Molecular and Serological Characterization of Hepatitis B Virus (HBV)-Positive Samples with Very Low or Undetectable Levels of HBV Surface Antigen
Abstract
:1. Introduction
2. Materials and Methods
2.1. Samples
2.2. Serologic and Molecular Assays
2.3. Sequencing of the PreS1–PreS2–S Gene Regions
2.4. Mutation Analysis
2.5. Statistical Analysis
3. Results
3.1. Incremental Detection by ARCHITECT HBsAgNx
3.2. HBV DNA and Anti-HBs Levels Relative to HBsAgNx Detection
3.3. Sequence Analysis of the PreS1, PreS2, and S Regions
3.4. PreS1, PreS2, and S Substitutions Unique to OBI or HSLD Samples
3.5. Detection of OBI and HSLD Samples by Novel Biomarker Assays
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Global Hepatitis Report; World Health Organization: Geneva, Switzerland, 2017; Available online: http//www.who.int/hepatitis/publications/global-hepatitis-report2017/en (accessed on 1 June 2021).
- Kuhns, M.C.; Kleinman, S.H.; McNamara, A.L.; Rawal, B.; Glynn, S.; Busch, M.P. Lack of correlation between HBsAg and HBV DNA levels in blood donors who test positive for HBsAg and anti-HBc: Implications for future HBV screening policy. Transfusion 2004, 44, 1332–1339. [Google Scholar] [CrossRef]
- Gerlich, W.H.; Bremer, C.; Saniewski, M.; Schüttler, C.G.; Wend, U.C.; Willems, W.R.; Glebe, D. Occult Hepatitis B Virus Infection: Detection and Significance. Dig. Dis. 2010, 28, 116–125. [Google Scholar] [CrossRef] [PubMed]
- European Association for the Study of the Liver. EASL 2017 Clinical Practice Guidelines on the management of hepatitis B virus infection. J. Hepatol. 2017, 67, 370–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerlich, W.H.; Glebe, D.; Schüttler, C.G. Hepatitis B viral safety of blood donations: New gaps identified. Ann. Blood 2018, 3, 38. [Google Scholar] [CrossRef]
- Yuen, M.F.; Chen, D.S.; Dusheiko, G.M.; Janssen, H.L.; Lau, D.T.; Locarnini, S.A.; Peters, M.G.; Lai, C.L. Hepatitis B virus infection. Nat. Rev. 2018, 4, 18035. [Google Scholar] [CrossRef]
- Locarnini, S.; Raimondo, G. How infectious is the hepatitis B virus? Readings from the occult. Gut 2019, 68, 182–183. [Google Scholar] [CrossRef]
- Wang, H.; Wang, M.; Huang, J.; Xu, R.; Liao, Q.; Shan, Z.; Zheng, Y.; Rong, X.; Tang, X.; Li, T.; et al. Novel hepatitis B virus surface antigen mutations associated with occult genotype B hepatitis B virus infection affect HBsAg detection. J. Viral Hepat. 2020, 27, 915–921. [Google Scholar] [CrossRef]
- Lou, S.; Taylor, R.; Pearce, S.; Kuhns, M.; Leary, T. An ultra-sensitive Abbott ARCHITECT ® assay for the detection of hepatitis B virus surface antigen (HBsAg). J. Clin. Virol. 2018, 105, 18–25. [Google Scholar] [CrossRef]
- Kuhns, M.C.; McNamara, A.L.; Holzmayer, V.; Cloherty, G.A. Molecular and serological characterization of hepatitis B vaccine breakthrough infections in serial samples from two plasma donors. Virol. J. 2019, 16, 43. [Google Scholar] [CrossRef]
- Kuhns, M.C.; Holzmayer, V.; McNamara, A.L.; Sickinger, E.; Schultess, J.; Cloherty, G.A. Improved detection of early acute, late acute, and occult Hepatitis B infections by an increased sensitivity HBsAg assay. J. Clin. Virol. 2019, 118, 41–45. [Google Scholar] [CrossRef]
- Sickinger, E.; Braun, H.-B.; Meyer, T.; Schmid, K.; Daghfal, D.; Oer, M.; Schultess, J. Performance characteristics of the high sensitivity Alinity i & ARCHITECT HBsAg Next Qualitative/Confirmatory assays. Diagn. Microbiol. Infect. Dis. 2020, 97, 115033. [Google Scholar] [CrossRef]
- Butler, E.K.; Gersch, J.; McNamara, A.; Luk, K.-C.; Holzmayer, V.; De Medina, M.; Schiff, E.; Kuhns, M.; Cloherty, G.A. Hepatitis B Virus Serum DNA and RNA Levels in Nucleos(t)ide Analog-Treated or Untreated Patients During Chronic and Acute Infection. Hepatology 2018, 68, 2106–2117. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Hayer, J.; Jadeau, F.; Deleage, G.; Kay, A.; Zoulim, F.; Combet, C. HBVdb: A knowledge database for Hepatitis B Virus. Nucleic Acids Res. 2013, 41, D566–D570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, C.-H.; Yuan, Q.; Chen, P.-J.; Zhang, Y.-L.; Chen, C.-R.; Zheng, Q.-B.; Yeh, S.-H.; Yu, H.; Xue, Y.; Chen, Y.-X.; et al. Influence of mutations in hepatitis B virus surface protein on viral antigenicity and phenotype in occult HBV strains from blood donors. J. Hepatol. 2012, 57, 720–729. [Google Scholar] [CrossRef]
- Warner, N.; Locarnini, S. The antiviral drug selected hepatitis B virus rtA181T/sW172* mutant has a dominant negative secretion defect and alters the typical profile of viral rebound. Hepatology 2008, 48, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Bi, X.; Tong, S. Impact of immune escape mutations and N-linked glycosylation on the secretion of hepatitis B virus virions and subviral particles: Role of the small envelope protein. Virology 2018, 518, 358–368. [Google Scholar] [CrossRef]
- Forbi, J.C.; Ben-Ayed, Y.; Xia, G.-L.; Vaughan, G.; Drobeniuc, J.; Switzer, W.M.; Khudyakov, Y.E. Disparate distribution of hepatitis B virus genotypes in four sub-Saharan African countries. J. Clin. Virol. 2013, 58, 59–66. [Google Scholar] [CrossRef] [Green Version]
- Gerlich, W.H.; Glebe, D.; Schüttler, C.G. Deficiencies in the standardization and sensitivity of diagnostic tests for hepatitis B virus. J. Viral Hepat. 2007, 14, 16–21. [Google Scholar] [CrossRef]
- Malagnino, V.; Salpini, R.; Maffongelli, G.; Battisti, A.; Fabeni, L.; Piermatteo, L.; Colagrossi, L.; Fini, V.; Ricciardi, A.; Sarrecchia, C.; et al. High rates of chronic HBV genotype E infection in a group of migrants in Italy from West Africa: Virological characteristics associated with poor immune response. PLoS ONE 2018, 13, e0195045. [Google Scholar] [CrossRef]
- Candotti, D.; Grabarczyk, P.; Ghiazza, P.; Roig, R.; Casamitjana, N.; Iudicone, P.; Schmidt, M.; Bird, A.; Crookes, R.; Brojer, E.; et al. Characterization of occult hepatitis B virus from blood donors carrying genotype A2 or genotype D strains. J. Hepatol. 2008, 49, 537–547. [Google Scholar] [CrossRef]
- Allain, J.-P.; Belkhiri, D.; Vermeulen, M.; Crookes, R.; Cable, R.; Amiri, A.; Reddy, R.; Bird, A.; Candotti, D. Characterization of occult hepatitis B virus strains in south african blood donors. Hepatology 2009, 49, 1868–1876. [Google Scholar] [CrossRef]
- Bes, M.; Vargas, V.; Piron, M.; Casamitjana, N.; Esteban, J.I.; Vilanova, N.; Pinacho, A.; Quer, J.; Puig, L.; Guardia, J.; et al. T cell responses and viral variability in blood donation candidates with occult hepatitis B infection. J. Hepatol. 2012, 56, 765–774. [Google Scholar] [CrossRef]
- Huang, X.; Ma, C.; Zhang, Q.; Shi, Q.; Huang, T.; Liu, C.; Li, J.; Hollinger, F.B. Impact of “a” determinant mutations on detection of hepatitis B surface antigen (HBsAg) in HBV strains from Chinese patients with occult hepatitis B. J. Med. Virol. 2017, 89, 1796–1803. [Google Scholar] [CrossRef]
- Kwei, K.; Tang, X.; Lok, A.S.; Sureau, C.; Garcia, T.; Li, J.; Wands, J.; Tong, S. Impared virion secretion by hepatitis B virus immune escape mutants and its rescue by wild-type envelope proteins or a second-site mutation. J. Virol. 2013, 87, 2352–2357. [Google Scholar] [CrossRef] [Green Version]
- Ito, K.; Qin, Y.; Guarnieri, M.; Garcia, T.; Kwei, K.; Mizokami, M.; Zhang, J.; Li, J.; Wands, J.R.; Tong, S. Impairment of Hepatitis B Virus Virion Secretion by Single-Amino-Acid Substitutions in the Small Envelope Protein and Rescue by a Novel Glycosylation Site. J. Virol. 2010, 84, 12850–12861. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaudhuri, V.; Tayal, R.; Nayak, B.; Acharya, S.K.; Panda, S.K. Occult hepatitis B virus infection in chronic liver disease: Full-length genome and analysis of mutant surface promoter. Gastroenterology 2004, 127, 1356–1371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pondé, R.A.A. Molecular mechanisms underlying HBsAg negativity in occult HBV infection. Eur. J. Clin. Microbiol. Infect. Dis. 2015, 34, 1709–1731. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.-L.; Li, X.; Li, J.; Zhang, Z.-H. Genetic variation of occult hepatitis B virus infection. World J. Gastroenterol. 2016, 22, 3531–3546. [Google Scholar] [CrossRef]
- Fu, S.; Zhang, J.; Yuan, Q.; Wang, Q.; Deng, Q.; Li, J.; Xia, N.; Wang, Y.; Wen, Y.; Tong, S. Lost small envelope protein expression from naturally occurring PreS1 deletion mutants of hepatitis B virus is often accompanied by increased HBx and core protein expression as well as genome replication. J. Virol. 2021, 95, e00660-21. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Gewaily, D.; Ahn, S.H.; Preskill, C.; Wang, Y.; Zong, L.; Zhang, J.; Han, K.H.; Wands, J.; Li, J.; et al. Sequence analysis and functional characterization of full-length hepatitis Bl virus genomes from Korean cirrhotic patients with or without liver cancer. Virus Res. 2017, 235, 86–95. [Google Scholar] [CrossRef]
- Märschenz, S.; Endres, A.S.; Brinckmann, A.; Heise, T.; Kristiansen, G.; Nürnberg, P.; Krüger, D.H.; Günther, S.; Meisel, H. Functional analysis of complex hepatitis B virus variants associated with development of liver cirrhosis. Gastroenterology 2006, 131, 765–780. [Google Scholar] [CrossRef]
- Pollicino, T.; Raimondo, G. Occult Hepatitis B infection. J. Hepatol. 2014, 61, 688–689. [Google Scholar] [CrossRef] [Green Version]
- Raimondo, G.; Locarnini, S.; Pollicino, T.; Levrero, M.; Zoulim, F.; Lok, A.S.; Taormina Workshop on Occult HBV Infection Faculty Members. Update of the statements on biology and clinical impact of occult hepatitis B virus infection. J. Hepatol. 2019, 71, 397–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warner, N.; Locarnini, S.; Xu, H. The role of hepatitis B surface antibodies in HBV infection, disease and clearance. Future Virol. 2020, 15, 293–306. [Google Scholar] [CrossRef]
- Maasoumy, B.; Wiegand, S.; Jaroszewicz, J.; Bremer, B.; Lehmann, P.; Deterding, K.; Taranta, A.; Manns, M.; Wedemeyer, H.; Glebe, D.; et al. Hepatitis B core-related antigen (HBcrAg) levels in the natural history of hepatitis B virus infection in a large European cohort predominantly infected with genotypes A and D. Clin. Microbiol. Infect. 2015, 21, 606.e1–606.e10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pronier, C.; Candotti, D.; Boizeau, L.; Bomo, J.; Laperche, S.; Thibault, V. The contribution of more sensitive hepatitis B surface antigen assays to detecting and monitoring hepatitis B infection. J. Clin. Virol. 2020, 129, 104507. [Google Scholar] [CrossRef] [PubMed]

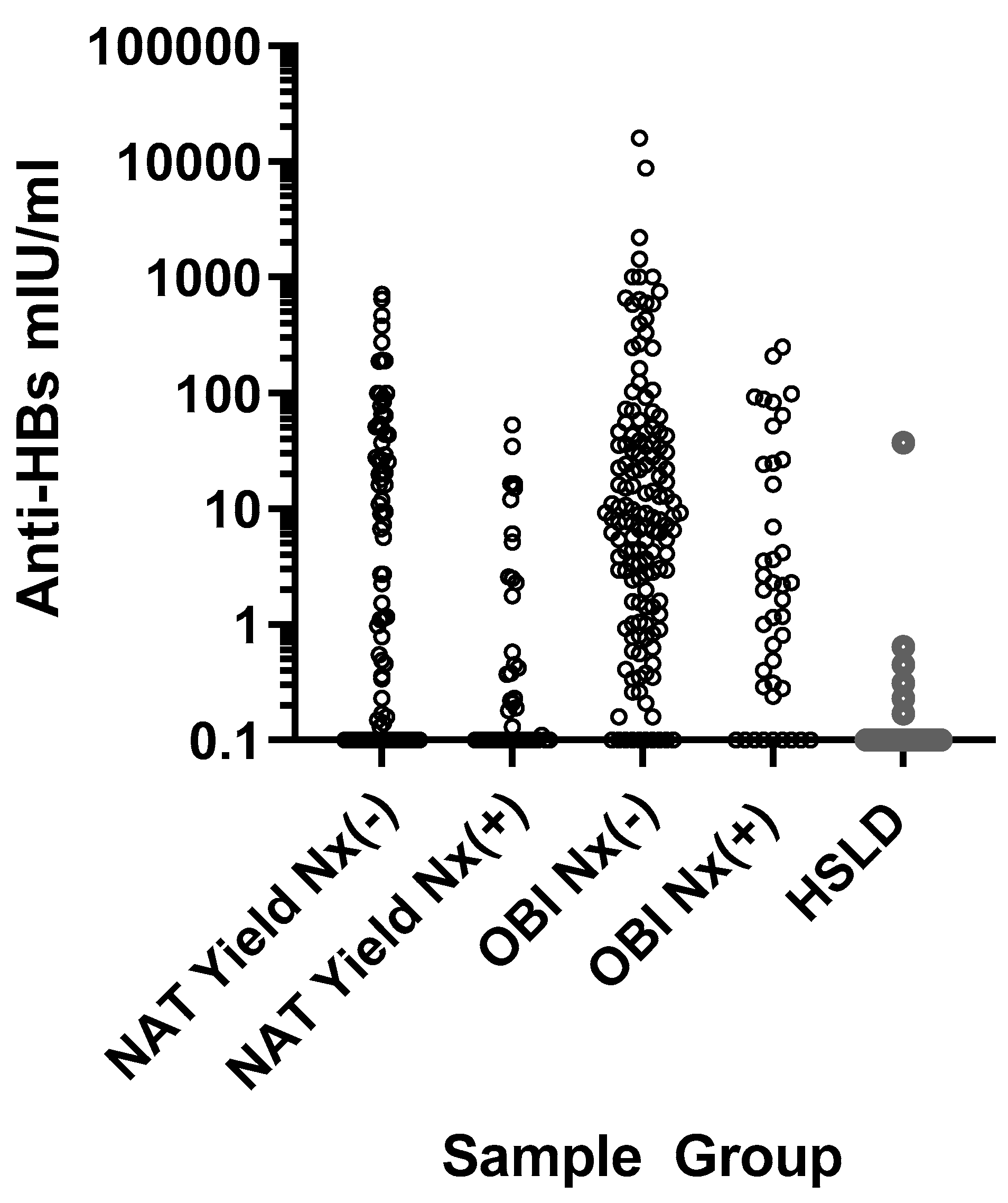
| Sample Group | |||||
|---|---|---|---|---|---|
| HBV NAT Yield HBsAg Qual II(−), HBV DNA(+), Anti-HBc(−) N = 131 | Occult HBV Infection (OBI) HBsAg Qual II(−), HBV DNA(+), Anti-HBc(+), N = 188 | HSLD (High HBsAg with Low HBV DNA Levels) Anti-HBc(+) N = 28 | |||
| HBsAg (IU/mL) | <0.005 a HBsAgNx(−) | 0.005–0.02 b HBsAgNx(+) | <0.005 a HBsAgNx(−) | 0.005–0.02 b HBsAgNx(+) | Mean = 10,104 c Median = 6050.5 c |
| N Samples (% of total) | 87 (66.4) | 44 (33.6) | 146 (77.7) | 42 (22.3) | 28 (100) |
| HBV DNA | |||||
| Mean IU/mL | 50.3 | 384.1 | 25.9 | 139.5 | 32.4 |
| Range | 1.2–676.1 | 38.0–1995.0 | 1.1–363.1 | 1.3–1995.0 | 2.0–85.0 |
| p < 0.0001 | p = 0.0005 | ||||
| Anti-HBs | |||||
| N ≥ 10 mIU/mL (%) | 36 (41.4) | 6 (13.6) | 66 (45.2) | 12 (28.6) | 1 (3.6) |
| p = 0.0014 | p = 0.0748 | ||||
| N sequenced | 45 | 43 | 34 | 27 | 28 |
| Genotypes | A1,A2,B,C,E,F,H | A1,A2,B,C,E | A1,A2,B,C,D,E | A1,A2,A7,B,C,D,E | B,C,E |
| N Samples with PreS1 Substitutions | 32 (71.1%) | 23 (53.5%) | 30 (88.2%) | 24 (89.9%) | 26 (92.9%) |
| Mean Substitutions (range) | 1.50 (1–3) | 1.70 (1–4) | 2.37 (1–6) | 1.75 (1–5) | 3.77 * (1–12) |
| N Samples with PreS2 Substitutions | 34 (75.6%) | 30 (69.8%) | 26 (76.5%) | 17 (63.0%) | 27 ** (96.4%) |
| Mean Substitutions (range) | 1.94 (1–4) | 2.10 (1–5) | 3.0 (1–8) | 2.65 (1–6) | 3.0 (1–8) |
| N Samples with S Substitutions | 25 (55.6%) | 21 (48.8%) | 27 (79.4%) | 21 (77.8%) | 24 (85.7%) |
| Mean Substitutions (range) | 1.24 (1–3) | 1.52 (1–5) | 7.44 (1–26) | 7.10 (1–18) | 3.46 *** (1–9) |
| N Samples with S Escape Mutation | 2 (4.4%) | 1 (2.3%) | 16 (47.1%) | 12 (44.4%) | 2 (7.1%) |
| N Samples with Substitutions in All Three Regions | 17 (37.8%) | 11 (25.6%) | 22 (64.7%) | 13 (48.1%) | 24 (85.7%) |
| Sample | Anti-HBs | HBsAgNx | Genotype | S Protein Amino Acid Substitutions |
|---|---|---|---|---|
| SANBS83 | − | − | A1 | T143M |
| 254 | − | − | C2 | Q101H, R160S |
| AM8.98000 | − | − | B4 | T131N |
| BM7.00531 | − | − | B4 | P127T |
| BM6.33816 | − | − | C1 | Y100C |
| 264 | − | + | E | I110IL |
| 97 | − | + | A2 | Y100S |
| 291 | − | + | A1 | A159G |
| 292 | − | + | C5 | L110I, R160K |
| AM9.92991 | − | + | B4 | A128V |
| BM9.02298 | − | + | B4 | I110L, T143M |
| BM3.32643 | − | + | B4 | I110L, S113T |
| 300 | + | − | H | S143T |
| Sample | Anti-HBs | HBsAgNx | Genotype | S Protein Amino Acid Substitutions |
|---|---|---|---|---|
| HBV0150 | − | − | A2 | Y100C, Q101R, M103I, I110F, S113P, S117G, T123P, T126I, G130GD, N131K, F134V, T143S, C147Y |
| 102 | − | − | A2 | M103I |
| ARC77 | − | − | A2 | Q101R, G145A |
| AM7.98526 | − | − | B4 | M133L |
| BM5.15420 | − | − | B4 | M133L |
| BM8.11637 | − | − | B4 | T123N, F134C |
| BM3.26184 | − | − | C1 | S154P |
| 270 | − | + | E | I110IL |
| 271 | − | + | E | I110L, C124Y |
| 274 | − | + | E | Q101R, L127P, D144E |
| HBV0316 | − | + | D3 | G119R, P120L, R122Q, C124S, M133I, T140S, K141N, P142L, D144G |
| ARC44 | − | + | A1 | V106I |
| ARC09 | − | + | B2 | L109M, M133L |
| ARC48 | − | + | B4 | L109M, M133L, F134V |
| ARC82 | − | + | D3 | G112N, A128V, T131A, M133I |
| BM2.11547 | − | + | B4 | Y100W, Q101QL, Q129H |
| BM7.00672 | − | + | B4 | S114A, G119R, P120Q, C124S, T126I, Q129P, G130K, T131N, S132F, M133T |
| BM9.06187 | − | + | C1 | Y100C, G145A |
| SANBS26 | + | − | A1 | Q101H, F134V, P142L, D144A |
| SANBS63 | + | − | A1 | N131H |
| SANBS96 | + | − | A1 | G112E, T118M, P120PT, M133T, T140I |
| HBV0003 | + | − | A1 | Q101QR, M103V, S117SN, T118TM, P120PS, A128V, M133MT, C139CY, D144E |
| OBI7096 | + | − | D1 | Q101L, S117T, P120S, M133T, Y134F, S136Y, D144A, S154P |
| OBI7063 | + | − | D2 | Y100C, T118A, P120Q, P127T, Q129R, S136Y, K160N |
| OBI7086 | + | − | D3 | Q101QR, G112R, S113T, T123A, P127I, A128V, G130GR, P142PL, D144E, C147CY |
| ARC50 | + | − | A2 | 122Ins 2aa |
| ARC56 | + | − | A2 | Y100C, Q101QC, M103I, N131K, M133I, D144E, S154P |
| AM8.89075 | + | − | B4 | Q101R, L104F, K141R, D144A, K160N |
| BM8.05932 | + | − | B4 | P120S, G130GR, T131TN, M133T |
| BM4.15392 | + | − | B4 | Y100F, Q101R, L109R, I110L, S113T, P120A, K122T, P127L, F134S, D144E, K160V |
| BM3.32210 | + | − | B4 | Y100YC, Q101R |
| 279 | + | + | A7 | Y100C |
| 262 | + | + | E | I110IL |
| ARC79 | + | + | A2 | D144N |
| BM2.27608 | + | + | B4 | P127A, Q129R, T131N, M133S, F134Y |
| AM9.94494 | + | + | B4 | A159GV |
| Genotype | NAT Yield and OBI | N High HBsAg–Low DNA (HSLD) (HBsAg ≥ 0.05 IU/mL) | |
|---|---|---|---|
| N HBsAgNx(−) (HBsAg < 0.005 IU/mL) | N HBsAgNx(+) Qual II (−) (HBsAg 0.005–0.02 IU/mL) | ||
| A1 | 12 | 14 | 0 |
| A2/A7 | 13 | 15 | 0 |
| B | 33 | 17 | 5 * |
| C | 12 | 10 | 3 |
| D | 4 | 2 | 0 |
| E | 3 | 12 | 20 |
| F1 | 1 | 0 | 0 |
| H | 1 | 0 | 0 |
| Total N sequenced | 79 | 70 | 28 |
| Sample Group | PreS1 | PreS2 |
|---|---|---|
| OBI HBsAgNx(−) | F25L(2), A28T, K57T, del 57-99, P65L, S78N, P89T, N98NK, N98NT, N98I, G102R | del 9-22, A11T(2), P36Q, P54Q |
| OBI HBsAgNx(+) | S5L, H50Q(3), del 66-76, del 94 | del 8-19, L20K, del 21-22, A24V, S28N, N55T |
| HSLD | del 7-29, H15Q, T18S(2), D27G(3), R34K(2), R38K, D49N, A62S(3), F62C, F67L, T67I, T67N, T68I, H70V, del 71-97, G72S(3), G73S, G73N(3), W76S(2), S77I, Q79L, del 84-87, K85N, K85Q(4), K85T(4), T96S, Q99R, Q99K, S101V, G101R, I107L(2), D113E, D114E(3), A119V | T6P, T6K, T6S(5), del 8-22, F8Y, del 19-22, V39A(5), A39V, S43L, T49I, P52L(4), P52H(2) |
| Sample Group | S Amino Acids 1–99 | S Amino Acids 100–226 |
|---|---|---|
| OBI HBsAgNx(−) | E2G(2), F19Y, T27A, P29PL, S31N, P62L, P62F, I92T, F93L, F93C(2), L94S, L97H, L98R | M103I(3), M103V, L104F, S113P, S113T(2), T118M, T118TM, T118A, K122ins., T123P, T123N, T123A, S136Y(2), C139CY, T140I, T143S, D144A(3), C147Y, C147CY, S167L, G185GE, F219S, C221Y |
| OBI HBsAgNx(+) | S6SL, S6T, T23NI, I28T, T45K, S55Y, I57IT, L84LF, I86T, L95S | G119R(2), C124Y, C124S(2), S132F, W182*, P188PL, S193L, M197MT |
| HSLD | L13R, A17E(2), L21S(2), L21W, S58L(5), R73H, M75T, F80S | T115N, T116N, T189I(2), F200Y(4), L216*(2), I218L |
| Sample Group | |||
|---|---|---|---|
| OBI | HSLD | ||
| HBsAg (IU/mL) | <0.005 a | 0.005–0.02 b | ≥0.05 c |
| HBV Pregenomic RNA (pgRNA) | |||
| N detected/N tested | 2 */14 (14.3%) | 0/9 (0%) | 12/26 (46.2%) |
| Log U/mL for detected samples | <1.65, 4.09 | All <1.65 d | |
| HBV core-related antigen (HBcrAg) | |||
| N detected/N tested | 2 */14 (14.3%) | 1/9 (11.1%) | 12/28 (42.9%) |
| Log U/mL for detected samples | 3.1, ≥6.8 | 3.0 | Mean = 3.5 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuhns, M.C.; Holzmayer, V.; Anderson, M.; McNamara, A.L.; Sauleda, S.; Mbanya, D.; Duong, P.T.; Dung, N.T.T.; Cloherty, G.A. Molecular and Serological Characterization of Hepatitis B Virus (HBV)-Positive Samples with Very Low or Undetectable Levels of HBV Surface Antigen. Viruses 2021, 13, 2053. https://doi.org/10.3390/v13102053
Kuhns MC, Holzmayer V, Anderson M, McNamara AL, Sauleda S, Mbanya D, Duong PT, Dung NTT, Cloherty GA. Molecular and Serological Characterization of Hepatitis B Virus (HBV)-Positive Samples with Very Low or Undetectable Levels of HBV Surface Antigen. Viruses. 2021; 13(10):2053. https://doi.org/10.3390/v13102053
Chicago/Turabian StyleKuhns, Mary C., Vera Holzmayer, Mark Anderson, Anne L. McNamara, Silvia Sauleda, Dora Mbanya, Pham T. Duong, Nguyen T. T. Dung, and Gavin A. Cloherty. 2021. "Molecular and Serological Characterization of Hepatitis B Virus (HBV)-Positive Samples with Very Low or Undetectable Levels of HBV Surface Antigen" Viruses 13, no. 10: 2053. https://doi.org/10.3390/v13102053
APA StyleKuhns, M. C., Holzmayer, V., Anderson, M., McNamara, A. L., Sauleda, S., Mbanya, D., Duong, P. T., Dung, N. T. T., & Cloherty, G. A. (2021). Molecular and Serological Characterization of Hepatitis B Virus (HBV)-Positive Samples with Very Low or Undetectable Levels of HBV Surface Antigen. Viruses, 13(10), 2053. https://doi.org/10.3390/v13102053





