A Comprehensive Coordinator Supported Hepatitis C Virus Testing and Linkage to Treatment Program at Kaiser Permanente Mid-Atlantic States
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. HCV Pathway
2.3. Variables
2.4. Statistical Analysis
3. Results
3.1. Demographics of Patients Tested for HCV
3.2. Demographics of Patients with Chronic HCV
3.3. Patients Completing Steps in the Testing Pathway and Accessing DAA Treatment
3.4. Event Completion and Time-to-Event Analyses
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schillie, S.; Wester, C.; Osborne, M.; Wesolowski, L.; Ryerson, A.B. CDC Recommendations for Hepatitis C Screening Among Adults—United States, 2020. MMWR. Recomm. Rep. 2020, 69, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hofmeister, M.G.; Rosenthal, E.M.; Barker, L.K.; Rosenberg, E.S.; Barranco, M.A.; Hall, E.W.; Edlin, B.R.; Mermin, J.; Ward, J.W.; Ryerson, A.B. Estimating Prevalence of Hepatitis C Virus Infection in the United States, 2013–2016. Hepatology 2019, 69, 1020–1031. [Google Scholar] [CrossRef] [PubMed]
- Ryerson, A.B.; Schillie, S.; Barker, L.K.; Kupronis, B.A.; Wester, C. Vital Signs: Newly Reported Acute and Chronic Hepatitis C Cases―United States, 2009–2018. Morb. Mortal. Wkly. Rep. 2020, 69, 399–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Centers for Disease Control and Prevention. Beyond Baby Boomers: Hepatitis C Now Heavily Impacting Multiple Generations. 2020. Available online: https://www.cdc.gov/nchhstp/newsroom/2020/hepatitis-c-impacting-multiple-generations-press-release.html (accessed on 15 October 2021).
- Denniston, M.M.; Klevens, R.M.; McQuillan, G.M.; Jiles, R.B. Awareness of infection, knowledge of hepatitis C, and medical follow-up among individuals testing positive for hepatitis C: National Health and Nutrition Examination Survey 2001–2008. Hepatology 2012, 55, 1652–1661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, B.D.; Morgan, R.L.; Beckett, G.A.; Falck-Ytter, Y.; Holtzman, D.; Teo, C.G.; Jewett, A.; Baack, B.; Rein, D.B.; Patel, N.; et al. Recommendations for the identification of chronic hepatitis C virus infection among persons born during 1945–1965. Morb. Mortal. Wkly. Rep. Recomm. Rep. 2012, 61, 1–32. [Google Scholar]
- US Preventive Services Task Force. Final Recommendation Statement—Hepatitis C Virus Infection in Adolescents and Adults: Screening. 2020. Available online: https://www.uspreventiveservicestaskforce.org/uspstf/recommendation/hepatitis-c-screening (accessed on 15 October 2021).
- Zibbell, J.E.; Asher, A.K.; Patel, R.C.; Kupronis, B.; Iqbal, K.; Ward, J.W.; Holtzman, D. Increases in Acute Hepatitis C Virus Infection Related to a Growing Opioid Epidemic and Associated Injection Drug Use, United States, 2004 to 2014. Am. J. Public Health 2018, 108, 175–181. [Google Scholar] [CrossRef] [PubMed]
- Tsay, C.J.; Lim, J.K. Assessing the Effectiveness of Strategies in US Birth Cohort Screening for Hepatitis C Infection. J. Clin. Transl. Hepatol. 2020, 8, 25–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konerman, M.A.; Thomson, M.; Gray, K.; Moore, M.; Choxi, H.; Seif, E.; Lok, A.S.F. Impact of an electronic health record alert in primary care on increasing hepatitis c screening and curative treatment for baby boomers. Hepatology 2017, 66, 1805–1813. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Centers for Disease Control and Prevention. Know More Hepatitis. Available online: https://www.cdc.gov/knowmorehepatitis/index.htm (accessed on 15 October 2021).
- Linas, B.P.; Barter, D.M.; Leff, J.A.; Assoumou, S.A.; Salomon, J.A.; Weinstein, M.C.; Kim, A.Y.; Schackman, B.R. The Hepatitis C Cascade of Care: Identifying Priorities to Improve Clinical Outcomes. PLoS ONE 2014, 9, e97317. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reader, S.W.; Kim, H.-S.; El-Serag, H.B.; Thrift, A.P. Persistent Challenges in the Hepatitis C Virus Care Continuum for Patients in a Central Texas Public Health System. Open Forum Infect. Dis. 2020, 7, ofaa322. [Google Scholar] [CrossRef] [PubMed]
- Saab, S.; Challita, Y.P.; Najarian, L.M.; Guo, R.; Saggi, S.S.; Choi, G. Hepatitis C Screening: Barriers to Linkage to Care. J. Clin. Transl. Hepatol. 2019, 7, 226–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez-Watson, C.; Rubenstein, K.B.; Jonas, M.C.; Sun, Y.; Horberg, M.; Loftus, B. Hepatitis C Care Pathway Associated With Increased Screening, Confirmation, and Diagnosis Communication to Patients. Clin. Gastroenterol. Hepatol. 2021, 19, 607–609.e2. [Google Scholar] [CrossRef] [PubMed]
- Jonas, M.C.; Rodriguez, C.V.; Redd, J.; Sloane, D.A.; Winston, B.J.; Loftus, B.C. Streamlining Screening to Treatment: The Hepatitis C Cascade of Care at Kaiser Permanente Mid-Atlantic States. Clin. Infect. Dis. 2016, 62, 1290–1296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horberg, M.A.; Hurley, L.B.; Klein, D.B.; Towner, W.J.; Kadlecik, P.; Antoniskis, D.; Mogyoros, M.; Brachman, P.S.; Remmers, C.L.; Gambatese, R.C.; et al. The HIV Care Cascade Measured Over Time and by Age, Sex, and Race in a Large National Integrated Care System. AIDS Patient Care STDs 2015, 29, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Horberg, M.A.; Certa, J.M.; Rubenstein, K.B.; Hurley, L.B.; Satre, D.D.; Kadlecik, P.M.; Silverberg, M.J. Beyond the HIV Care Continuum and Viral Suppression: Broadening the Scope of Quality Metrics for Total HIV Patient Care. AIDS Patient Care STDs 2020, 34, 461–469. [Google Scholar] [CrossRef] [PubMed]
- Lin, D.Y.; Wei, L.J. The robust inference for the Cox proportional hazards model. J. Am. Stat. Assoc. 1989, 84, 1074–1078. [Google Scholar] [CrossRef]
- Therneau, T. A Package for Survival Analysis in R; CRAN, 2020. Available online: https://CRAN.R-project.org/package=survival (accessed on 1 September 2020).
- Dore, G.J.; Martinello, M.; Alavi, M.; Grebely, J. Global elimination of hepatitis C virus by 2030: Why not? Nat. Med. 2020, 26, 157–160. [Google Scholar] [CrossRef] [PubMed]
| HCV Pathway | Usual Care | p-Value | ||
|---|---|---|---|---|
| Total 2015–2018 | Patient | 131,176 (100.0%) | 128,311 (100.0%) | |
| Age group | 18–19 | 1145 (0.9%) | 3987 (3.1%) | <0.001 |
| 20–29 | 10,804 (8.2%) | 34,088 (26.6%) | <0.001 | |
| 30–39 | 9862 (7.5%) | 27,794 (21.7%) | <0.001 | |
| 40–49 | 8550 (6.5%) | 19,107 (14.9%) | <0.001 | |
| 50–59 | 50,164 (38.2%) | 22,030 (17.2%) | <0.001 | |
| 60–69 | 43,926 (33.5%) | 16,663 (13.0%) | <0.001 | |
| 70–79 | 6481 (4.9%) | 3907 (3.0%) | <0.001 | |
| 80–89 | 232 (0.2%) | 693 (0.5%) | <0.001 | |
| 90–99 | 12 (0.0%) | 41 (0.0%) | <0.001 | |
| 100+ | 0 | 1 (0.0%) | 0.312 | |
| Baby boomer | Baby boomer | 98,331 (75.0%) | 39,134 (30.5%) | <0.001 |
| Not baby boomer | 32,845 (25.0%) | 89,177 (69.5%) | <0.001 | |
| Sex | Female | 71,593 (54.6%) | 86,575 (67.5%) | <0.001 |
| Male | 59,583 (45.4%) | 41,736 (32.5%) | <0.001 | |
| Pregnancy | Pregnancy during testing | 403 (0.6%) | 3459 (4.0%) | <0.001 |
| Race | American Indian/Alaskan native | 714 (0.5%) | 764 (0.6%) | 0.084 |
| Asian | 18,813 (14.3%) | 15,950 (12.4%) | <0.001 | |
| Black/African American | 54,201 (41.3%) | 58,420 (45.5%) | <0.001 | |
| Native Hawaiian/ Pacific islander | 388 (0.3%) | 346 (0.3%) | 0.21 | |
| Unknown or not reported | 17,022 (13.0%) | 18,316 (14.3%) | <0.001 | |
| White/ Caucasian | 39,111 (29.8%) | 33,167 (25.8%) | <0.001 | |
| Other | 927 (0.7%) | 1348 (1.1%) | <0.001 | |
| Insurance | Commercial | 96,326 (73.4%) | 103,012 (80.3%) | <0.001 |
| Medicaid | 9131 (7.0%) | 11,356 (8.9%) | <0.001 | |
| Medicare | 24,790 (18.9%) | 12,459 (9.7%) | <0.001 | |
| Other | 64 (0.0%) | 120 (0.1%) | <0.001 | |
| Unknown | 865 (0.7%) | 1364 (1.1%) | <0.001 | |
| IV drug use | No or unknown | 131,087 (99.9%) | 128,291 (100%) | <0.001 |
| Yes | 89 (0.1%) | 20 (0.0%) | <0.001 | |
| Sexual history: men who have sex with men (MSM) | No or unknown | 128,481 (97.9%) | 125,040 (97.5%) | <0.001 |
| Yes | 2695 (2.1%) | 3271 (2.5%) | <0.001 |
| HCV Pathway | Usual Care | p-Value | ||
|---|---|---|---|---|
| Total 2015–2018 | Patients | 2122 (100.0%) | 620 (100.0%) | |
| 18–19 | 3 (0.1%) | 1 (0.2%) | 0.909 | |
| 20–29 | 86 (4.1%) | 33 (5.3%) | 0.172 | |
| 30–39 | 133 (6.3%) | 64 (10.3%) | 0.001 | |
| 40–49 | 155 (7.3%) | 85 (13.7%) | <0.001 | |
| 50–59 | 757 (35.7%) | 203 (32.7%) | 0.178 | |
| 60–69 | 892 (42.0%) | 190 (30.6%) | <0.001 | |
| 70–79 | 86 (4.1%) | 37 (6.0%) | 0.043 | |
| 80–89 | 8 (0.4%) | 7 (1.1%) | 0.026 | |
| 90–99 | 2 (0.1%) | 0 (0%) | 0.444 | |
| 100+ | 0 (0%) | 0 (0%) | ||
| Baby boomer | Baby boomer | 1646 (77.6%) | 400 (64.5%) | <0.001 |
| Not baby boomer | 476 (22.4%) | 220 (35.5%) | <0.001 | |
| Sex | Female | 803 (37.8%) | 249 (40.2%) | 0.296 |
| Male | 1319 (62.2%) | 371 (59.8%) | 0.296 | |
| Pregnancy | Pregnancy during testing | 11 (1.4%) | 8 (3.2%) | 0.042 |
| Race | American Indian/Alaskan native | 11 (0.5%) | 6 (1.0%) | 0.21 |
| Asian | 114 (5.4%) | 71 (11.5%) | <0.001 | |
| Black/African American | 1346 (63.4%) | 339 (54.7%) | <0.001 | |
| Native Hawaiian/ Pacific islander | 3 (0.1)% | 0 (0%) | 0.349 | |
| Unknown or not reported | 68 (3.2%) | 26 (4.2%) | 0.234 | |
| White/Caucasian | 574 (27.0%) | 174 (28.1%) | 0.618 | |
| Other | 6 (0.3%) | 4 (0.6%) | 0.188 | |
| Insurance | Commercial | 1251 (59.0%) | 405 (65.3%) | 0.004 |
| Medicaid | 384 (18.1%) | 83 (13.4%) | 0.006 | |
| Medicare | 463 (21.8%) | 125 (20.2%) | 0.376 | |
| Other | 5 (0.2%) | 1 (0.2%) | 0.727 | |
| Unknown | 19 (0.9%) | 6 (1.0%) | 0.868 | |
| IV drug use | No or unknown | 2085 (98.3%) | 618 (99.7%) | 0.009 |
| Yes | 37 (1.7%) | 2 (0.3%) | 0.009 | |
| Sexual history: men who have sex with men (MSM) | No or unknown | 2107 (99.3%) | 603 (97.3%) | <0.001 |
| Yes | 15 (0.7%) | 17 (2.7%) | <0.001 | |
| DAA treatment 1 | No treatment | 924 (43.5%) | 370 (59.7%) | <0.001 |
| treatment | 1198 (56.5%) | 250 (40.3%) | <0.001 |
| HCV Pathway | Usual Care | p-Value | |
|---|---|---|---|
| Total Tested | 131,176 | 128,311 | <0.001 |
| HCV Antibody-positive 1 | 4127 (3.1%) 1 | 2220 (1.7%) 1 | <0.001 |
| Chronic HCV and HIV co-infected 2 | 23 (0.6%) 2 | 0 2 | <0.001 |
| Chronic HCV mono-infected (HCV Ab-positive, HCV RNA-positive) 3 | 2122 (51.4%) 3 | 620 (27.9%) 3 | <0.001 |
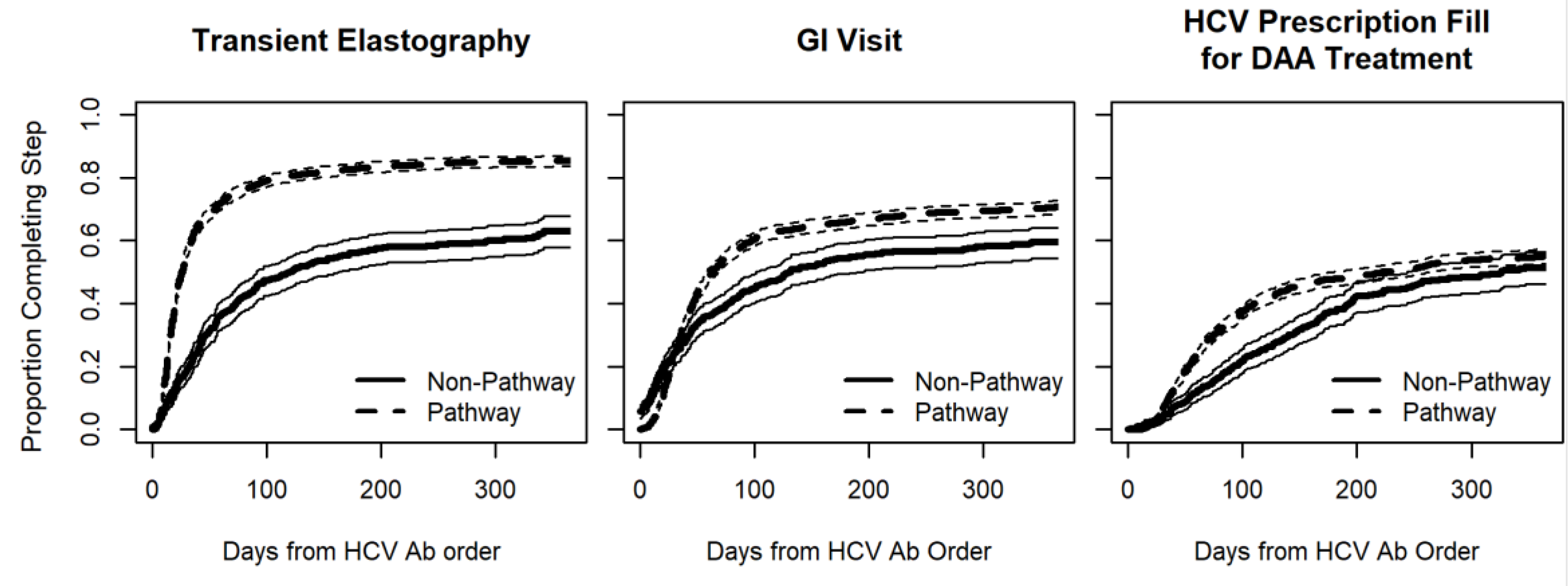
| Hepatic transient elastography 3 | 1753 (82.6%) 3 | 283 (45.6%) 3 | <0.001 |
| GI visit 3 | 1532 (72.2%) 3 | 288 (46.5%) 3 | <0.001 |
| DAA treatment 3 | 1198 (56.5%) 3 | 250 (40.3%) 3 | <0.001 |
| Hazard Ratio 1 | 95% Lower Confidence Limit | 95% Upper Confidence Limit | p-Value | |
|---|---|---|---|---|
| Hepatic transient elastography | ||||
| HCV pathway (compared to usual care) | 2.43 | 2.15 | 2.75 | <0.001 |
| Gastroenterology visit | ||||
| HCV pathway (compared to usual care) | 1.36 | 1.18 | 1.56 | <0.001 |
| HCV prescription fill | ||||
| HCV pathway (compared to usual care) | 1.34 | 1.16 | 1.54 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jonas, M.C.; Rubenstein, K.; Watson, E.; Basra, S.; Horberg, M. A Comprehensive Coordinator Supported Hepatitis C Virus Testing and Linkage to Treatment Program at Kaiser Permanente Mid-Atlantic States. Viruses 2021, 13, 2140. https://doi.org/10.3390/v13112140
Jonas MC, Rubenstein K, Watson E, Basra S, Horberg M. A Comprehensive Coordinator Supported Hepatitis C Virus Testing and Linkage to Treatment Program at Kaiser Permanente Mid-Atlantic States. Viruses. 2021; 13(11):2140. https://doi.org/10.3390/v13112140
Chicago/Turabian StyleJonas, Mary Cabell, Kevin Rubenstein, Eric Watson, Sundeep Basra, and Michael Horberg. 2021. "A Comprehensive Coordinator Supported Hepatitis C Virus Testing and Linkage to Treatment Program at Kaiser Permanente Mid-Atlantic States" Viruses 13, no. 11: 2140. https://doi.org/10.3390/v13112140
APA StyleJonas, M. C., Rubenstein, K., Watson, E., Basra, S., & Horberg, M. (2021). A Comprehensive Coordinator Supported Hepatitis C Virus Testing and Linkage to Treatment Program at Kaiser Permanente Mid-Atlantic States. Viruses, 13(11), 2140. https://doi.org/10.3390/v13112140







