76.16 ± 0.76 b | 73.10 ± 1.15 b | |
| rR2B-FPCS-CIAV | 57.67 ± 1.52 a | 61.53 ± 1.85 c | 59.26 ± 0.64 c | 59.20 ± 1.31 c | 57.00 ± 0.90 c | |
| CIAV-Inactivated | 54.0± 2.60 a | 58.16 ± 1.25 c | 60.20 ± 0.72 c | 60.00 ± 0.80 c | 59.03 ± 0.95 c | |
| Hb (g %) | Control unchallenged | 9.77 ± 0.20 a | 11.33 ± 1.52 a | 9.76 ± 0.15 a | 9.60 ± 0.10 a | 9.70 ± 0.10 a |
| Control challenged | 9.80 ± 0.10 b | 8.23 ± 0.15 b | 7.60 ± 0.10 b | 8.20 ± 0.10 b | 8.50 ± 0.30 b | |
| rR2B-FPCS-CIAV | 10.30 ± 0.20 b | 10.16 ± 0.76 c | 11.20 ± 0.20 c | 10.23 ± 0.25 c | 10.36 ± 0.32 c | |
| CIAV-Inactivated | 10.20 ± 0.20 b | 9.80 ± 0.10 c | 9.70 ± 0.15 d | 9.60 ± 0.10 c | 9.50 ± 0.20 c | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chellappa, M.M.; Dey, S.; Pathak, D.C.; Singh, A.; Ramamurthy, N.; Ramakrishnan, S.; Mariappan, A.K.; Dhama, K.; Vakharia, V.N. Newcastle Disease Virus Vectored Chicken Infectious Anaemia Vaccine Induces Robust Immune Response in Chickens. Viruses 2021, 13, 1985. https://doi.org/10.3390/v13101985
Chellappa MM, Dey S, Pathak DC, Singh A, Ramamurthy N, Ramakrishnan S, Mariappan AK, Dhama K, Vakharia VN. Newcastle Disease Virus Vectored Chicken Infectious Anaemia Vaccine Induces Robust Immune Response in Chickens. Viruses. 2021; 13(10):1985. https://doi.org/10.3390/v13101985
Chicago/Turabian StyleChellappa, Madhan Mohan, Sohini Dey, Dinesh Chandra Pathak, Asmita Singh, Narayan Ramamurthy, Saravanan Ramakrishnan, Asok Kumar Mariappan, Kuldeep Dhama, and Vikram N. Vakharia. 2021. "Newcastle Disease Virus Vectored Chicken Infectious Anaemia Vaccine Induces Robust Immune Response in Chickens" Viruses 13, no. 10: 1985. https://doi.org/10.3390/v13101985








