Feasibility and Effectiveness Assessment of SARS-CoV-2 Antigenic Tests in Mass Screening of a Pediatric Population and Correlation with the Kinetics of Viral Loads
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Participants
2.2. Procedures and Measurements
Testing for SARS-CoV-2 with Antigenic and Qualitative Real Time RT-PCR
- -
- DiaSorin Molecular Simplexa™ COVID-19 Direct assay system (Diasorin Cypress, CA, USA), which rapidly amplifies two targets of the SARS-CoV-2 genome (the S gene and the ORF1ab gene) without RNA extraction. The assay also reveals the presence of host mRNA in the same reaction to confirm the correct execution of the test. Viral RNA was detected via Liaison®MDX plus Direct amplification disc. The final Limit of Detection (LoD) of the test was 500 copies/mL with a Negative Percent Agreement (NPA) and a Positive Percent Agreement (PPA) of 100% [11,12].
- -
- Xpert® Xpress SARS-CoV-2 (Cepheid, Sunnyvale, CA, USA), which rapidly amplifies two targets of the SARS-CoV-2 genome (the E and N2 genes), without RNA extraction, using a proprietary cartridge technology. The LoD was 0.0050 PFU/mL and 0.0200 PFU/mL for N2 and E, respectively, with a PPA of 97.8% (95%CI: 88.4–99.6%) and an NPA of 95.6% (95% CI: 85.2–98.8%). The assay also reveals the presence of host mRNA in the same reaction to confirm the correct execution of the test.
2.3. Quantitative Real Time RT-PCR
2.4. Statistical Analysis
2.5. Ethics
3. Results
3.1. COVID-19 Ag FIA Performance
3.2. AFIAS COVID-19 Ag Performance
3.3. Comparison between Antigenic Test and Quantitative Real Time RT-PCR
4. Discussion
- -
- In that period of time, the prevalence of the infection fluctuated but remained lower than 10%;
- -
- This fluctuation prevented the examiners from drawing conclusions about statistical and epidemiological data;
- -
- The number of children tested with either Ag-RDT was unbalanced due to the different manufactures’ supply. This circumstance gave more statistic impact to the AFIAS COVID-19 Ag kit;
- -
- Only few positive cases (about 6.8% of the total for COVID-19 Ag FIA and 7.7% for AFIAS COVID-19 Ag) have emerged in our cohort; such a small sample prevented us from drawing conclusions on the actual infectivity in our community.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- European Centre for Disease Prevention and Control. COVID-19 Testing Strategies and Objectives; ECDC: Stockholm, Sweden, 2020; Available online: https://www.ecdc.europa.eu/en/publications-data/covid-19-testing-strategies-and-objectives (accessed on 1 July 2021).
- WHO. Laboratory Testing for Coronavirus Disease (COVID-19) in Suspected Human Cases: Interim Guidance, 19 March 2020. Available online: https://apps.who.int/iris/handle/10665/331501 (accessed on 1 July 2021).
- European Centre for Disease Prevention and Control/European Agency for Safety and Health at Work. Considerations on the Use of Rapid Antigen Detection (Including Self) Tests for SARS-CoV-2 in Occupational Settings; ECDC/EU-OSHA: Stockholm, Sweden; Bilbao, Spain, 6 May 2021; Available online: https://www.ecdc.europa.eu/en/publications-data/considerations-use-rapid-antigen-detection-including-self-tests-sars-cov-2 (accessed on 1 July 2021).
- European Centre for Disease Prevention and Control. Options for the Use of Rapid Antigen Tests for COVID-19 in the EU/EEA and the UK; ECDC: Stockholm, Sweden, 2020; Available online: https://www.ecdc.europa.eu/en/publications-data/options-use-rapid-antigen-tests-covid-19-eueea-and-uk (accessed on 1 July 2021).
- Aggiornamento Della Definizione di Caso COVID-19 e Strategie di Testing e Screening—AGENAS. Available online: https://www.agenas.gov.it/comunicazione/primo-piano/1795-aggiornamento-definizione-caso-strategie-testing-screening (accessed on 1 July 2021).
- Aggiornamento Sull’uso dei Test Antigenici e Molecolari per la Rilevazione di SARS-CoV-2 ISS. Available online: https://www.trovanorme.salute.gov.it/norme/renderNormsanPdf?anno=2021&codLeg=78828&parte=1%20&serie=null (accessed on 1 July 2021).
- Colavita, F.; Vairo, F.; Meschi, S.; Valli, M.B.; Lalle, E.; Castilletti, C.; Fusco, D.; Spiga, G.; Bartoletti, P.; Ursino, S.; et al. COVID-19 Rapid Antigen Test as Screening Strategy at Points of Entry: Experience in Lazio Region, Central Italy, August–October 2020. Biomolecules 2021, 11, 425. [Google Scholar] [CrossRef] [PubMed]
- Masetti, R.; Corsini, I.; Leardini, D.; Lanari, M.; Pession, A. Presentations to the emergency department in Bologna, Italy, during COVID-19 outbreak. BMJ Paediatr. Open 2020, 4, e000748. [Google Scholar] [CrossRef] [PubMed]
- Lazzerini, M.; Barbi, E.; Apicella, A.; Marchetti, F.; Cardinale, F.; Trobia, G. Delayed access or provision of care in Italy resulting from fear of COVID-19. Lancet Child Adolesc. Health 2020, 4, e10–e11. [Google Scholar] [CrossRef]
- Rauschenberg, C.; Schick, A.; Goetzl, C.; Roehr, S.; Riedel-Heller, S.G.; Koppe, G.; Durstewitz, D.; Krumm, S.; Reininghaus, U. Social isolation, mental health, and use of digital interventions in youth during the COVID-19 pandemic: A nationally representative survey. Eur. Psychiatry 2021, 64, 1–32. [Google Scholar] [CrossRef] [PubMed]
- Cui, J.; Li, F.; Shi, Z.-L. Origin and evolution of pathogenic coronaviruses. Nat. Rev. Microbiol. 2019, 17, 181–192. [Google Scholar] [CrossRef] [Green Version]
- Available online: https://www.fda.gov/media/136286/download (accessed on 1 July 2021).
- Piralla, A.; Ricchi, M.; Cusi, M.G.; Prati, P.; Vicari, N.; Scarsi, G.; Gandolfo, C.; Anichini, G.; Terrosi, C.; Percivalle, E.; et al. Residual SARS-CoV-2 RNA in nasal swabs of convalescent COVID-19 patients: Is prolonged quarantine always justified? Int. J. Infect. Dis. 2021, 102, 299–302. [Google Scholar] [CrossRef]
- La Scola, B.; Le Bideau, M.; Andreani, J.; Hoang, V.T.; Grimaldier, C.; Colson, P.; Gautret, P.; Raoult, D. Viral RNA load as determined by cell culture as a management tool for discharge of SARS-CoV-2 patients from infectious disease wards. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 1059–1061. [Google Scholar] [CrossRef]
- Chiereghin, A.; Zagari, R.M.; Galli, S.; Moroni, A.; Gabrielli, L.; Venturoli, S.; Bon, I.; Rossini, G.; Saracino, I.M.; Pavoni, M.; et al. Recent Advances in the Evaluation of Serological Assays for the Diagnosis of SARS-CoV-2 Infection and COVID-19. Front. Public Health 2021, 8, 620222. [Google Scholar] [CrossRef]
- Kretzschmar, M.; Rozhnova, G.; Bootsma, M.C.J.; van Boven, M.; van de Wijgert, J.H.H.M.; Bonten, M.J.M. Impact of delays on effectiveness of contact tracing strategies for COVID-19: A modelling study. Lancet Public Health 2020, 5, e452–e459. [Google Scholar] [CrossRef]
- Van Kampen, J.J.A.; Van De Vijver, D.A.M.C.; Fraaij, P.L.A.; Haagmans, B.L.; Lamers, M.M.; Okba, N.; van den Akker, J.P.C.; Endeman, H.; Gommers, D.A.M.P.J.; Cornelissen, J.J.; et al. Duration and key determinants of infectious virus shedding in hospitalized patients with coronavirus disease-2019 (COVID-19). Nat. Commun. 2021, 12, 267. [Google Scholar] [CrossRef]
- Liverpool COVID-19 Community Testing Pilot: Interim Evaluation Report Summary. GOV.UK. Available online: https://www.gov.uk/government/publications/liverpool-covid-19-community-testing-pilot-interim-evaluation-report-summary/liverpool-covid-19-community-testing-pilot-interim-evaluation-report-summary (accessed on 1 July 2021).
- Wölfel, R.; Corman, V.M.; Guggemos, W.; Seilmaier, M.; Zange, S.; Müller, M.A.; Niemeyer, D.; Jones, T.C.; Vollmar, P.; Rothe, C.; et al. Virological assessment of hospitalized patients with COVID-2019. Nature 2020, 581, 465–469. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez-Grande, C.; Adán-Jiménez, J.; Catalán, P.; Alcalá, L.; Estévez, A.; Muñoz, P.; Pérez-Lago, L.; de Viedma, D.G. Inference of Active Viral Replication in Cases with Sustained Positive Reverse Transcription-PCR Results for SARS-CoV-2. J. Clin. Microbiol. 2021, 59, e02277-20. [Google Scholar] [CrossRef] [PubMed]
- Taleghani, N.; Taghipour, F. Diagnosis of COVID-19 for controlling the pandemic: A review of the state-of-the-art. Biosens. Bioelectron. 2021, 174, 112830. [Google Scholar] [CrossRef] [PubMed]
- Mina, M.J.; Peto, T.; García-Fiñana, M.; Semple, M.G.; Buchan, I. Clarifying the evidence on SARS-CoV-2 antigen rapid tests in public health responses to COVID-19. Lancet 2021, 397, 1425–1427. [Google Scholar] [CrossRef]
- Armstrong, S. COVID-19: Tests on students are highly inaccurate, early findings show. BMJ 2020, 371, m4941. [Google Scholar] [CrossRef] [PubMed]
- García-Fiñana, M.; Hughes, D.M.; Cheyne, C.P.; Burnside, G.; Stockbridge, M.; Fowler, T.; Fowler, V.L.; Wilcox, M.H.; Semple, M.G.; Buchan, I. Performance of the Innova SARS-CoV-2 antigen rapid lateral flow test in the Liverpool asymptomatic testing pilot: Population based cohort study. BMJ 2021, 374, n1637. [Google Scholar] [CrossRef] [PubMed]
- Dust, K.; Hedley, A.; Nichol, K.; Stein, D.; Adam, H.; Karlowsky, J.A.; Bullard, J.; Van Caeseele, P.; Alexander, D.C. Comparison of commercial assays and laboratory developed tests for detection of SARS-CoV-2. J. Virol. Methods 2020, 285, 113970. [Google Scholar] [CrossRef] [PubMed]
- Drain, P.K.; Ampajwala, M.; Chappel, C.; Gvozden, A.B.; Hoppers, M.; Wang, M.; Rosen, R.; Young, S.; Zissman, E.; Montano, M. A Rapid, High-Sensitivity SARS-CoV-2 Nucleocapsid Immunoassay to Aid Diagnosis of Acute COVID-19 at the Point of Care: A Clinical Performance Study. Infect. Dis. Ther. 2021, 10, 753–761. [Google Scholar] [CrossRef] [PubMed]
- Park, M.; Won, J.; Choi, B.Y.; Lee, C.J. Optimization of primer sets and detection protocols for SARS-CoV-2 of coronavirus disease 2019 (COVID-19) using PCR and real-time PCR. Exp. Mol. Med. 2020, 52, 963–977. [Google Scholar] [CrossRef] [PubMed]
- European Centre for Disease Prevention and Control. Contact Tracing: Public Health Management of Persons, including Healthcare Workers, Who Have Had Contact with COVID-19 Cases in the European Union—Third Update; ECDC: Stockholm, Sweden, 2020; Available online: https://www.ecdc.europa.eu/en/covid-19-contact-tracing-public-health-management (accessed on 10 July 2021).
- Schools COVID-19 Operational Guidance (Applies until Step 4). GOV.UK. Available online: https://www.gov.uk/government/publications/actions-for-schools-during-the-coronavirus-outbreak/schools-coronavirus-covid-19-operational-guidance (accessed on 1 July 2021).
- European Centrefor Disease Prevention and Control. COVID-19 in Children and the Role of School Settings in Transmission-Second Update; ECDC: Stockholm, Sweden, 2021; Available online: https://www.ecdc.europa.eu/en/publications-data/children-and-school-settings-covid-19-transmission (accessed on 10 July 2021).
- European Centre for Disease Prevention and Control. Population-Wide Testing of SARS-CoV-2: Country Experiences and Potential Approaches in the EU/EEA and the UK.; ECDC: Stockholm, Sweden, 2020; Available online: https://www.ecdc.europa.eu/en/publications-data/population-wide-testing-sars-cov-2-country-experiences-and-potential-approaches (accessed on 10 July 2021).

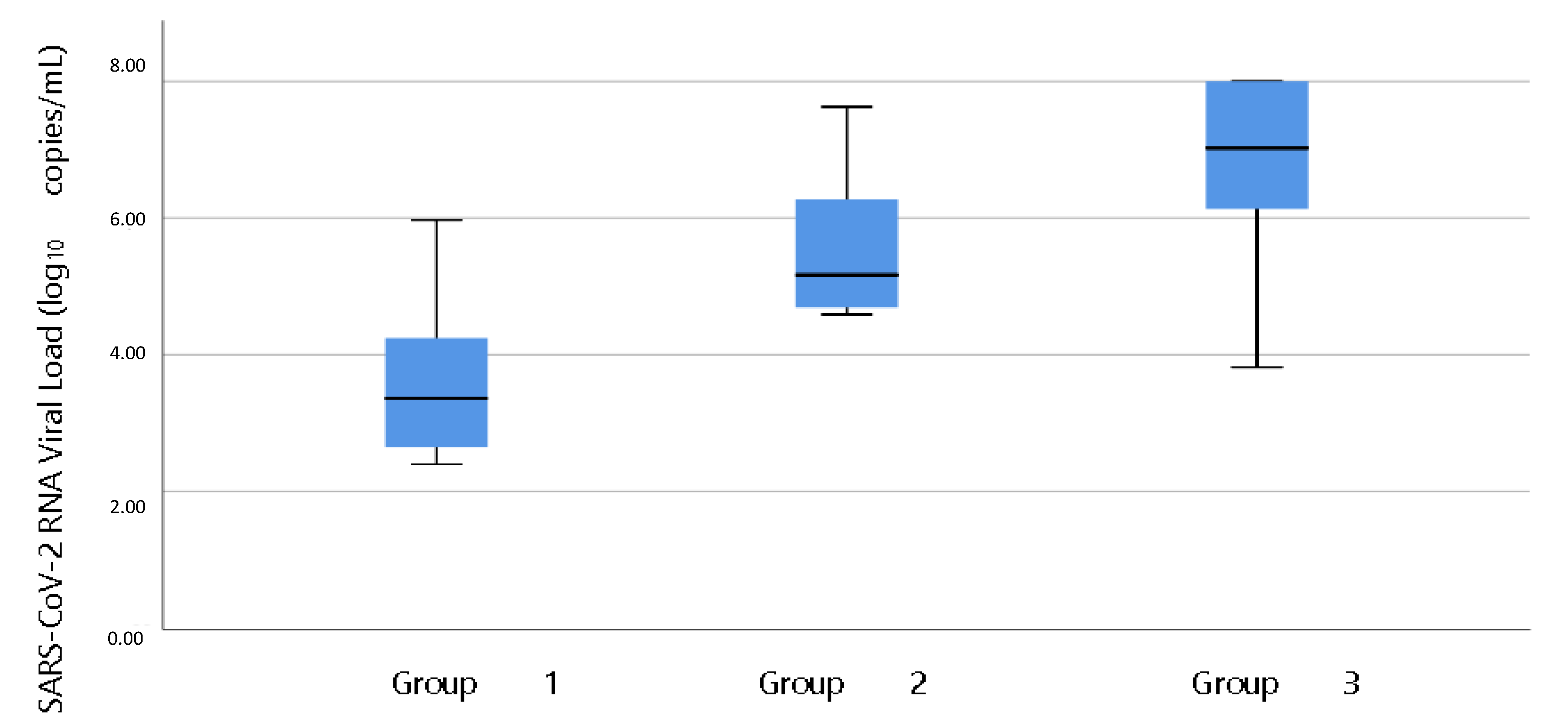
| Characteristic | COVID-19 Ag FIA | AFIAS COVID-19 Ag |
|---|---|---|
| Sex: | ||
| Male | 206 | 450 |
| Female | 180 | 342 |
| Mean age (SD) | 4.6 (4.5) | 6.8 (7.26) |
| Infection-related symptoms: | 280 | 494 |
| Fever | 198 | 345 |
| Pharyngitis | 114 | 83 |
| Cough | 94 | 112 |
| Nasal discharge | 87 | 111 |
| Diarrhea | 47 | 74 |
| Headache | 21 | 54 |
| Anosmia/Ageusia | 2 | 5 |
| Asthenia | 14 | 19 |
| Noninfection-related symptoms: | 106 | 298 |
| Results: | ||
| Positive | 15 | 63 |
| Negative | 367 | 725 |
| Invalid | 4 | 4 |
| Patient | Ag-RDT Kit | Qualitative Real Time RT-PCR | Quantitative Real Time RT-PCR | Epidemiological Link | ||||
|---|---|---|---|---|---|---|---|---|
| COI | CT-Value | Copies/mL | ||||||
| S * | ORF1ab * | E ° | N2 ° | RdRp&ORF8 § | ||||
| 1 | COVID-19 Ag FIA | 0.24 | 34 | 35 | - | - | NA | 11 days from previous positive result |
| 2 | COVID-19 Ag FIA | 0.3 | - | - | 34 | 35 | NA | 10 days from previous positive result |
| 3 | COVID-19 Ag FIA | 0 | - | - | 38 | 39 | ND | 11 days from previous positive result |
| 4 | COVID-19 Ag FIA | 0.07 | - | - | 35 | 0 | 2.3 × 103 | 13 days from previous positive result |
| 5 | COVID-19 Ag FIA | 0.24 | 38 | 0 | - | - | ND | 23 days from previous positive result |
| 6 | COVID-19 Ag FIA | 0.03 | 37 | 39 | - | - | 1 × 103 | 8 days from onset of symptoms |
| 7 | COVID-19 Ag FIA | 0.05 | 35 | 0 | - | - | NA | 0 days from onset of symptoms |
| 8 | COVID-19 Ag FIA | 0.25 | 32 | 35 | - | - | 2.3 × 104 | 2 days from last contact with a confirmed COVID-19 |
| 9 | COVID-19 Ag FIA | 0.01 | - | - | 26 | 26 | 9 × 105 | 21 days from last contact with a confirmed COVID-19 |
| 10 | COVID-19 Ag FIA | 0.22 | 38 | 0 | - | - | 4.5 × 102 | no contact history nor COVID-19 symptoms |
| 11 | COVID-19 Ag FIA | 0.12 | 38 | 0 | - | - | ND | no contact history nor COVID-19 symptoms |
| 12 | COVID-19 Ag FIA | 0.26 | - | - | 35 | 0 | NA | no contact history nor COVID-19 symptoms |
| 13 | AFIAS COVID-19 Ag | 0.49 | - | - | 35 | 36 | NA | 25 days from previous positive result |
| 14 | AFIAS COVID-19 Ag | 0.55 | - | - | 35 | 0 | detected < 250 | 15 days from onset of symptoms |
| 15 | AFIAS COVID-19 Ag | 0.41 | - | - | 37 | 0 | ND | 22 days from onset of symptoms |
| 16 | AFIAS COVID-19 Ag | 0.35 | - | - | 37 | 0 | 1.7 × 104 | 0 days from onset of symptoms |
| 17 | AFIAS COVID-19 Ag | 0.32 | 38 | 0 | NA | 3 days from onset of symptoms | ||
| 18 | AFIAS COVID-19 Ag | 0.63 | 35 | 35 | 1.1 × 104 | no contact history nor COVID-19 symptoms | ||
| 19 | AFIAS COVID-19 Ag | 0.86 | - | - | 0 | 32 | NA | no contact history nor COVID-19 symptoms |
| 20 | AFIAS COVID-19 Ag | 0.61 | 0 | 35 | NA | no contact history nor COVID-19 symptoms | ||
| A | B | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Real Time RT-PCR | Real Time RT-PCR | ||||||||
| Positive | Negative | tot | Positive | Negative | tot | ||||
| Ag-RDT | Positive | 14 | 1 | 15 | Ag-RDT | Positive | 14 | 1 | 15 |
| Negative | 12 | 354 | 366 | Negative | 2 | 354 | 356 | ||
| tot | 26 | 355 | 381 | tot | 16 | 355 | 371 | ||
| Sensitivity = 53.8% (CI 35.4–71.4%), Specificity = 99.7% (CI 98.4–100%), PPV= 93.3% (CI 65.7–99%), NPV=96.7% (CI 96.7–98.2%). | Sensitivity = 87.5% (CI 61.6–98.4%), Specificity = 99.7% (CI 98.4–100%), PPV = 93.3% (CI 66.2–98%), NPV = 99.4% (CI 98–99.8%). | ||||||||
| A | B | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Real Time RT-PCR | Real Time RT-PCR | ||||||||
| Positive | Negative | tot | Positive | Negative | tot | ||||
| Ag-RDT | Positive | 51 | 12 | 63 | Ag-RDT | Positive | 51 | 12 | 63 |
| Negative | 8 | 694 | 702 | Negative | 1 | 694 | 695 | ||
| tot | 59 | 706 | 765 | tot | 52 | 706 | 758 | ||
| Sensitivity = 86.4% (CI 75–93.9%), Specificity = 98.3% (CI 97.1–99.1%), PPV = 81% (CI 70.6–88.3%), NPV = 98.9% (CI 97.9–99.4%) | Sensitivity = 98.1% (CI 89.7–99.9%), Specificity = 98.3% (CI 97.1–99.1%), PPV = 81% (CI 70.8–88.2%), NPV = 99.9% (CI 99–100%) | ||||||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lanari, M.; Biserni, G.B.; Pavoni, M.; Borgatti, E.C.; Leone, M.; Corsini, I.; Lazzarotto, T. Feasibility and Effectiveness Assessment of SARS-CoV-2 Antigenic Tests in Mass Screening of a Pediatric Population and Correlation with the Kinetics of Viral Loads. Viruses 2021, 13, 2071. https://doi.org/10.3390/v13102071
Lanari M, Biserni GB, Pavoni M, Borgatti EC, Leone M, Corsini I, Lazzarotto T. Feasibility and Effectiveness Assessment of SARS-CoV-2 Antigenic Tests in Mass Screening of a Pediatric Population and Correlation with the Kinetics of Viral Loads. Viruses. 2021; 13(10):2071. https://doi.org/10.3390/v13102071
Chicago/Turabian StyleLanari, Marcello, Giovanni Battista Biserni, Matteo Pavoni, Eva Caterina Borgatti, Marta Leone, Ilaria Corsini, and Tiziana Lazzarotto. 2021. "Feasibility and Effectiveness Assessment of SARS-CoV-2 Antigenic Tests in Mass Screening of a Pediatric Population and Correlation with the Kinetics of Viral Loads" Viruses 13, no. 10: 2071. https://doi.org/10.3390/v13102071
APA StyleLanari, M., Biserni, G. B., Pavoni, M., Borgatti, E. C., Leone, M., Corsini, I., & Lazzarotto, T. (2021). Feasibility and Effectiveness Assessment of SARS-CoV-2 Antigenic Tests in Mass Screening of a Pediatric Population and Correlation with the Kinetics of Viral Loads. Viruses, 13(10), 2071. https://doi.org/10.3390/v13102071







