Genetic Characterization and Pathogenesis of Three Novel Reassortant H5N2 Viruses in South Korea, 2018
Abstract
:1. Introduction
2. Materials and Methods
2.1. Collection of Feces Sample
2.2. Virus Isolation from the Feces Sample
2.3. RNA Extraction for Sequencing
2.4. Next Generation Sequencing (NGS) by Illumina Hiseq X Method
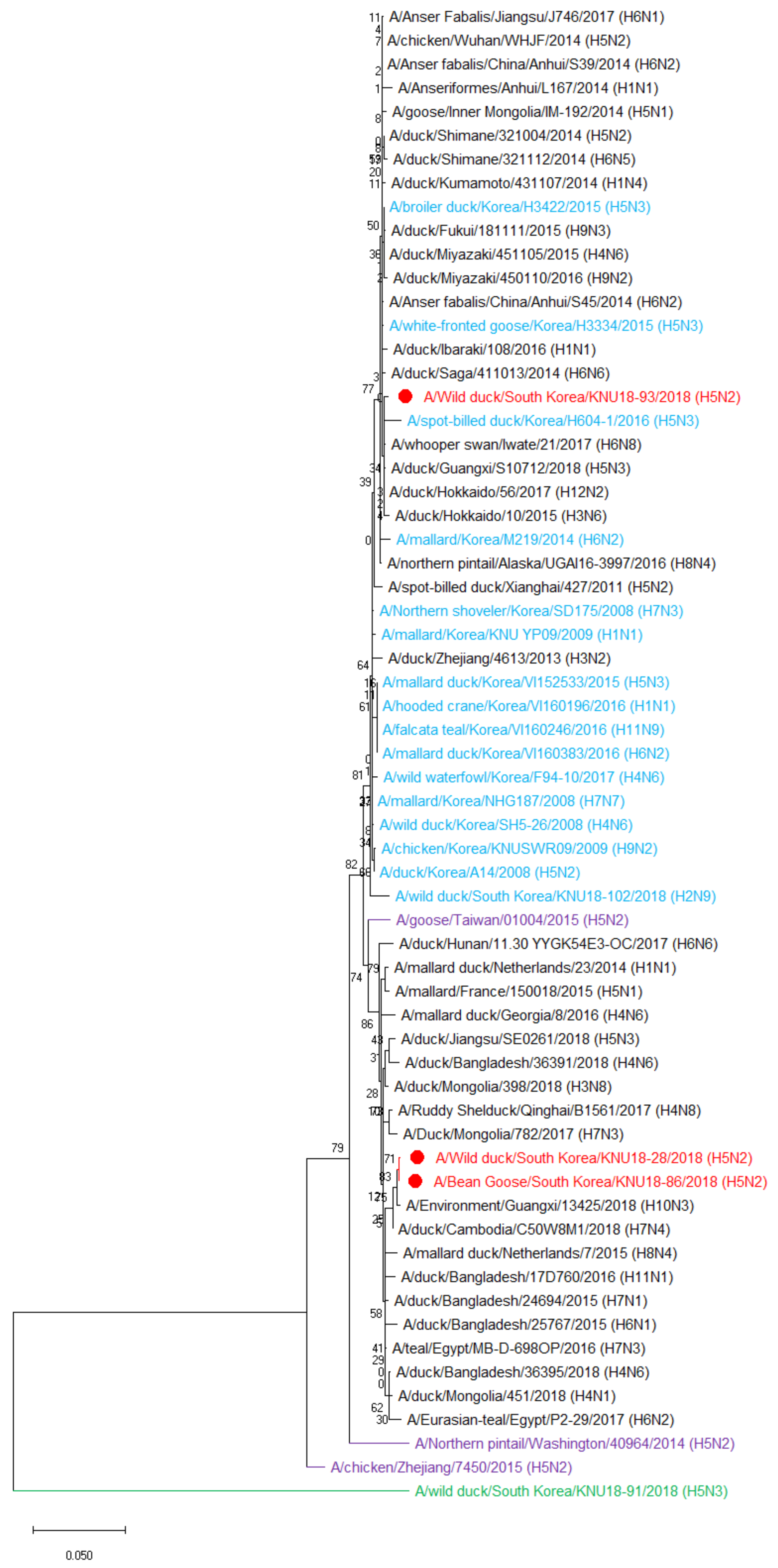
2.5. Phylogenetic Tree Analysis
2.6. Viral Replication Kinetics in MDCK Cells
2.7. Determination of Infectious Doses–EID50 and TCID50
2.8. Adaptation in Mice
2.9. Statistical Analysis
3. Results
3.1. Genetic Characterization of Three Novel Avian Influenza A (H5N2) Viruses
3.2. Hypothesis of the Reassortment Event of the Three Novel H5N2 Isolated Strains (KNU18-28, KNU18-86, and KNU18-93)
3.3. Molecular Characterization
3.4. Viral Replication Kinetics in MDCK Cells
3.5. Adaptation in Mice
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Fouchier, R.A.M.; Munster, V.; Wallensten, A.; Bestebroer, T.M.; Herfst, S.; Smith, D.; Rimmelzwaan, G.F.; Olsen, B.; Osterhaus, A.D.M.E. Characterization of a Novel Influenza A Virus Hemagglutinin Subtype (H16) Obtained from Black-Headed Gulls. J. Virol. 2005, 79, 2814–2822. [Google Scholar] [CrossRef] [Green Version]
- Tong, S.; Li, Y.; Rivailler, P.; Conrardy, C.; Castillo, D.A.A.; Chen, L.-M.; Recuenco-Cabrera, S.; Ellison, J.; Davis, C.T.; York, I.; et al. A distinct lineage of influenza A virus from bats. Proc. Natl. Acad. Sci. USA 2012, 109, 4269–4274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verhagen, J.H.; Munster, V.; Fouchier, R.A. Ecology and Evolution of Avian Influenza Viruses. In Genetics and Evolution of Infectious Disease; Tibayrenc, M., Ed.; Elsevier: London, UK, 2011; pp. 729–749. [Google Scholar]
- Horimoto, T.; Kawaoka, Y. Pandemic Threat Posed by Avian Influenza A Viruses. Clin. Microbiol. Rev. 2001, 14, 129–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryan, J.R. Chapter 9—Recent Animal Disease Outbreaks and Lessons Learned. In Biosecurity and Bioterrorism, 2nd ed.; Ryan, J.R., Ed.; Butterworth-Heinemann: Oxford, UK, 2016; pp. 217–239. [Google Scholar]
- Reuters. Russia Reports Outbreak of Highly Pathogenic H5N2 Bird Flu; OIE: Paris, France, 2017. [Google Scholar]
- Okamatsu, M.; Saito, T.; Yamamoto, Y.; Mase, M.; Tsuduku, S.; Nakamura, K.; Tsukamoto, K.; Yamaguchi, S. Low pathogenicity H5N2 avian influenza outbreak in Japan during the 2005–2006. Veter. Microbiol. 2007, 124, 35–46. [Google Scholar] [CrossRef]
- Kim, H.-R.; Park, C.-K.; Oem, J.-K.; Bae, Y.-C.; Choi, J.-G.; Lee, O.-S.; Lee, Y.-J. Characterization of H5N2 influenza viruses isolated in South Korea and their influence on the emergence of a novel H9N2 influenza virus. J. Gen. Virol. 2010, 91, 1978–1983. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.H.; Pascua, P.N.Q.; Song, M.-S.; Baek, Y.H.; Kim, C.-J.; Choi, H.-W.; Sung, M.-H.; Webby, R.J.; Webster, R.G.; Poo, H.; et al. Isolation and Genetic Characterization of H5N2 Influenza Viruses from Pigs in Korea. J. Virol. 2009, 83, 4205–4215. [Google Scholar] [CrossRef] [Green Version]
- Yeo, S.J.; Hoang, V.T.; Duong, T.B.; Nguyen, N.M.; Tuong, H.T.; Azam, M.; Sung, H.W.; Park, H. Emergence of a Novel Reassortant H5N3 Avian Influenza Virus in Korean Mallard Ducks in 2018. Intervirology 2021, 1–16. [Google Scholar] [CrossRef]
- World Health Organization. CDC Protocol of Realtime RTPCR for Influenza A(H1N1); World Health Organization (WHO): Geneva, Switzerland, 2009. [Google Scholar]
- Hebert, P.D.N.; Stoeckle, M.Y.; Zemlak, T.S.; Francis, C.M. Identification of Birds through DNA Barcodes. PLoS Biol. 2004, 2, e312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ambardar, S.; Gupta, R.; Trakroo, D.; Lal, R.; Vakhlu, J. High Throughput Sequencing: An Overview of Sequencing Chemistry. Indian J. Microbiol. 2016, 56, 394–404. [Google Scholar] [CrossRef] [Green Version]
- World Health Organization. Manual for the Laboratory Diagnosis and Virological Surveillance of Influenza; World Health Organization (WHO): Geneva, Switzerland, 2011. [Google Scholar]
- Reed, L.J.; Muench, H. A simple method of estimating fifty per cent endpoints. Am. J. Epidemiol. 1938, 27, 493–497. [Google Scholar] [CrossRef]
- Su, Y.; Yang, H.-Y.; Zhang, B.-J.; Jia, H.-L.; Tien, P. Analysis of a point mutation in H5N1 avian influenza virus hemagglutinin in relation to virus entry into live mammalian cells. Arch. Virol. 2008, 153, 2253–2261. [Google Scholar] [CrossRef] [PubMed]
- Henningson, J.N.; Rajao, D.S.; Kitikoon, P.; Lorusso, A.; Culhane, M.; Lewis, N.S.; Anderson, T.; Vincent, A.L. Comparative virulence of wild-type H1N1pdm09 influenza A isolates in swine. Veter. Microbiol. 2015, 176, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Carbone, V.; Schneider, E.K.; Rockman, S.; Baker, M.; Huang, J.X.; Ong, C.; Cooper, M.A.; Yuriev, E.; Li, J.; Velkov, T. Molecular Characterisation of the Haemagglutinin Glycan-Binding Specificity of Egg-Adapted Vaccine Strains of the Pandemic 2009 H1N1 Swine Influenza A Virus. Molecules 2015, 20, 10415–10434. [Google Scholar] [CrossRef] [PubMed]
- Auewarakul, P.; Suptawiwat, O.; Kongchanagul, A.; Sangma, C.; Suzuki, Y.; Ungchusak, K.; Louisirirotchanakul, S.; Lerdsamran, H.; Pooruk, P.; Thitithanyanont, A.; et al. An Avian Influenza H5N1 Virus That Binds to a Human-Type Receptor. J. Virol. 2007, 81, 9950–9955. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elderfield, R.A.; Watson, S.J.; Godlee, A.; Adamson, W.; Thompson, C.I.; Dunning, J.; Fernández-Alonso, M.; Blumenkrantz, D.; Hussell, T.; Zambon, M.; et al. Accumulation of Human-Adapting Mutations during Circulation of A(H1N1)pdm09 Influenza Virus in Humans in the United Kingdom. J. Virol. 2014, 88, 13269–13283. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Lu, R.; Peng, X.; Peng, X.; Cheng, L.; Liu, F.; Wu, N. Characterization of Novel Reassortant Influenza A (H5N2) Viruses Isolated from Poultry in Eastern China, 2015. Front. Microbiol. 2017, 8, 741. [Google Scholar] [CrossRef]
- Matrosovich, M.; Zhou, N.; Kawaoka, Y.; Webster, R. The Surface Glycoproteins of H5 Influenza Viruses Isolated from Humans, Chickens, and Wild Aquatic Birds Have Distinguishable Properties. J. Virol. 1999, 73, 1146–1155. [Google Scholar] [CrossRef] [Green Version]
- Xu, L.; Bao, L.; Li, F.; Lv, Q.; Ma, Y.; Zhou, J.; Xu, Y.; Deng, W.; Zhan, L.; Zhu, H.; et al. Adaption of Seasonal H1N1 Influenza Virus in Mice. PLoS ONE 2011, 6, e28901. [Google Scholar] [CrossRef]
- Zhao, Y.; Yu, Z.; Liu, L.; Wang, T.; Sun, W.; Wang, C.; Xia, Z.; Gao, Y.; Zhou, B.; Qian, J.; et al. Adaptive amino acid substitutions enhance the virulence of a novel human H7N9 influenza virus in mice. Veter. Microbiol. 2016, 187, 8–14. [Google Scholar] [CrossRef]
- Chen, H.; Bright, R.A.; Subbarao, K.; Smith, C.; Cox, N.J.; Katz, J.M.; Matsuoka, Y. Polygenic virulence factors involved in pathogenesis of 1997 Hong Kong H5N1 influenza viruses in mice. Virus Res. 2007, 128, 159–163. [Google Scholar] [CrossRef]
- Li, J.; Ishaq, M.; Prudence, M.; Xi, X.; Hu, T.; Liu, Q.; Guo, D. Single mutation at the amino acid position 627 of PB2 that leads to increased virulence of an H5N1 avian influenza virus during adaptation in mice can be compensated by multiple mutations at other sites of PB2. Virus Res. 2009, 144, 123–129. [Google Scholar] [CrossRef]
- Prokopyeva, E.; Sobolev, I.; Prokopyev, M.; Shestopalov, A. Adaptation of influenza A(H1N1)pdm09 virus in experimental mouse models. Infect. Genet. Evol. 2016, 39, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Salomon, R.; Franks, J.; Govorkova, E.A.; Ilyushina, N.A.; Yen, H.L.; Hulse-Post, D.J.; Humberd, J.; Trichet, M.; Rehg, J.E.; Webby, R.J.; et al. The polymerase complex genes contribute to the high virulence of the human H5N1 influenza virus isolate A/Vietnam/1203/04. J. Exp. Med. 2006, 203, 689–697. [Google Scholar] [CrossRef] [Green Version]
- Govorkova, E.; Rehg, J.E.; Krauss, S.; Yen, H.-L.; Guan, Y.; Peiris, M.; Nguyen, T.D.; Hanh, T.H.; Puthavathana, P.; Long, H.T.; et al. Lethality to Ferrets of H5N1 Influenza Viruses Isolated from Humans and Poultry in 2004. J. Virol. 2005, 79, 2191–2198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gabriel, G.; Abram, M.; Keiner, B.; Wagner, R.; Klenk, H.-D.; Stech, J. Differential Polymerase Activity in Avian and Mammalian Cells Determines Host Range of Influenza Virus. J. Virol. 2007, 81, 9601–9604. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hulse-Post, D.J.; Franks, J.; Boyd, K.; Salomon, R.; Hoffmann, E.; Yen, H.-L.; Webby, R.J.; Walker, D.; Nguyen, T.D.; Webster, R.G. Molecular Changes in the Polymerase Genes (PA and PB1) Associated with High Pathogenicity of H5N1 Influenza Virus in Mallard Ducks. J. Virol. 2007, 81, 8515–8524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wei, K.; Sun, H.; Sun, Z.; Sun, Y.; Kong, W.; Pu, J.; Ma, G.; Yin, Y.; Yang, H.; Guo, X.; et al. Influenza A Virus Acquires Enhanced Pathogenicity and Transmissibility after Serial Passages in Swine. J. Virol. 2014, 88, 11981–11994. [Google Scholar] [CrossRef] [Green Version]
- Xu, C.; Hu, W.-B.; Xu, K.; He, Y.-X.; Wang, T.-Y.; Chen, Z.; Li, T.-X.; Liu, J.-H.; Buchy, P.; Sun, B. Amino acids 473V and 598P of PB1 from an avian-origin influenza A virus contribute to polymerase activity, especially in mammalian cells. J. Gen. Virol. 2012, 93, 531–540. [Google Scholar] [CrossRef] [PubMed]
- Yamayoshi, S.; Yamada, S.; Fukuyama, S.; Murakami, S.; Zhao, D.; Uraki, R.; Watanabe, T.; Tomita, Y.; Macken, C.; Neumann, G.; et al. Virulence-Affecting Amino Acid Changes in the PA Protein of H7N9 Influenza A Viruses. J. Virol. 2014, 88, 3127–3134. [Google Scholar] [CrossRef] [Green Version]
- Leung, B.W.; Chen, H.; Brownlee, G.G. Correlation between polymerase activity and pathogenicity in two duck H5N1 influenza viruses suggests that the polymerase contributes to pathogenicity. Virology 2010, 401, 96–106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeo, S.-J.; Than, D.-D.; Park, H.-S.; Sung, H.W.; Park, H. Molecular Characterization of a Novel Avian Influenza A (H2N9) Strain Isolated from Wild Duck in Korea in 2018. Viruses 2019, 11, 1046. [Google Scholar] [CrossRef] [Green Version]
- Mei, K.; Liu, G.; Chen, Z.; Gao, Z.; Zhao, L.; Jin, T.; Yu, X.; Chen, Q. Deep sequencing reveals the viral adaptation process of environment-derived H10N8 in mice. Infect. Genet. Evol. 2015, 37, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Lycett, S.J.; Ward, M.J.; Lewis, F.I.; Poon, A.; Pond, S.L.K.; Brown, A.J.L. Detection of Mammalian Virulence Determinants in Highly Pathogenic Avian Influenza H5N1 Viruses: Multivariate Analysis of Published Data. J. Virol. 2009, 83, 9901–9910. [Google Scholar] [CrossRef] [Green Version]
- Wu, W.L.; Chen, Y.; Wang, P.; Song, W.; Lau, S.-Y.; Rayner, J.M.; Smith, G.J.; Webster, R.G.; Peiris, J.S.M.; Lin, T.; et al. Antigenic Profile of Avian H5N1 Viruses in Asia from 2002 to 2007. J. Virol. 2008, 82, 1798–1807. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Peng, X.; Peng, X.; Cheng, L.; Jin, C.; Lu, X.; Xie, T.; Yao, H.; Wu, N. Multiple amino acid substitutions involved in the adaptation of avian-origin influenza A (H10N7) virus in mice. Arch. Virol. 2015, 161, 977–980. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Cheng, K.; Sun, W.; Zhang, X.; Xia, X.; Gao, Y. PB2 and HA mutations increase the virulence of highly pathogenic H5N5 clade 2.3.4.4 avian influenza virus in mice. Arch. Virol. 2018, 163, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Zou, X.; Zhou, J.; Tang, J.; Shu, Y. Residues 41V and/or 210D in the NP protein enhance polymerase activities and potential replication of novel influenza (H7N9) viruses at low temperature. Virol. J. 2015, 12, 71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Q.; Chen, H.; Huang, J.; Chen, Y.; Gu, M.; Wang, X.; Hu, S.; Liu, X.; Liu, X. A nonpathogenic duck-origin H9N2 influenza A virus adapts to high pathogenicity in mice. Arch. Virol. 2014, 159, 2243–2252. [Google Scholar] [CrossRef]
- Li, J.; Zheng, W.; Hou, L.; Chen, C.; Fan, W.; Qu, H.; Jiang, J.; Liu, J.; Gao, G.F.; Zhou, J.; et al. Differential nucleocytoplasmic shuttling of the nucleoprotein of influenza a viruses and association with host tropism. Cell. Microbiol. 2016, 19, e12692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peng, X.; Wu, H.; Peng, X.; Wu, X.; Cheng, L.; Liu, F.; Ji, S.; Wu, N. Amino acid substitutions occurring during adaptation of an emergent H5N6 avian influenza virus to mammals. Arch. Virol. 2016, 161, 1665–1670. [Google Scholar] [CrossRef] [PubMed]
- Katz, J.M.; Lu, X.; Tumpey, T.M.; Smith, C.B.; Shaw, M.W.; Subbarao, K. Molecular Correlates of Influenza A H5N1 Virus Pathogenesis in Mice. J. Virol. 2000, 74, 10807–10810. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.; Deng, M.; Lin, Y.; Chang, C.; Shieh, H.K.; Shiau, J.; Huang, C. Characterization of an H5N1 avian influenza virus from Taiwan. Veter. Microbiol. 2007, 124, 193–201. [Google Scholar] [CrossRef]
- Fan, S.; Deng, G.; Song, J.; Tian, G.; Suo, Y.; Jiang, Y.; Guan, Y.; Bu, Z.; Kawaoka, Y.; Chen, H. Two amino acid residues in the matrix protein M1 contribute to the virulence difference of H5N1 avian influenza viruses in mice. Virology 2009, 384, 28–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Z.; Jiang, Y.; Jiao, P.; Wang, A.; Zhao, F.; Tian, G.; Wang, X.; Yu, K.; Bu, Z.; Chen, H. The NS1 Gene Contributes to the Virulence of H5N1 Avian Influenza Viruses. J. Virol. 2006, 80, 11115–11123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hassan, K.E.; Saad, N.; Abozeid, H.H.; Shany, S.; El-Kady, M.F.; Arafa, A.; El-Sawah, A.A.; Pfaff, F.; Hafez, H.M.; Beer, M.; et al. Genotyping and reassortment analysis of highly pathogenic avian influenza viruses H5N8 and H5N2 from Egypt reveals successive annual replacement of genotypes. Infect. Genet. Evol. 2020, 84, 104375. [Google Scholar] [CrossRef]
- Hassan, K.; King, J.; El-Kady, M.; Afifi, M.; Abozeid, H.; Pohlmann, A.; Beer, M.; Harder, T. Novel Reassortant Highly Pathogenic Avian Influenza A(H5N2) Virus in Broiler Chickens, Egypt. Emerg. Infect. Dis. 2020, 26, 129–133. [Google Scholar] [CrossRef] [PubMed]
- Pasick, J.; Berhane, Y.; Joseph, T.; Bowes, V.; Hisanaga, T.; Handel, K.; Alexandersen, S. Reassortant Highly Pathogenic Influenza A H5N2 Virus Containing Gene Segments Related to Eurasian H5N8 in British Columbia, Canada, 2014. Sci. Rep. 2015, 5, srep09484. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hill, N.J.; Hussein, I.T.; Davis, K.R.; Ma, E.J.; Spivey, T.J.; Ramey, A.M.; Puryear, W.B.; Das, S.R.; Halpin, R.A.; Lin, X.; et al. Reassortment of Influenza A Viruses in Wild Birds in Alaska before H5 Clade 2.3.4.4 Outbreaks. Emerg. Infect. Dis. 2017, 23, 654–657. [Google Scholar] [CrossRef] [Green Version]
- Zhao, G.; Gu, X.; Lu, X.; Pan, J.; Duan, Z.; Zhao, K.; Gu, M.; Liu, Q.; He, L.; Chen, J.; et al. Novel Reassortant Highly Pathogenic H5N2 Avian Influenza Viruses in Poultry in China. PLoS ONE 2012, 7, e46183. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Peng, X.; Xu, L.; Jin, C.; Cheng, L.; Lu, X.; Xie, T.; Yao, H.; Wu, N. Characterization of a novel highly pathogenic H5N2 avian influenza virus isolated from a duck in eastern China. Arch. Virol. 2014, 159, 3377–3383. [Google Scholar] [CrossRef]
- Gu, M.; Huang, J.; Chen, Y.; Chen, J.; Wang, X.; Liu, X. Genome Sequence of a Natural Reassortant H5N2 Avian Influenza Virus from Domestic Mallard Ducks in Eastern China. J. Virol. 2012, 86, 12463–12464. [Google Scholar] [CrossRef] [Green Version]
- Wang, W.-C.; Kuan, C.-Y.; Tseng, Y.-J.; Chang, C.-H.; Liu, Y.-C.; Chang, Y.-C.; Hsu, Y.-C.; Hsieh, M.-K.; Ou, S.-C.; Hsu, W.-L. The Impacts of Reassortant Avian Influenza H5N2 Virus NS1 Proteins on Viral Compatibility and Regulation of Immune Responses. Front. Microbiol. 2020, 11, 280. [Google Scholar] [CrossRef]
- Hatta, M.; Gao, P.; Halfmann, P.; Kawaoka, Y. Molecular Basis for High Virulence of Hong Kong H5N1 Influenza A Viruses. Science 2001, 293, 1840–1842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Finkelstein, D.B.; Mukatira, S.; Mehta, P.K.; Obenauer, J.C.; Su, X.; Webster, R.G.; Naeve, C.W. Persistent Host Markers in Pandemic and H5N1 Influenza Viruses. J. Virol. 2007, 81, 10292–10299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foeglein, Á.; Loucaides, E.M.; Mura, M.; Wise, H.M.; Barclay, W.; Digard, P. Influence of PB2 host-range determinants on the intranuclear mobility of the influenza A virus polymerase. J. Gen. Virol. 2011, 92, 1650–1661. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chen, H.; Jiao, P.; Deng, G.; Tian, G.; Li, Y.; Hoffmann, E.; Webster, R.G.; Matsuoka, Y.; Yu, K. Molecular Basis of Replication of Duck H5N1 Influenza Viruses in a Mammalian Mouse Model. J. Virol. 2005, 79, 12058–12064. [Google Scholar] [CrossRef] [Green Version]
- Nam, J.-H.; Shim, S.-M.; Song, E.-J.; Españo, E.; Jeong, D.-G.; Song, D.; Kim, J.-K. Rapid virulence shift of an H5N2 avian influenza virus during a single passage in mice. Arch. Virol. 2017, 162, 3017–3024. [Google Scholar] [CrossRef] [PubMed]





| Gene Segment | Genbank ID | Reference Strain Accession ID | Highest Similarly Reference Strain | Identity (%) |
|---|---|---|---|---|
| PB2 | MT477766.1 | EPI965204 EPI965285 EPI1332121 | A/duck/Bangladesh/24697/2015 (A/H15N9) A/duck/Bangladesh/24704/2015 (A/H15N9) A/Environment/Jiangxi/22207/2014 (A/H4N2) | 99.12 (2260/2280) 99.08 (2259/2280) 98.86 (2254/2280) |
| PB1 | MT477767.1 | EPI1549213 EPI1549229 EPI1549221 | A/wild bird/Eastern China/1754/2017 (A/H5N3) A/wild bird/Eastern China/1759/2017 (A/H5N3) A/wild bird/Eastern China/1758/2017 (A/H5N3) | 99.34 (2259/2274) 99.30 (2258/2274) 99.30 (2258/2274) |
| PA | MT477768.1 | EPI1619612 EPI1848485 EPI1513958 | A/Anser Fabalis/Jiangsu/J746/2017 (A/H6N1) A/Environment/Guangxi/13425/2018 (A/H10N3) A/mallard/Korea/H15-1/2017 (A/H5N2) | 99.44 (2139/2151) 99.40 (2138/2151) 99.40 (2138/2151) |
| HA | MT477769.1 | EPI1513834 EPI1528984 EPI1514074 | A/mallard/Korea/H50-4/2016 (A/H5N3) A/duck/Jiangsu/SE0261/2018 (A/H5N3) A/spot-billed duck/Korea/H10-1/2017 (A/H5N3) | 99.35 (1684/1695) 99.29 (1683/1695) 99.29 (1683/1695) |
| NP | MT477770.1 | EPI1567180 EPI1062334 EPI1062329 | A/wild waterfowl/Korea/F14-5/2016 (A/H6N1) A/Bean goose/Hubei/CH-i177/2017_H7N7 (A/H7N7) A/Bean goose/Hubei/CH-I299/2017_H7N7 (A/H7N7) | 99.60 (1491/1497) 99.60 (1491/1497) 99.60 (1491/1497) |
| NA | MT477771.1 | EPI1513961 EPI1514049 EPI1514041 | A/mallard/Korea/H15-1/2017 (A/H5N2) A/bean goose/Korea/H112/2017 (A/H5N2) A/spot-billed duck/Korea/H51/2017 (A/H5N2) | 99.01 (1396/1410) 98.72 (1392/1410) 98.72 (1392/1410) |
| M | MT477772.1 | EPI1567198 EPI1567190 EPI1098997 | A/bean goose/Korea/F54-8/2017 (A/H6N1) A/bean goose/Korea/F27-6/2017 (A/H6N1) A/duck/Bangladesh/31227/2016 (A/H6N2) | 99.80 (980/982) 99.80 (980/982) 99.80 (980/982) |
| NS | MT477773.1 | EPI1848486 EPI1635085 EPI1635077 | A/Environment/Guangxi/13425/2018 (A/H10N3) A/duck/Cambodia/C50W8M1/2018 (A/H7N4) A/duck/Cambodia/12T-24-1-D17/2018 (A/H7N4) | 99.52 (834/838) 99.52 (834/838) 99.28 (832/838) |
| Gene Segment | Genbank ID | Reference Strain Accession ID | Highest Similarly Reference Strain | Identity (%) |
|---|---|---|---|---|
| PB2 | MT477790.1 | EPI965204 EPI965285 EPI1332121 | A/duck/Bangladesh/24697/2015 (A/H15N9) A/duck/Bangladesh/24704/2015 (A/H15N9) A/Environment/Jiangxi/22207/2014 (A/H4N2) | 99.08 (2259/2280) 99.04 (2258/2280) 98.82 (2259/2280) |
| PB1 | MT477791.1 | EPI1549213 EPI1549229 EPI1549221 | A/wild bird/Eastern China/1754/2017 (A/H5N3) A/wild bird/Eastern China/1759/2017 (A/H5N3) A/wild bird/Eastern China/1758/2017 (A/H5N3) | 99.38 (2260/2274) 99.34 (2259/2274) 99.34 (2259/2274) |
| PA | MT477792.1 | EPI1619612 EPI1513958 EPI1848485 | A/Anser Fabalis/Jiangsu/J746/2017 (A/H6N1) A/mallard/Korea/H15-1/2017 (A/H5N2) A/Environment/Guangxi/13425/2018 (A/H10N3) | 99.21 (2136/2153) 99.16 (2135/2153) 99.07 (2133/2153) |
| HA | MT477793.1 | EPI1514074 EPI1513954 EPI1513946 | A/spot-billed duck/Korea/H10-1/2017 (A/H5N3) A/mallard/Korea/A44-5/2017 (A/H5N3) A/mallard/Korea/A33-5/2017 (A/H5N2) | 99.23 (1682/1695) 99.23 (1682/1695) 99.17 (1681/1695) |
| NP | MT477794.1 | EPI1567180 EPI1062334 EPI1062329 | A/wild waterfowl/Korea/F14-5/2016 (A/H6N1) A/Bean goose/Hubei/CH-i177/2017_H7N7 (A/H7N7) A/Bean goose/Hubei/CH-I299/2017_H7N7 (A/H7N7) | 99.53 (1490/1497) 99.53 (1490/1497) 99.53 (1490/1497) |
| NA | MT477795.1 | EPI1513961 EPI1514041 EPI1514049 | A/mallard/Korea/H15-1/2017 (A/H5N2) A/spot-billed duck/Korea/H51/2017 (A/H5N2) A/bean goose/Korea/H112/2017 (A/H5N2) | 99.22 (1399/1410) 98.65 (1391/1410) 98.58 (1390/1410) |
| M | MT477796.1 | EPI1567198 EPI1567190 EPI1098997 | A/bean goose/Korea/F54-8/2017 (A/H6N1) A/bean goose/Korea/F27-6/2017 (A/H6N1) A/duck/Bangladesh/31227/2016 (A/H6N2) | 99.80 (980/982) 99.80 (980/982) 99.80 (980/982) |
| NS | MT477797.1 | EPI1848486 EPI1635085 EPI1635077 | A/Environment/Guangxi/13425/2018 (A/H10N3) A/duck/Cambodia/C50W8M1/2018 (A/H7N4) A/duck/Cambodia/12T-24-1-D17/2018 (A/H7N4) | 99.64 (835/838) 99.64 (835/838) 99.40 (833/838) |
| Gene Segment | Genbank ID | Reference Strain Accession ID | Highest Similarly Reference Strain | Identity (%) |
|---|---|---|---|---|
| PB2 | MT477798.1 | EPI965204 EPI965285 EPI1332121 | A/duck/Bangladesh/24697/2015 (A/H15N9) A/duck/Bangladesh/24704/2015 (A/H15N9) A/Environment/Jiangxi/22207/2014 (A/H4N2) | 99.04 (2258/2280) 98.99 (2257/2280) 98.77 (2252/2280) |
| PB1 | MT477799.1 | EPI1549213 EPI1549229 EPI1549221 | A/wild bird/Eastern China/1754/2017 (A/H5N3) A/wild bird/Eastern China/1759/2017 (A/H5N3) A/wild bird/Eastern China/1758/2017 (A/H5N3) | 99.30 (2258/2274) 99.25 (2257/2274) 99.25 (2257/2274) |
| PA | MT477800.1 | EPI1619612 EPI1513958 EPI867668 | A/Anser Fabalis/Jiangsu/J746/2017 (A/H6N1) A/mallard/Korea/H15-1/2017 (A/H5N2) A/duck/Gunma/3/2016 (A/H3N8) | 99.49 (2140/2151) 99.44 (2139/2151) 99.12 (2132/2151) |
| HA | MT477801.1 | EPI1514074 EPI1513954 EPI1513946 | A/spot-billed duck/Korea/H10-1/2017 (A/H5N3) A/mallard/Korea/A44-5/2017 (A/H5N3) A/mallard/Korea/A33-5/2017 (A/H5N2) | 99.23 (1682/1695) 99.23 (1682/1695) 99.17 (1681/1695) |
| NP | MT477802.1 | EPI1567180 EPI1062334 EPI1062329 | A/wild waterfowl/Korea/F14-5/2016 (A/H6N1) A/Bean goose/Hubei/CH-i177/2017_H7N7 (A/H7N7) A/Bean goose/Hubei/CH-I299/2017_H7N7 (A/H7N7) | 99.40 (1488/1497) 99.40 (1488/1497) 99.40 (1488/1497) |
| NA | MT477803.1 | EPI1513961 EPI1514041 EPI1514049 | A/mallard/Korea/H15-1/2017 (A/H5N2) A/spot-billed duck/Korea/H51/2017 (A/H5N2) A/bean goose/Korea/H112/2017 (A/H5N2) | 98.94 (1395/1410) 98.37 (1387/1410) 98.30 (1386/1410) |
| M | MT477804.1 | EPI1567198 EPI1567190 EPI1098997 | A/bean goose/Korea/F54-8/2017 (A/H6N1) A/bean goose/Korea/F27-6/2017 (A/H6N1) A/duck/Bangladesh/31227/2016 (A/H6N2) | 99.80 (980/982) 99.80 (980/982) 99.80 (980/982) |
| NS | MT477805.1 | EPI1521597 EPI1619617 EPI1567328 | A/duck/Hokkaido/56/2017 (A/H12N2) A/Anser Fabalis/Jiangsu/J746/2017 (A/H6N1) A/whooper swan/Iwate/21/2017 (A/H6N8) | 99.76 (836/838) 99.64 (836/838) 99.64 (836/838) |
| Virus Strain | HA Receptor-Binding Residues (H5 Numbering) | NA | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cleavage Sites | D/E94N | I116M | S121N | A134V | G139R | S142G | D221G/N | Q222L | Deleted Range from 50–70 | M26I | I106V | T223I | K/S373A/N | |
| KNU18-28 (H5N2) | PQRETR↓GLF | D | I | S | A | G | S | G | Q | No deletion | I | I | I | S |
| KNU18-86 (H5N2) | PQRETR↓GLF | D | I | S | A | G | S | G | Q | No deletion | I | I | I | S |
| KNU18-93 (H5N2) | PQRETR↓GLF | D | I | S | A | G | S | G | Q | No deletion | I | I | I | S |
| KNU18-91 (H5N3) | PQRETR↓GLF | D | I | S | A | G | S | G | Q | No deletion | I | I | I | S |
| KA14 (H5N2) | PQRETR↓GLF | D | I | S | A | G | S | G | Q | No deletion | I | I | I | S |
| Z7450 (H5N2) | RERRRKR↓GLF | T | I | S | A | G | S | G | Q | No deletion | I | I | I | S |
| Viral Protein | Amino Acid | KA14 | Z7450 | KNU18-91 | KNU18-28 | KNU18-86 | KNU18-93 | Phenotype | References |
|---|---|---|---|---|---|---|---|---|---|
| PB2 | L89V | V | V | V | V | V | V | Enhanced polymerase activity, increased virulence in mice | [26] |
| K251R | R | R | R | R | R | R | Increased virulence in mice | [27] | |
| T309D | D | D | D | D | D | D | Enhanced polymerase activity, increased virulence in mice | [26] | |
| T339K | K | K | K | K | K | K | Enhanced polymerase activity, increased virulence in mice | [26] | |
| Q368R | R | R | R | R | R | R | Increased polymerase activity, increased virulence in mammals | [28] | |
| H447Q | Q | Q | Q | Q | Q | Q | Increased polymerase activity, increased virulence in mammals | [28] | |
| R477G | G | G | G | G | G | G | Enhanced polymerase activity, increased virulence in mice | [26] | |
| I495V | V | V | V | V | V | V | Enhanced polymerase activity, increased virulence in mice | [26] | |
| A676T | T | T | T | T | T | T | Enhanced polymerase active, increased virulence in mice | [26] | |
| PB1 | D/A3V | V | V | V | V | V | V | Increased polymerase activity, increased virulence in mammals | [28,29] |
| L13P | P | P | P | P | P | P | Increased polymerase activity, increased virulence in mammals | [30] | |
| R207K | K | K | K | K | K | K | Increased polymerase activity in mammalian cells | [31] | |
| K328N | N | N | N | N | N | N | Increased polymerase activity, increased virulence in mammals | [28,29] | |
| H436Y | Y | Y | Y | Y | Y | Y | Increased polymerase activity and virulence in mallards, ferrets, and mice | [31] | |
| A469T | T | T | T | T | T | T | Conferred in contact transmissibility in guinea pigs | [32] | |
| L473V | V | V | V | V | V | V | Increased polymerase activity and replication efficiency | [33] | |
| V652A | A | A | A | A | A | A | Increased virulence in mice | [27] | |
| PA | S37A | A | A | A | A | A | A | Significantly increased viral growth and polymerase activity in mammalian cells | [34] |
| H266R | R | R | R | R | R | R | Increased polymerase activity, increased virulence in mammals and birds | [35,36] | |
| F277S | S | S | S | S | S | S | Contributed to the virulence and mammalian adaptation | [36] | |
| C278Q | Q | Q | Q | Q | Q | Q | Adapt to mammalian hosts | [37] | |
| S/A515T | T | T | T | T | T | T | Increased polymerase activity, increased virulence in mammals and birds | [31] | |
| HA | A/I/P/S/T86V | A | T | V | V | V | V | Increased virulence in mammals | [38,39] |
| Q/H/I138L/N | Q | N | N | N | N | N | Increased virulence in mammals | [38,39] | |
| K212E/R/G | K | E | E | E | E | E | Increased virulence in mammals | [38,39] | |
| G395E | E | E | E | E | E | E | Enhanced polymerase activity, increased virulence in mice | [40] | |
| F427L | L | L | L | L | L | L | Important for adaptation of H5N5 AIVs to mammals | [41] | |
| NP | V41I | I | I | I | I | I | I | Might contribute to viral transmissibility | [42] |
| V105M | M | M | M | M | M | M | Contribute to the increased virulence of the H9N2 | [43] | |
| D210E | E | E | E | E | E | E | Might contribute to viral transmissibility | [42] | |
| F253I | I | I | I | I | I | I | Results in attenuated pathogenicity of the virus in mice | [44] | |
| I353V | V | V | V | V | V | I | Increased virulence in mice | [27] | |
| NA | M26I | I | I | I | I | I | I | Increased virulence in mice | [24] |
| R143K | R | K | K | K | K | K | Increased virulence in mammals and mice | [45] | |
| T223I | I | I | I | I | I | I | Increased virulence in mammals | [25,46,47] | |
| M1 | N30D | D | D | D | D | D | D | Increased virulence in mammals | [48] |
| A166V | A | V | V | V | V | V | Contribute to the increased virulence of the H9N2. | [43] | |
| NS1 | A/P42S | S | S | A | S | S | S | Increased virulence in mammals, antagonism of IFN induction | [38] |
| T/D/V/R/A127N | N | N | R | N | N | N | Increased virulence in mammals | [38] | |
| V149A | A | A | A | A | A | A | Pathogenicity in mice, antagonism of IFN induction | [49] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nguyen, A.T.V.; Hoang, V.T.; Sung, H.W.; Yeo, S.-J.; Park, H. Genetic Characterization and Pathogenesis of Three Novel Reassortant H5N2 Viruses in South Korea, 2018. Viruses 2021, 13, 2192. https://doi.org/10.3390/v13112192
Nguyen ATV, Hoang VT, Sung HW, Yeo S-J, Park H. Genetic Characterization and Pathogenesis of Three Novel Reassortant H5N2 Viruses in South Korea, 2018. Viruses. 2021; 13(11):2192. https://doi.org/10.3390/v13112192
Chicago/Turabian StyleNguyen, Anh Thi Viet, Vui Thi Hoang, Haan Woo Sung, Seon-Ju Yeo, and Hyun Park. 2021. "Genetic Characterization and Pathogenesis of Three Novel Reassortant H5N2 Viruses in South Korea, 2018" Viruses 13, no. 11: 2192. https://doi.org/10.3390/v13112192






