Abstract
At the end of December 2019, an outbreak of COVID-19 occurred in Wuhan city, China. Modelling plays a crucial role in developing a strategy to prevent a disease outbreak from spreading around the globe. Models have contributed to the perspicacity of epidemiological variations between and within nations and the planning of desired control strategies. In this paper, a literature review was conducted to summarise knowledge about COVID-19 disease modelling in three countries—China, the UK and Australia—to develop a robust research framework for the regional areas that are urban and rural health districts of New South Wales, Australia. In different aspects of modelling, summarising disease and intervention strategies can help policymakers control the outbreak of COVID-19 and may motivate modelling disease-related research at a finer level of regional geospatial scales in the future.
1. Introduction
Over the last few decades, the world faced a massive challenge in controlling infectious disease outbreaks in several areas [1]. Recently, a new infectious disease, SARS-CoV-2 named COVID-19, a virus of coronaviridae family and genus beta coronavirus, has emerged globally, and almost all countries and territories are now fighting against this newly appeared infectious disease [2]. The Municipal Commission in Wuhan, China, reported a cluster of pneumonia cases that had an unfamiliar etiology on 12th December 2019. COVID-19 was first identified in Wuhan city, Hubei Province of China, on 31st December 2019, and it spread so fast that within only five months, nearly two million people were infected in 185 countries around the world [3]. On 11th March 2020, the World Health Organization (WHO) announced the transmission of COVID-19 as a global pandemic because of the rapid increment of its infection rate [4]. Following SARS-CoV, which originated in China in 2003, and MERS-CoV, which originated in Saudi Arabia in 2013, SARS-CoV-2 seems to have become the third most significant public health concern of its type. The current fatality rate for COVID-19 cases is about 3.4%, significantly less than SARS and MERS, but potentially higher than those reported for endemic human non-SARS CoV infections [5].
The number of cases quickly rose to 44, with 11 of these patients in severe condition on 3rd January 2020. The COVID-19 virus spread across mainland China with over 30 thousand confirmed cases and over 600 deaths within only one month [6]. The World Health Organization (WHO) published an online resource that presented countries with guidance on detecting, testing and controlling possible cases on 10th January 2020 [7]. The first case outside of China was reported on 13 January 2020. Then, by 11th March 2020, the WHO declared COVID-19 to be a pandemic, based on its fast spread outside China. As of 11th November 2020, over 51.3 million people have been infected globally, with a 2.5% death rate [8]. Currently, almost 47.7% of the total global infections are in three countries—the United States (US), India, and Brazil. Together, deaths in these countries make up around 41.7% of global deaths [8]. According to the Worldometer estimation, up to the date 20th July 2021, nearly 191.7 million people have been identified as infected, with more than 4 million deaths, and about 174.5 million individuals have recovered in 213 countries and territories around the globe [9].
In the US, state and local governments, following the Center for Disease Control (CDC) guidance, started monitoring all individuals who had been in close proximity with confirmed COVID-19 cases. As a result, by 26th February 2020, 12 travel-related positive cases and three positive cases with no travel history were documented [10]. Specifically, the latter category of infections was a cause for concern since it indicated a significantly higher presence of the virus in the United States. In worldwide COVID-19 deaths, the US has been severely burdened by the disease and it alone accounts for about 18.9% of the global deaths, followed by Brazil and India with about 12.8% and 10.0% of global deaths, respectively [4].
The first cases of COVID-19 were linked to a live animal market in Wuhan, China [11]; however, the current rapid spread is via human-to-human transmission. Once infected, the individual will first undergo a period without visible clinical symptoms, called a latent SARS-CoV-2 infection. People with latent SARS-CoV-2 can become infectious one to two days before the onset of symptoms and continue to be infectious up to seven days after that [12]. Therefore, after a certain period, the latent SARS-CoV-2 infection progresses to an active COVID-19 infection. The disease spreads quickly from a person with active COVID-19 infection to another person when the infectious and susceptible persons are close [13]. The spread of COVID-19 depends on the length of exposure of susceptible people to the infected person [14]. It is, in turn, dependent on many factors, such as the crowdedness of the environment, any super-spreading events, the prevailing climatic conditions and the immune status of the exposed individual [15].
Despite extensive epidemiological research on various coronaviruses, there are still many unknowns about this new disease. It is thought that COVID-19 primarily spreads via respiratory droplets and aerosol and has an incubation period of up to 14 days, with symptom onset generally occurring at around days 5–6, similar to SARS-CoV, the cause of the severe acute respiratory syndrome (SARS) epidemic in 2002 [16,17,18]. However, unlike SARS-CoV, which resulted in high viral loads in the lower respiratory tract and led to viral shedding with symptom onset, SARS-CoV-2 has been shown to result in viral shedding due to asymptomatic infection from the upper respiratory tract and making it problematic to organisation preventative procedures that depend on symptomatology [6,19]. As a result, it led to an extreme contact rate from infectious persons to susceptible individuals, and while SARS was basically under control within eight months, the nature of COVID-19 is resembled differently due to the several variants [20]. COVID-19 has various signs and symptoms, varying from a mild cough and fever to a shortness of breath, pain, and even anosmia [21]. The disease is also severely prevalent, with most affected persons being asymptomatic or presenting only mild symptoms. However, the other critical forms of COVID-19 require hospitalisations and, in many cases, prolonged intubations. Treatment for the COVID-19 generally focused on supportive capacities, with only limited antiviral medicine (and announced vaccines in all nations that are open or ready for extensive use to remarkably reduce the number of people dying from COVID-19 through vaccination), presenting some promise at that moment [22,23,24,25,26].
A recent study on risk factors conducted by the Oxford Royal College of General Practitioners Research and the Surveillance Centre primary care network investigated severe disease combined infection rate and disease rate and showed a higher probability of infection for older people, men, people of ethnicity other than white, as well as people from areas with a higher socio-economically deprivation or population density [27]. In addition, initial studies showed COVID-19 to be associated with older age, ethnicity, high population density, and comorbidities such as respiratory infections, hypertension, diabetes, and cardiovascular diseases [19,21,28,29,30]. Notwithstanding significant improvements in science and technology, our perception of the pathogenesis of COVID-19 still seems to be rudimentary, with new (and sometimes conflicting) data emerging almost daily to address the pandemic more efficiently and a race to possible intervention strategy selections.
Modelling has been used as a tool to address gaps in knowledge and to inform health policies for the prevention and control of COVID-19 [31,32,33,34]. Currently, researchers have developed different types of modelling approaches to estimate the relationship between COVID-19 and various risk factors in different sociodemographic and geospatial settings [21,33,35,36,37]. In addition, modelling studies also explore the impact of different intervention strategies to identify the most effective ones. In this study, we carry out a literature review on COVID-19 and infectious disease modelling strategies to develop a robust research framework for the regional areas of New South Wales (NSW), Australia. We believe this may help improve the control strategy for COVID-19 epidemics at the regional level in NSW, and the prospective modelling outcomes will be helpful to decision-makers.
2. Modelling Experience from three Countries for COVID-19
In this section, we appraise different modelling strategies used for the COVID-19 outbreak in three countries—China, the UK, and Australia. Within Australia, we will focus on the transmission dynamics modelling approach considered in NSW.
2.1. Models with Single and Multiple Interventions
A mathematical model is an essential tool to determine which combination interventions would be most effective for reducing the outbreak of COVID-19. Prem et al. [34] developed a modified SEIR model to investigate the impact of physical distancing and population mixing on the progression of COVID-19 in Wuhan, China. In this study, the authors applied synthetic location-specific contact patterns in Wuhan and adjusted these for school closures, extended workplace closures, and decreasing mixing in the general population. They also considered predicting the impact of lifting control measures by permitting people to return to work in their offices. This study found that physical distancing measures were the most useful for controlling COVID-19 in Wuhan. However, implementing physical distancing measures produced varying results, with the duration of infectiousness and the adaptation of school and workplace closures during COVID-19 outbreaks. This study suggests that the premature and sudden lifting of restrictions could lead to a secondary outbreak. Nevertheless, the risk of a secondary outbreak could be minimised or controlled by relaxing restrictions systematically. The limitations of this study are statistical uncertainties about measures of the basic reproduction number and the continuation of infectiousness.
Most of the mathematical modelling studies focus on the transmission dynamics of COVID-19 and do not consider the changing epidemiology and temporal and spatial transmission heterogeneity. Hou et al. [38] developed a modified multi-stage SEIR model to describe the transmission dynamics of COVID-19 in Wuhan at different spatio-temporal scales. In this study, the authors consider the variation in infectivity and introduce the control, the basic reproduction number, by assuming the exposed population to be infectious and simulate the future spread of COVID-19 across Wuhan. The authors also built a novel source-tracing algorithm to infer the initial exposed number of individuals and to estimate the number of infections during the epidemic. The significant findings of this study are that the spatial patterns of COVID-19 spread are heterogeneous, and the infectivity is significantly more remarkable for the exposed population than the infectious population. However, in this study, the predicted exposed population is much greater than the officially reported size of the infectious population in Wuhan.
Due to the insufficient number of COVID-19 vaccines in the early stage, in many countries, lockdown is one of the most effective measures to control the spread of infection and to evaluate the influence of non-pharmaceutical interventions, including the reopening of schools and workplaces, as well as household contacts, and the broader relaxation of physical distancing. Panovaka-Griffiths et al. [39] develop a stochastic individual-based model for the transmission dynamics of COVID-19 in the UK to estimate the impact of school reopening strategies and contact tracing–testing scenarios. The results showed that increasing testing levels and effective contact tracing coupled with isolation might control COVID-19 in the UK. However, without raising testing levels and widespread contact tracing, the reopening of schools together with the gradual relaxing of lockdown measures are likely to cause secondary outbreaks of COVID-19. This study suggests that for preventing secondary spikes in COVID-19 in the UK, the relaxation of physical distancing such as the reopening of schools must be followed by large-scale, effective contact tracing, supported by isolation and the testing of symptomatic individuals [39].
Despite the first confirmed case of COVID-19 in the UK occurring on 30th January 2020, the UK government waited until lab-confirmed cases reached 11,080 before initiating a lockdown on 24th March [40]. How and when to make public health decisions during epidemics are challenging questions to answer. The appropriate policy response should be based on scientific evidence, which depends on good data and modelling. Modelling is the most effective way of measuring and controlling the current outbreak of COVID-19. The critical parameter for explaining the spread of COVID-19 is the basic reproduction number, which is the expected number of secondary cases caused by a single infectious individual introduced into a susceptible population. If the basic reproduction number is less than one, the disease is endemic; otherwise, it is an epidemic. In looking at the effect of the basic reproduction number on the dynamics of the outbreak of COVID-19 in the UK, Wang et al. [41] considered the SIR and SEIR model. Here, the authors defined four types of populations; susceptible (S)—those who are not in contact with the virus but might be infected as a result of transmission from an infected individual; Exposed (E)—those who are infected but not infectious; Infected (I)—those who are infected and infectious; Removed (R)—those who were previously infected but are now free of the disease. The results showed that the basic reproduction number plays a crucial role in explaining the dynamics of the outbreak of COVID-19 in the UK, but due to the novel nature of COVID-19, there is still a challenge to evaluate the epidemiological implications. Therefore, further research is urgently required to fill the gaps.
COVID-19 spreads quickly from one person with the virus to another person when the infectious person coughs and the susceptible person comes into physical contact [13]. Stutt et al. [42] developed a mathematical model to show the effect of wearing facemasks with or without lockdown times on the transmission dynamics of COVID-19 in the UK. The results showed that when the public adopts wearing facemasks most of the time, the effective reproduction number can be reduced to below one, leading towards the elimination of epidemic spread. Furthermore, when lockdown times are implemented in combination with facemask use, there is a lesser spread of the disease, and the secondary peak is not as high. This study suggested that a combination of strategies, including wearing facemasks and social distancing or lockdowns, may constitute a satisfactory policy for controlling COVID-19.
COVID-19 has placed significant extra pressure on hospital intensive care services in many countries, including Australia [43]. Mathematical modelling can provide important insights into the likely cause of the epidemic—these insights are valuable for the intensive care services during the epidemic. Adekunle et al. [36] developed a stochastic metapopulation model to describe the effect of travel bans imposed globally and within Australia on international flight travel volumes. The results showed that travel bans on international passengers arriving from different countries, including Iran, Italy and South Korea, had no significant impact on decreasing the outbreak of COVID-19 cases. However, in the case of a ban on travellers from China, it did have a significant impact. The authors mentioned that one reason for this was that the prevalence of the disease in countries like Iran, Italy and South Korea was lower than in China, and Italy had previously implemented a lockdown by the time Australia implemented restrictions on travellers coming from Italy. Thus, they suggested that the travel ban is very efficient in delaying the extensive transmission of COVID-19. A similar conclusion was drawn by Ip et al. [44] who evaluated various mitigation policies implemented by the state and federal governments of Australia using a generalised space–time autoregressive model. They found that both international and interstate border controls helped to reduce the number of new COVID-19 cases in Australia.
Kang et al. [6] explained the spatio-temporal pattern and explored the spatial relationship of the COVID-19 epidemic in mainland China. This study found that most of the models, except medical-care-based connection models, showed a significant spatial relationship of COVID-19 infections, which means that the management of the spatial spread in the early stage of COVID-19 is very significant for the control of the further transmission. However, although this study has incorporated the spatial aspect of COVID-19, it has some limitations. Firstly, this study did not take into account the number of suspected cases. Therefore, it is a challenge to understand the spatio-temporal transmission of COVID-19. Secondly, this study did not incorporate the urban–rural connection, which might have an important impact on transmission. Therefore, further research is needed to include the most critical factors and to explore the spatial spread of COVID-19.
Costantino et al. [45] developed a deterministic model to further explore the effectiveness of full and partial travel bans in Australia for travellers from China against the spread of COVID-19. They modelled three basic scenarios—no ban, the current ban, followed by a full or partial lifting to examine the influence of travel bans on the dynamics of COVID-19 outbreak control. Moreover, they used COVID-19 incidence data from China and details of passenger flights between China and Australia during and after the outbreak in China, obtained from incoming passenger arrival record cards. The results show that without a travel ban, an increase of more than 2000 cases and around 400 deaths would have occurred. The complete travel ban decreased the number of cases by more than 86%, while the partial travel ban reduced the number of cases by 50%. These figures indicate the efficacy of policy decisions. This study suggests that imposing travel restrictions with a country (China) experiencing an epidemic peak is highly effective. Further tabulated information of the key literature review on COVID-19 modelling in China, the UK, and Australia is summarised in Table 1, which follows.

Table 1.
Summary of the key findings of some important literature about COVID-19 modelling in China, the UK, and Australia.
2.2. Models with Age Structure and Vaccination
Age is one of the significant factors which can influence the occurrence and severity of the COVID-19 disease. Chang et al. [59] developed an agent-based model for transmission dynamics of the ongoing COVID-19 outbreak in Australia. The authors applied the model to compare several intervention strategies, including travel restrictions, case isolation, school closures, social distancing, and home quarantine. The results showed that the rate of symptomatic cases in children is one-fifth of the rate for adults. This study also shows that the intervention of school closures alone was not effective unless coupled with a high level of social distancing. Therefore, the authors asserted that the combination of social distancing with effective isolation and international travel restrictions was the most effective way to control the outbreak of COVID-19.
Vaccination is often considered the best way to prevent or control outbreaks of infectious diseases including COVID-19 [64]. In addition, in the cases of some infectious diseases, there is no specific treatment except vaccination. Although the exploration of vaccines for COVID-19 was a great challenge, different types of vaccines are now available to combat the spread of COVID-19. The European Medicines Agency and the Italian Medicines Agency have approved Pfizer, Moderna, AstraZeneca AZD1222 and J&J Ad26.COV2.S on 13th March 2021 [65].
Table 2 presents a tabulated summary of the current models that include the vaccination strategies specifically focused on China, the UK, and Australia. For instance, McBryde et al. (2021) developed a COVID-19 model with a vaccination to explore the direct and indirect effects of vaccination by vaccine type, age strategy, and coverage in Australia [66]. The model incorporated some crucial factors, including age-specific mixing, infectiousness, susceptibility and severity, to examine the epidemic under different intervention scenarios. The authors found that the current mixed program, including vaccination with AstraZeneca and Pfizer, would not achieve herd immunity unless 85% of Australia is covered, including 5–16 years of age and considering the effective reproduction number for Delta variant is 5. However, when the value of the effective reproduction number is 3, the mixed program can achieve herd immunity at 60–70% coverage without vaccinating 5–15 years of age. The general finding of this study was that vaccination can prevent 85% of death compared to without vaccination [66].

Table 2.
Some current models that include vaccination strategies in China, the UK, and Australia.
In 2021, with numerous vaccines becoming available in Australia, Maclntyre et al. (2021) developed a compartmental COVID-19 model to explore the vaccine’s effectiveness for target groups, including health workers, young people and older adults, and mass vaccination in NSW Australia [67]. For the target group, results showed that health worker vaccination is necessary for health system resilience. Furthermore, age-based policies with restricted doses of the vaccine can reduce a small amount of infections, but vaccinating older people reduces the prevalence of death. On the other hand, mass vaccination, including 66% of the NSW population, can achieve herd immunity. However, this study also found that slower vaccination rates can lead to a prolonging of the COVID-19 pandemic, and a higher number of cases and deaths in the population [67].
Besides, to measure the optimal vaccine prioritisation of COVID-19 transmission, Han et al. (2011) developed a data-driven mechanistic model in China [68]. In this model, they considered 17 age groups and divided the population into five compartments: the unvaccinated susceptible population (S); persons who received at least the first dose of vaccine but have yet to develop protection (V); persons who received the second dose of the vaccine but failed in protection (U); infectious individuals including asymptomatic and symptomatic infections (I); and recovered or immune individuals (R). The result showed that a time-varying vaccination program for the different age groups is the most effective means of reducing deaths and infections. Furthermore, this study recommended that, to minimise the number of deaths and ICU admissions, people over 65 years of age should be vaccinated before moving to other groups such as younger and middle-aged people. Finally, the early phase of high vaccination capacity is the key to achieving significant success of policy measures and implementations [68].
Moreover, a mathematical model with different age groups in the UK was proposed by Moore et al. (2021) to investigate different COVID-19 vaccination scenarios and the age-specific vaccine efficacy [69]. A modified SEIR-type model was considered with a force of infection determined by age-dependent social contact matrices. The authors assumed that the new secondary infections increase due to the first infections within a household. However, the secondary household contacts were to be quarantined and subsequently performed no additional role for the outbreak of COVID-19. The result showed that vaccination is the most effective for the elderly and vulnerable population, which helped reduce the number of deaths and healthcare demands [69]. Modelling vaccination with non-pharmaceutical interventions is necessary to investigate significant variations in behaviours associated with COVID-19 prevention, detection and treatment than a single intervention. Furthermore, Moore et al. [70] proposed another age-structured model-integrated two-dose vaccination and non-pharmaceutical interventions in the UK. The finding showed that vaccination alone is not sufficient to contain the outbreak of COVID-19. In the absence of non-pharmaceutical interventions, the vaccine will prevent 85% of infections in the population. Combining vaccination and non-pharmaceutical interventions can eliminate the COVID-19 outbreak in the UK [70].
Statistically, modelling plays a vital role in efforts that focus on predicting, assessing, and controlling potential outbreaks of different kinds of infectious diseases. Modelling can also be used to explore the contagious disease dynamics that impact numerous variables ranging from the micro host–pathogen level to host-to-host interactions and dominant ecological, social, economic, and geographical factors across the globe. Additionally, Table 3 discusses some key literature for different infectious disease modelling approaches and their control strategies. For instance, Kanyiri et al. (2018) provide modelling results of the transmission dynamics of influenza by incorporating the aspect of drug resistance and using dynamical systems and sensitivity analysis [71]. Overall, the findings of Table 3 studies reveal some consistencies and disparities between the modelling tools and techniques, as well as the diseases and the nature of infections. Indeed, the knowledge of these modelling approaches would help develop a contemporary and robust research framework, which may specifically focus on different spatial levels within a region. Location-specific knowledge is required to develop an appropriate model for a particular area such as regional areas in NSW, Australia.

Table 3.
Review of key literature for other infectious diseases modelling.
3. Developing Models with a Regional Focus
COVID-19 poses a significant challenge for the government healthcare system in regional areas of NSW. One of the most significant challenges is the demand for hospitals to treat critically ill COVID-19 patients [60]. Current knowledge from the outbreak in Italy suggests that a severe demand for intensive care support can occur at the peak of an epidemic. The shortage of intensive care support often leads to preventable deaths due to the lack of accessible intensive care units (ICU) and healthcare workers [81]. The epidemic trajectory of COVID-19 in NSW seems delayed by many weeks compared to several states, including Victoria, due to the travel bans implemented at the beginning of the epidemic. The situation is changing very quickly, and NSW government policy has recently focussed on prevention rather than lockdowns or eliminating COVID-19 infection from the community [60]. Nonetheless, unless an effective vaccine is produced, it seems possible that the outbreak of this disease will transmit quickly within the general population [82]. The effectiveness of current and prospective non-pharmaceutical intervention strategies, including social distancing, is unpredictable or highly reliant on the extent to which they are implemented.
Mathematical modelling is one of the most effective ways to gain insights into the dynamics of an epidemic and to assist in the allocation of resources, including intensive care resources, during different stages of the pandemic. Fox et al. [60] developed a modified SEIR model to estimate hospitalised cases and ICU cases per 100,000 population in NSW. This study considers two scenarios; one is no intervention within a basic reproduction number of 2.4, and the other is social distancing strategies leading to a basic reproduction number of 1.6. The results showed that without social distancing measures, the peak of the COVID-19 cases for hospitalisation would be 450 per 100,000, with about 150 people needing intensive care. According to the scenario without intervention, the outbreak infection peak would be late June and hospital usage in early July. Under the second scenario with social distancing, around 180 people would be hospitalised per 100,000, with 65 people needing intensive care. The outbreak will move to early October, and peak ICU usage will move to mid-November. The authors suggested that the social distancing intervention strategy would be partially effective for the delay of the epidemic peak by around 12 weeks. However, this study did not estimate the effect of suppression strategies, which would reduce the peak of ICU demand. Therefore, further modelling is required to explore the impact of suppression strategies at the time of the epidemic in NSW, including on ICU demand. Such modelling strategies will help to notify the public concerning the timing, severity, and continuation of mitigation policies.
Weather variables including temperature, humidity and rainfall are critical determinants for the outbreak of COVID-19 in NSW [83] and other states and countries [84]. To explore the association between meteorological variables and the number of COVID-19 cases, Ward et al. [83] used a time series analysis in NSW. They used a multivariate generalised additive model (GAM) where a correlation matrix was used to select a weather variable to avoid multicollinearity in the analysis. The best model was selected based on the backward algorithm and the Akaike information criteria (AIC) value. Weather variables were analysed through a 14-day interval based on the incubation time, and the natural splines function with two degrees of freedom is used for the model trend and seasonality. The results showed that temperature and rainfall have no relationship with COVID-19 in NSW, while low temperature and low humidity are suitable for the survival and spread of the virus, because they dry out the mucous membrane, reduce the function of cilia and facilitate the spread of suspended matter in the atmosphere [84,85]. Some modelling studies suggested that lower temperatures may increase the number of COVID-19 cases [84,86]. Therefore, more research is needed to explore the association between temperature and the number of COVID-19 cases.
In the future, we propose to develop a comprehensive model of COVID-19 transmission dynamics over time to infer the impact of mitigation, suppression and multiple interventions and their cost-effective analysis for controlling COVID-19 outbreaks in NSW. We will develop a modified SEIR model to account for the following mutually exclusive compartments: Susceptible , uninfected individuals who are susceptible to the COVID-19 infection; Exposed , representing those who are infected and have not yet developed active COVID-19; Infectious , comprising individuals with active COVID-19; the Recovered , who were previously infected and successfully treated, or death . For estimating healthcare needs, we will categorise the infectious group into two sub-cases: Mild and Critical ; where Mild cases do not require hospital beds; and Critical cases need hospital beds. A flow diagram of our proposed model is presented in Figure 1.
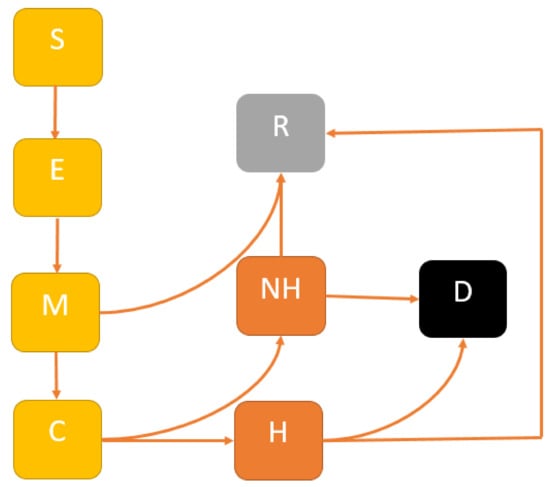
Figure 1.
Extended SEIR model structure: The population is divided into the following six classes: susceptible, exposed (and not yet symptomatic), infectious (symptomatic), i.e., mild (mild or moderate symptom) and critical (severe symptoms), death and recovered (i.e., isolation, recovered, or otherwise non-infectious).
To the best of our knowledge, in previous modelling studies, many mathematical models have been investigated, focusing on mysterious transmission dynamics of COVID-19 using different types of intervention strategies. However, none of them have used a cost-effective analysis for the economy in NSW, Australia. This model will consider a set of non-linear differential equations and will distinguish two essential features—the direct link between the Exposed and Recovered population and the practical healthcare demand resulting from the separation of infections into mild and critical cases. First, we will use a next-generation matrix to determine the basic reproduction number of COVID-19, where is the estimated number of secondary cases produced by single infectious cases and exclusively the susceptible population. Then, to supplement and validate the model structure, we will calibrate the number of cases from the COVID-19 data in NSW. Following this, we will perform a sensitivity analysis to explore the impact of parameters on the model outcomes. Finally, we will incorporate the economic compartment into our proposed model to explore the financial consequences of different interventions and their impact on the dynamics of COVID-19 in NSW, Australia.
4. Conclusions
COVID-19 has had more attention from the government and media than any previous infectious disease, including influenza. Modelling studies can contribute to developing novel control methods, improving computational tools, and public data sharing. For example, modelling studies strongly advised border closures, and China first imposed an internal travel lockdown on Wuhan, which delayed the epidemic peak of COVID-19 within China but had a more significant impact on other countries [35,87,88]. Statistical modelling has also projected the shifting of outbreaks from one country to another, based on these locations’ connectedness [89].
Age is a significant risk factor that can increase the severity of the outbreak of COVID-19. Mixing models can examine age-specific contact patterns and infection risk and use relative infectiousness [90]. Modelling studies have found that children are less likely to acquire an infection, and when infected, they are much less likely to show symptoms. This information will assist policymakers in strategy development. In addition, mixing models have showed that school lockdowns have a modest impact on COVID-19 transmission, encouraging authorities to re-open schools or to avoid school lockdowns completely [34,91].
Mathematical models can estimate the potential epidemic outbreak of COVID-19. One of the essential components for the modelling studies is the basic reproduction number, which is the expected secondary cases caused by a single infectious case in a susceptible population. Modelling studies have shown that implementing suppression (i.e., immediate lockdown) strategies will decrease the reproduction number to less than one, which means that the disease dies out gradually without the need to take any further action. Furthermore, any deficiencies in performing mitigation strategies will increase the risk of having a reproduction number greater than one, which indicates that the disease persists in the population, and governments need to take more actions to control the disease [92]. Intervention strategies and government-imposed constraints on human migration have started to decrease the spread. Models presenting variations in transmission rates over time have been influential tools, helping decision-makers to implement improvements in outbreak control within public health strategies [93].
It is well known that vaccines are very effective for infectious disease control [94,95]. Therefore, for the elimination of COVID-19, a vaccine is urgently needed for global-scale use. There are many clinical trials of COVID-19 vaccines underway, though a few countries claimed success in efficacy trials at their local or national scale. Modelling studies are beneficial in evaluating the effectiveness of vaccines within clinical trials and for reducing biases [96,97]. Modelling can also assist in evaluating the possible effectiveness of vaccination policies, including location-specific ring-vaccination, age-specific vaccination, and the socioeconomic and geopolitical advantages of vaccination. However, for COVID-19, the situation is even more challenging as the disease affects different age groups differently. There is also a greater risk of co-infection and mortality with other diseases, especially in the older age group.
The information generated from the models of the COVID-19 pandemic allows collaborative involvement between decision-makers and researchers. Policymakers can provide researchers with a clear outlook of the policy settings, while researchers can construct models that assist in decision-making. Decision-makers can then plan the policy aims and the intervention strategies and should ideally build a setting where decision-makers and modellers work in combination on an ongoing basis.
Modelling studies may also perform a crucial role in expanding the scope of limited resources under discussion. For instance, a modelling study infers that UK health officials did not examine a policy that included testing due to a limited testing capacity [98]. Modellers also advise using suppression strategies in China rather than mitigation, as the results reduce exposure in China and reduce the number of global cases [99]. Modelling may also provide more optimal scenarios for different intervention strategies with significant benefits at a low cost. For example, in Australia, mitigation strategies are commonly considered rather than suppression strategies (except in Melbourne recently during the second wave of COVID-19 outbreaks) [61]. If modelling studies show that suppression strategies would provide better results, these actions can be implemented early in Australia, including in NSW. Our future application paper will consider this in the context of analysing epidemiological surveillance data to develop an optimal strategy to control COVID-19-type outbreaks in urban and rural health districts of NSW efficiently.
Non-pharmaceutical interventions and vaccination strategies are implemented to prevent and control COVID-19 in most countries in the world. Modelling can assess the potential impact of different interventions measures for mitigating the burden of COVID-19 across the globe [100]. Vaccination is the best way to prevent or control outbreaks of COVID-19. Mathematical models can examine the impact of vaccination on death if herd immunity is not achieved, and it also explores the direct effects of vaccination on reducing death are very good for which vaccines. Therefore, the steps for future research in modelling will be models with a combination of control strategies.
In this review, we have discussed some important COVID-19 models and have attempted to classify them by their structures (including some core assumptions). In addition, we summarise the model outcomes and distinctive features, including the impact of different intervention strategies and their cost, stability, and sensitivity analysis to identify the most impelling risk factors addressing model biases. In doing so, we have identified some open challenges and encouraging prospects for upcoming COVID-19 modelling-related research.
Finally, every study has its limitations. For future research, it is prudent to note those limitations that have posed a challenge to the findings of this study. This study’s specific limitation is the reliance on previously published research regarding mathematical modelling of COVID-19 in three countries, including Australia, China, and the UK, from 2019 to 2021. In addition, the quality of information obtained might not always be reliable, e.g., incidence, prevalence, health demand, etc., which may contaminate findings.
Author Contributions
A.R. planned the study, A.R. and M.A.K. analysed and prepared the manuscript. R.H.L.I. and M.B. helped in the preparation and review of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This paper was supported by Charles Sturt University COVID-19 Research Grant No. 57. Finally, the authors acknowledge an additional financial support provided by Charles Sturt University.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to thank Dmitry Demskoy for providing some feedback on an earlier version and colleagues in the Research Office at Charles Sturt University for their excellent services. We also acknowledge Charles Sturt University’s Institute for Land, Water and Society (ILWS) for providing financial support to enable the publication of this paper.
Conflicts of Interest
The authors declare that they have no competing interests.
References
- Harbeck, M.; Seifert, L.; Hänsch, S.; Wagner, D.; Birdsell, D.; Parise, K.; Wiechmann, I.; Grupe, G.; Thomas, A.; Keim, P.; et al. Yersinia pestis DNA from skeletal remains from the 6th century AD reveals insights into Justinianic Plague. PLoS Pathog. 2013, 9, e1003349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- World Health Organization (WHO). Middle East Respiratory Syndrome Coronavirus (MERS-CoV). Available online: https://www.who.int/news-room/fact-sheets/detail/middle-east-respiratory-syndrome-coronavirus-(mers-cov) (accessed on 28 October 2021).
- Li, Q.; Guan, X.; Wu, P.; Wang, X. Early transmission dynamics in Wuhan, China, of novel coronavirus—Infected pneumonia. N. Engl. J. Med. 2020, 382, 1199–1207. [Google Scholar] [CrossRef]
- Fang, Y.; Nie, Y.; Penny, M. Transmission dynamics of the COVID-19 outbreak and effectiveness of government interventions: A data-driven analysis. J. Med. Virol. 2020, 92, 645–659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raoult, D.; Zumla, A.; Locatelli, F.; Ippolito, G.; Kroemer, G. Coronavirus infections: Epidemiological, clinical and immunological features and hypotheses. Cell Stress 2020, 4, 66. [Google Scholar] [CrossRef]
- Kang, D.; Choi, H.; Kim, J.; Choi, J. Spatial epidemic dynamics of the COVID-19 outbreak in China. Int. J. Infect. Dis. 2020, 94, 96–102. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Laboratory testing of human suspected cases of novel coronavirus (nCoV) infection. Available online: https://apps.who.int/iris/bitstream/handle/10665/330374/WHO-2019-nCoV-laboratory-2020.1-eng.pdf (accessed on 28 October 2021).
- John Hopkins University. COVID-19 dashboard by the center for systems science and engineering. Available online: https://publichealthupdate.com/jhu/ (accessed on 28 October 2021).
- Worldometers. Coronavirus. 2021. Available online: https://www.worldometers.info/coronavirus/ (accessed on 28 October 2021).
- Burke, R.M.; Midgley, C.M.; Dratch, A.; Fenstersheib, M.; Haupt, T.; Holshue, M.; Ghinai, I.; Jarashow, M.C.; Lo, J.; McPherson, T.D.; et al. Active Monitoring of Persons Exposed to Patients with Confirmed COVID-19-United States, January–February 2020. MMWR Morb. Mortal. Wkly. Rep. 2020, 69, 245–246. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.; Ali, A.; Siddique, R.; Nabi, G. Novel coronavirus is putting the whole world on alert. J. Hosp. Infect. 2020, 104, 252–253. [Google Scholar] [CrossRef] [Green Version]
- Cevik, M.; Tate, M.; Lloyd, O.; Maraolo, A.; Schafers, J.; Ho, A. SARS-CoV-2, SARS-CoV, and MERS-CoV viral load dynamics, duration of viral shedding, and infectiousness: A systematic review and meta-analysis. Lancet Microbe 2020, 2, e13–e22. [Google Scholar] [CrossRef]
- Ghinai, I.; McPherson, T.; Hunter, J.; Kirking, H.; Christiansen, D.; Joshi, K.; Rubin, R.; Morales-Estrada, S.; Black, S.; Pacilli, M.; et al. First known person-to-person transmission of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) in the USA. Lancet 2020, 395, 1137–1144. [Google Scholar] [CrossRef]
- McBryde, E. The value of early transmission dynamic studies in emerging infectious diseases. Lancet Infect. Dis. 2020, 20, 512–513. [Google Scholar] [CrossRef] [Green Version]
- Hui, D.S.; Azhar, E.; Kim, Y.; Memish, Z.; Oh, M.; Zumla, A. Middle East respiratory syndrome coronavirus: Risk factors and determinants of primary, household, and nosocomial transmission. Lancet Infect. Dis. 2018, 18, e217–e227. [Google Scholar] [CrossRef] [Green Version]
- Khan, S.; Liu, J.; Xue, M. Transmission of SARS-CoV-2, Required Developments in Research and Associated Public Health Concerns. Front. Med. 2020, 7, 310. [Google Scholar] [CrossRef]
- Shah, V.K.; Firmal, P.; Alam, A.; Ganguly, D.; Chattopadhyay, S. Overview of Immune Response During SARS-CoV-2 Infection: Lessons From the Past. Front. Immunol. 2020, 11, 1949. [Google Scholar] [CrossRef] [PubMed]
- Baraniuk, C. Covid-19: What do we know about airborne transmission of SARS-CoV-2? BMJ 2021, 373, n1030. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.; Lagniton, P.; Ye, S.; Li, E.; Xu, R. COVID-19: What has been learned and to be learned about the novel coronavirus disease. Int. J. Biol. Sci. 2020, 16, 1753–1766. [Google Scholar] [CrossRef] [PubMed]
- Wilder-Smith, A.; Chiew, C.J.; Lee, V.J. Can we contain the COVID-19 outbreak with the same measures as for SARS? Lancet Infect. Dis. 2020, 20, e102–e107. [Google Scholar] [CrossRef] [Green Version]
- Wang, K.-w.; Gao, J.; Wang, H.; Wu, X.; Yuan, Q.; Guo, F.; Zhang, Z.; Cheng, Y. Epidemiology of 2019 novel coronavirus in Jiangsu Province, China after wartime control measures: A population-level retrospective study. Travel Med. Infect. Dis. 2020, 35, 101654. [Google Scholar] [CrossRef] [PubMed]
- Borriello, A.; Master, D.; Pellegrini, A.; Rose, J. Preferences for a COVID-19 vaccine in Australia. Vaccine 2021, 39, 473–479. [Google Scholar] [CrossRef]
- Wang, J.; Jing, R.; Lai, X.; Zhang, H.; Lyu, Y.; Knoll, M.; Fang, H. Acceptance of COVID-19 Vaccination during the COVID-19 Pandemic in China. Vaccines 2020, 8, 482. [Google Scholar] [CrossRef] [PubMed]
- Loomba, S.; Figueiredo, A.; Piatek, S.; de Graaf, K.; Larson, H. Measuring the impact of COVID-19 vaccine misinformation on vaccination intent in the UK and USA. Nat. Hum. Behav. 2021, 5, 337–348. [Google Scholar] [CrossRef]
- Sallam, M. COVID-19 vaccine hesitancy worldwide: A concise systematic review of vaccine acceptance rates. Vaccines 2021, 9, 160. [Google Scholar] [CrossRef] [PubMed]
- Chard, A.N.; Cacic-Dobo, M.; Diallo, M.; Sodha, S.; Wallace, A. Routine vaccination coverage-Worldwide, 2019. Morb. Mortal. Wkly. Rep. 2020, 69, 1706–1710. [Google Scholar] [CrossRef]
- De Lusignan, S.; Dorward, J.; Correa, A.; Jones, N.; Akinyemi, O.; Amirthalingam, G.; Andrews, N.; Byford, R.; Dabrera, G.; Elliot, A.; et al. Risk factors for SARS-CoV-2 among patients in the Oxford Royal College of General Practitioners Research and Surveillance Centre primary care network: A cross-sectional study. Lancet Infect. Dis. 2020, 20, 1034–1042. [Google Scholar] [CrossRef]
- Smith, S.; Morbey, R.; de Lusignan, S.; Pebody, R.; Smith, G.; Elliot, A. Investigating regional variation of respiratory infections in a general practice syndromic surveillance system. J. Public Health 2020, 43, e153–e160. [Google Scholar] [CrossRef] [Green Version]
- Pareek, M.; Bangash, M.; Pareek, N.; Pan, D.; Sze, S.; Minhas, J.; Hanif, W.; Khunti, K. Ethnicity and COVID-19: An urgent public health research priority. Lancet 2020, 395, 1421–1422. [Google Scholar] [CrossRef]
- Yang, J.; Zheng, Y.; Gou, X.; Pu, K.; Chen, Z.; Guo, Q.; Ji, R.; Wang, H.; Wang, Y.; Zhou, Y. Prevalence of comorbidities and its effects in patients infected with SARS-CoV-2: A systematic review and meta-analysis. Int. J. Infect. Dis. 2020, 94, 91–95. [Google Scholar] [CrossRef]
- Kucharski, A.J.; Russell, T.; Diamond, C.; Liu, Y.; Edmunds, J.; Funk, S.; Eggo, R. Early dynamics of transmission and control of COVID-19: A mathematical modelling study. Lancet Infect. Dis. 2020, 20, 553–558. [Google Scholar] [CrossRef] [Green Version]
- Tuite, A.R.; Fisman, D.N.; Greer, A.L. Mathematical modelling of COVID-19 transmission and mitigation strategies in the population of Ontario, Canada. CMAJ 2020, 192, E497–E505. [Google Scholar] [CrossRef] [Green Version]
- Panovska-Griffiths, J. Can mathematical modelling solve the current Covid-19 crisis? BMC Public Health 2020, 20, 551. [Google Scholar] [CrossRef] [PubMed]
- Prem, K.; Liu, Y.; Russell, T.; Kucharski, A.; Eggo, R.; Davies, N. The effect of control strategies to reduce social mixing on outcomes of the COVID-19 epidemic in Wuhan, China: A modelling study. Lancet Public Health 2020, 5, e261–e270. [Google Scholar] [CrossRef] [Green Version]
- Adekunle, A.; Meehan, M.; Rojas-Alvarz, D.; Trauer, J.; McBryde, E. Delaying the COVID-19 epidemic in Australia: Evaluating the effectiveness of international travel bans. Australian and New Zealand. J. Public Health 2020, 44, 257–259. [Google Scholar]
- Rahman, A.; Kuddus, M.A. Modelling the Transmission Dynamics of COVID-19 in Six High-Burden Countries. BioMed Res. Int. 2020, 2021, 5089184. [Google Scholar]
- Kuddus, M.A.; Rahman, A. Analysis of COVID-19 using a modified SLIR model with nonlinear incidence. Results Phys. 2021, 27, 104478. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Hong, J.; Ji, B.; Dong, B.; Chen, Y.; Ward, M.; Tu, W.; Jin, Z.; Hu, J.; Su, Q. Changing transmission dynamics of COVID-19 in China: A nationwide population-based piecewise mathematical modelling study. medRxiv 2020. preprint. [Google Scholar] [CrossRef]
- Panovska-Griffiths, J.; Kerr, C.; Stuart, R.; Mistry, D.; Klein, D.; Viner, R.; Bonell, C. Determining the optimal strategy for reopening schools, work and society in the UK: Balancing earlier opening and the impact of test and trace strategies with the risk of occurrence of a secondary COVID-19 pandemic wave. medRxiv 2020, preprint. [Google Scholar] [CrossRef]
- UK Government. Coronavirus (COVID-19) in the UK. 2020. Available online: https://coronavirus.data.gov.uk/ (accessed on 28 October 2021).
- Wang, N.; Fu, Y.; Zhang, H.; Shi, H. An evaluation of mathematical models for the outbreak of COVID-19. Precis. Clin. Med. 2020, 3, 85–93. [Google Scholar] [CrossRef]
- Stutt, R.O.; Retkute, R.; Bradley, M.; Gilligan, C.; Colvin, J. A modelling framework to assess the likely effectiveness of facemasks in combination with ‘lock-down’ in managing the COVID-19 pandemic. Proc. R. Soc. A 2020, 476, 20200376. [Google Scholar] [CrossRef] [PubMed]
- Australia Health Sector Emergency Response Plan for Novel Coronavirus (COVID-19); Australia Government, Department of Health: Canberra, Australia, 2020.
- Ip, R.H.L.; Demskoi, D.; Rahman, A.; Zheng, L. Evaluation of COVID-19 mitigation policies in Australia ssing generalised space-time autoregressive intervention models. Int. J. Environ. Res. Public Health 2021, 18, 7474. [Google Scholar] [CrossRef] [PubMed]
- Costantino, V.; Heslop, D.J.; MacIntyre, C.R. The effectiveness of full and partial travel bans against COVID-19 spread in Australia for travellers from China during and after the epidemic peak in China. J. Travel Med. 2020, 27, taaa081. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.; Chen, H. Modeling the epidemic dynamics and control of COVID-19 outbreak in China. Quant. Biol. 2020, 11, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, M.; Ning, J.; Du, Y.; Cao, J.; Zhang, D.; Wang, J.; Chen, M. Modelling the evolution trajectory of COVID-19 in Wuhan, China: Experience and suggestions. Public Health 2020, 183, 76–80. [Google Scholar] [CrossRef] [PubMed]
- Hao, X.; Cheng, S.; Wu, D.; Wu, T.; Lin, X.; Wang, C. Reconstruction of the full transmission dynamics of COVID-19 in Wuhan. Nature 2020, 584, 420–424. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.T.; Leung, K.; Bushman, M.; Kishore, N.; Niehus, R.; Salazar, P.; Cowling, B.; Lipsitch, M.; Leung, G. Estimating clinical severity of COVID-19 from the transmission dynamics in Wuhan, China. Nat. Med. 2020, 26, 506–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mizumoto, K.; Kagaya, K.; Chowell, G. Effect of the Wet Market on the coronavirus disease (COVID-19) transmission dynamics in China, 2019–2020. Int. J. Infect. Dis. 2020, 97, 96–101. [Google Scholar] [CrossRef]
- Zhang, J.; Litvinova, M.; Liang, Y.; Wang, Y.; Wang, W.; Zhao, S.; Wu, Q.; Merler, S.; Viboud, C.; Vespignani, A.; et al. Changes in contact patterns shape the dynamics of the COVID-19 outbreak in China. Science 2020, 368, 1481–1486. [Google Scholar] [CrossRef] [PubMed]
- Pang, L.; Liu, S.; Zhang, X.; Tian, T.; Zhao, Z. Transmission dynamics and control strategies of covid-19 in Wuhan, China. J. Biol. Syst. 2020, 28, 543–560. [Google Scholar] [CrossRef]
- Yang, P.; Qi, J.; Zhang, S.; Wang, X.; Bi, G.; Yang, Y.; Sheng, B.; Mao, X. Feasibility of Controlling COVID-19 Outbreaks in the UK by Rolling Interventions. medRxiv 2020. preprint. [Google Scholar] [CrossRef]
- Davies, N.G.; Kucharski, A.; Eggo, R.; Gimma, A.; Edmunds, W. Effects of non-pharmaceutical interventions on COVID-19 cases, deaths, and demand for hospital services in the UK: A modelling study. Lancet Public Health 2020, 5, e375–e385. [Google Scholar] [CrossRef]
- Booton, R.D.; MacGregor, L.; Vas, L.; Looker, K.; Hyams, C.; Bright, P.; Hading, I.; Lazarus, R.; Hamiton, F.; Lawson, D.; et al. Estimating the COVID-19 epidemic trajectory and hospital capacity requirements in South West England: A mathematical modelling framework. BMJ Open 2021, 11, e041536. [Google Scholar] [CrossRef] [PubMed]
- Rawson, T.; Brewer, T.; Veltcheva, D.; Huntingford, C.; Bonsall, M. How and when to end the COVID-19 lockdown: An optimization approach. Front. Public Health 2020, 8, 262. [Google Scholar] [CrossRef]
- Thompson, R.N. Epidemiological models are important tools for guiding COVID-19 interventions. BMC Med. 2020, 18, 152. [Google Scholar] [CrossRef] [PubMed]
- Peiliang, S.; Li, K. An SEIR Model for Assessment of Current COVID-19 Pandemic Situation in the UK. medRxiv 2020. preprint. [Google Scholar] [CrossRef]
- Chang, S.L.; Harding, N.; Zachreson, C.; Cliff, O.; Prokopenko, M. Modelling transmission and control of the COVID-19 pandemic in Australia. Nat. Comm. 2020, 11, 5710. [Google Scholar] [CrossRef]
- Fox, G.J.; Trauer, J.M.; McBryde, E. Modelling the impact of COVID-19 on intensive care services in New South Wales. Med J. Aust. 2020, 212, 468–469. [Google Scholar] [CrossRef]
- Moss, R.; Wood, J.; Brown, D.; Shearer, F.; Black, A.; Cheng, A.; McCaw, J.; McVernon, J. Modelling the impact of COVID-19 in Australia to inform transmission reducing measures and health system preparedness. medRxiv 2020. preprint. [Google Scholar] [CrossRef] [Green Version]
- Milne, G.J.; Xie, S. The effectiveness of social distancing in mitigating COVID-19 spread: A modelling analysis. medRxiv 2020. preprint. [Google Scholar] [CrossRef] [Green Version]
- Price, D.J.; Shearer, F.; Meehan, M.; McBryde, E.; Moss, R.; Golding, N.; Conway, E.; Dawson, P.; Cromer, D.; Wood, J.; et al. Early analysis of the Australian COVID-19 epidemic. eLife 2020, 9, e58785. [Google Scholar] [CrossRef]
- Bubar, K.M.; Reinholt, K.; Kissler, S.M.; Lipsitch, M.; Cobey, S.; Grad, Y.H.; Larremore, D.B. Model-informed COVID-19 vaccine prioritization strategies by age and serostatus. Science 2021, 371, 916–921. [Google Scholar]
- Giordano, G.; Colaneri, M.; Fillippo, A.; Blanchini, F.; Bolzern, P.; Nicolao, G.; Sacchi, P.; Colaneri, P.; Bruno, R. Modeling vaccination rollouts, SARS-CoV-2 variants and the requirement for non-pharmaceutical interventions in Italy. Nat. Med. 2021, 27, 993–998. [Google Scholar] [CrossRef] [PubMed]
- McBryde, E.; Meehan, M.; Caldwell, J.; Adekunle, A.; Ogunlade, S.; Kuddus, M.; Ragonnet, R.; Jayasundara, P.; Trauer, J.; Cope, R. Modelling direct and herd protection effects of vaccination against the SARS-CoV-2 Delta variant in Australia. Med. J. Aust. 2021, 34477236. [Google Scholar] [CrossRef]
- MacIntyre, C.R.; Costantino, V.; Trent, M. Modelling of COVID-19 vaccination strategies and herd immunity, in scenarios of limited and full vaccine supply in NSW, Australia. Vaccine in press. 2021. [Google Scholar] [CrossRef]
- Han, S.; Cai, J.; Yang, J.; Zhang, J.; Wu, Q.; Zheng, W.; Shi, H.; Ajelli, M.; Zhou, X.; Yu, H. Time-varying optimization of COVID-19 vaccine prioritization in the context of limited vaccination capacity. Nat. Commun. 2021, 12, 4673. [Google Scholar] [CrossRef]
- Moore, S.; Hill, E.; Dyson, L.; Tildesley, M.; Keeling, M. Modelling optimal vaccination strategy for SARS-CoV-2 in the UK. PLoS Comput. Biol. 2021, 17, e1008849. [Google Scholar] [CrossRef] [PubMed]
- Moore, S.; Hill, E.; Tildesley, M.; Dyson, L.; Keeling, M. Vaccination and non-pharmaceutical interventions for COVID-19: A mathematical modelling study. Lancet Infect. Dis. 2021, 21, 793–802. [Google Scholar] [CrossRef]
- Kanyiri, C.W.; Mark, K.; Luboobi, L. Mathematical Analysis of Influenza A Dynamics in the Emergence of Drug Resistance. Comput. Math. Methods Med. 2018, 2018, 2434560. [Google Scholar] [CrossRef]
- Wu, N.; Green, B.; Ben, X.; O’Banion, S. Deep Transformer Models for Time Series Forecasting: The Influenza Prevalence Case. arXiv 2020, arXiv:2001.08317. [Google Scholar]
- Lewnard, J.A.; Ndeffo, M.; Alfaro-Murillo, J.; Altice, F.; Bawo, L.; Nyenswah, T.; Galvani, A. Dynamics and control of Ebola virus transmission in Montserrado, Liberia: A mathematical modelling analysis. Lancet Infect. Dis. 2014, 14, 1189–1195. [Google Scholar] [CrossRef] [Green Version]
- Kucharski, A.J.; Funk, S.; Eggo, R.; Mallet, H.; Edmunds, W.; Nilles, E. Transmission dynamics of Zika virus in island populations: A modelling analysis of the 2013–2014 French Polynesia outbreak. PLoS Negl. Trop. Dis. 2016, 10, e0004726. [Google Scholar] [CrossRef] [Green Version]
- Farah, M.; Birrell, P.; Conti, S.; Angelis, D. Bayesian emulation and calibration of a dynamic epidemic model for A/H1N1 influenza. J. Am. Stat. Assoc. 2014, 109, 1398–1411. [Google Scholar] [CrossRef]
- Łuksza, M.; Lässig, M. A predictive fitness model for influenza. Nature 2014, 507, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Agusto, F.; Khan, M. Optimal control strategies for dengue transmission in Pakistan. Math. Biosci. 2018, 305, 102–121. [Google Scholar] [CrossRef]
- Kuddus, M.A.; Meehan, M.; White, L.; McBryde, E.; Adekunle, A. Modeling drug-resistant tuberculosis amplification rates and intervention strategies in Bangladesh. PLoS ONE 2020, 15, e0236112. [Google Scholar] [CrossRef] [PubMed]
- Rahman, A.; Kuddus, M.A. Cost-effective modeling of the transmission dynamics of malaria: A case study in Bangladesh. Commun. Stat. Case Stud. Data Anal. Appl. 2020, 6, 270–286. [Google Scholar] [CrossRef]
- Bhunu, C.; Mushayabasa, S.; Tchuenche, J. A theoretical assessment of the effects of smoking on the transmission dynamics of tuberculosis. Bull. Math. Biol. 2011, 73, 1333–1357. [Google Scholar] [CrossRef]
- Livingston, E.; Bucher, K. Coronavirus disease 2019 (COVID-19) in Italy. JAMA 2020, 323, 1335. [Google Scholar] [CrossRef] [Green Version]
- Hunter, P. The spread of the COVID-19 coronavirus: Health agencies worldwide prepare for the seemingly inevitability of the COVID-19 coronavirus becoming endemic. EMBO Rep. 2020, 21, e50334. [Google Scholar] [CrossRef]
- Ward, M.P.; Xiao, S.; Zhang, Z. The Role of Climate During the COVID-19 epidemic in New South Wales, Australia. Transbound. Emerg. Dis. 2020, 67, 2313–2317. [Google Scholar] [CrossRef] [PubMed]
- Qi, H.; Xiao, S.; Shi, R.; Ward, M.; Chen, Y.; Tu, W.; Su, Q.; Wang, W.; Wang, X.; Zhang, Z. COVID-19 transmission in Mainland China is associated with temperature and humidity: A time-series analysis. Sci. Total. Environ. 2020, 728, 138778. [Google Scholar] [CrossRef]
- Jamil, T.; Alam, I.; Gojobori, T.; Duarte, C. No evidence for temperature-dependence of the COVID-19 epidemic. Front. Public Health 2020, 8, 436. [Google Scholar] [CrossRef] [PubMed]
- Shi, P.; Dong, Y.; Yan, H.; Zhao, C.; Li, X.; Liu, W.; He, M.; Tang, S.; Xi, S. Impact of temperature on the dynamics of the COVID-19 outbreak in China. Sci. Total. Environ. 2020, 728, 138890. [Google Scholar] [CrossRef] [PubMed]
- Hou, J.; Hong, J.; Ji, B.; Dong, B.; Chen, Y.; Ward, M.; Tu, W.; Jin, Z.; Hu, J.; Su, Q.; et al. Changed transmission epidemiology of COVID-19 at early stage: A nationwide population-based piecewise mathematical modelling study. Travel Med. Infect. Dis. 2021, 39, 101918. [Google Scholar] [CrossRef]
- Bhatia, S.; Imao, N.; Cuomo-Dannenburg, G.; Baguelin, M.; Boonyasiri, A.; Cori, A.; Cucunuba, Z.; Dorigatti, I.; Fitzjohn, R.; Fu, H.; et al. Report 6: Relative Sensitivity of International Surveillance; Imperial College London: London, UK, 2020. [Google Scholar]
- Adegboye, O.; Adekunle, A.; Pak, A.; Gayawan, E.; Leung, D.; Rojas, D.; Elfaki, F.; McBryde, E.; Eisen, D. Change in outbreak epicenter and its impact on the importation risks of COVID-19 progression: A modelling study. Travel Med. Infect. Dis. 2021, 40, 101988. [Google Scholar] [CrossRef]
- Mossong, J.; Hens, N.; Jit, M.; Beutels, P.; Auranen, K.; Mikolajczzyk, R.; Massari, M.; Salmaso, S.; Tomba, G.; Wallinga, J.; et al. Social contacts and mixing patterns relevant to the spread of infectious diseases. PLoS Med. 2008, 5, e74. [Google Scholar] [CrossRef] [PubMed]
- McBryde, E.S.; Trauer, J.; Adekunle, A.; Ragonnet, R.; Meehan, M. Stepping out of lockdown should start with school re-openings while maintaining distancing measures. Insights from mixing matrices and mathematical models. medRxiv 2020. preprint. [Google Scholar] [CrossRef]
- Ferguson, N.; Laydon, D.; Nedjati-Gilani, G.; Imai, N.; Ainslie, K.; Baguelin, M.; Bhatia, S.; Boonyasiri, A.; Cucunuba, Z.; Cuomo-Dannenburg, G.; et al. Report 9: Impact of Non-Pharmaceutical Interventions (NPIs) to Reduce COVID19 Mortality and Healthcare Demand; Imperial College London: London, UK, 2020. [Google Scholar] [CrossRef]
- Taylor, C. How New Zealand’s ’eliminate’ Strategy Brought New Coronavirus Cases down to Zero. CNBC. 2020. Available online: www.cnbc.com (accessed on 28 October 2021).
- Brooks, J.V.; Frank, A.; Keen, M.; Bellisle, J.; Orme, I. Boosting vaccine for tuberculosis. Infect. Immun. 2001, 69, 2714–2717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drape, R.J.; Macklin, M.; Barr, L.; Jones, S.; Haynes, J.; Dean, H. Epidermal DNA vaccine for influenza is immunogenic in humans. Vaccine 2006, 24, 4475–4481. [Google Scholar] [CrossRef]
- Ragonnet, R.; Trauer, J.; Denholm, J.; Geard, N.; Hellard, M.; McBryde, E. Vaccination programs for endemic infections: Modelling real versus apparent impacts of vaccine and infection characteristics. Sci. Rep. 2015, 5, 15468. [Google Scholar] [CrossRef]
- Wu, Y.; Marsh, J.; McBryde, E.; Snelling, T. The influence of incomplete case ascertainment on measures of vaccine efficacy. Vaccine 2018, 36, 2946–2952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adam, D. Special report: The simulations driving the world’s response to COVID-19. Nature 2020, 580, 316. [Google Scholar] [CrossRef] [Green Version]
- Sridhar, D.; Majumder, M.S. Modelling the pandemic. BMJ 2020, 369, m1567. [Google Scholar] [CrossRef] [Green Version]
- Abdulla, F.; Nain, Z.; Karimuzzaman, M.; Hossain, M.M.; Rahman, A. A non-linear biostatistical graphical modeling of preventive actions and healthcare factors in controlling COVID-19 pandemic. Int. J. Environ. Res. Public Health 2021, 18, 4491. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).