Favipiravir Inhibits Mayaro Virus Infection in Mice
Abstract
:1. Introduction
2. Materials and Methods
2.1. Virus, Cells and Compounds
2.2. Ethics Statement
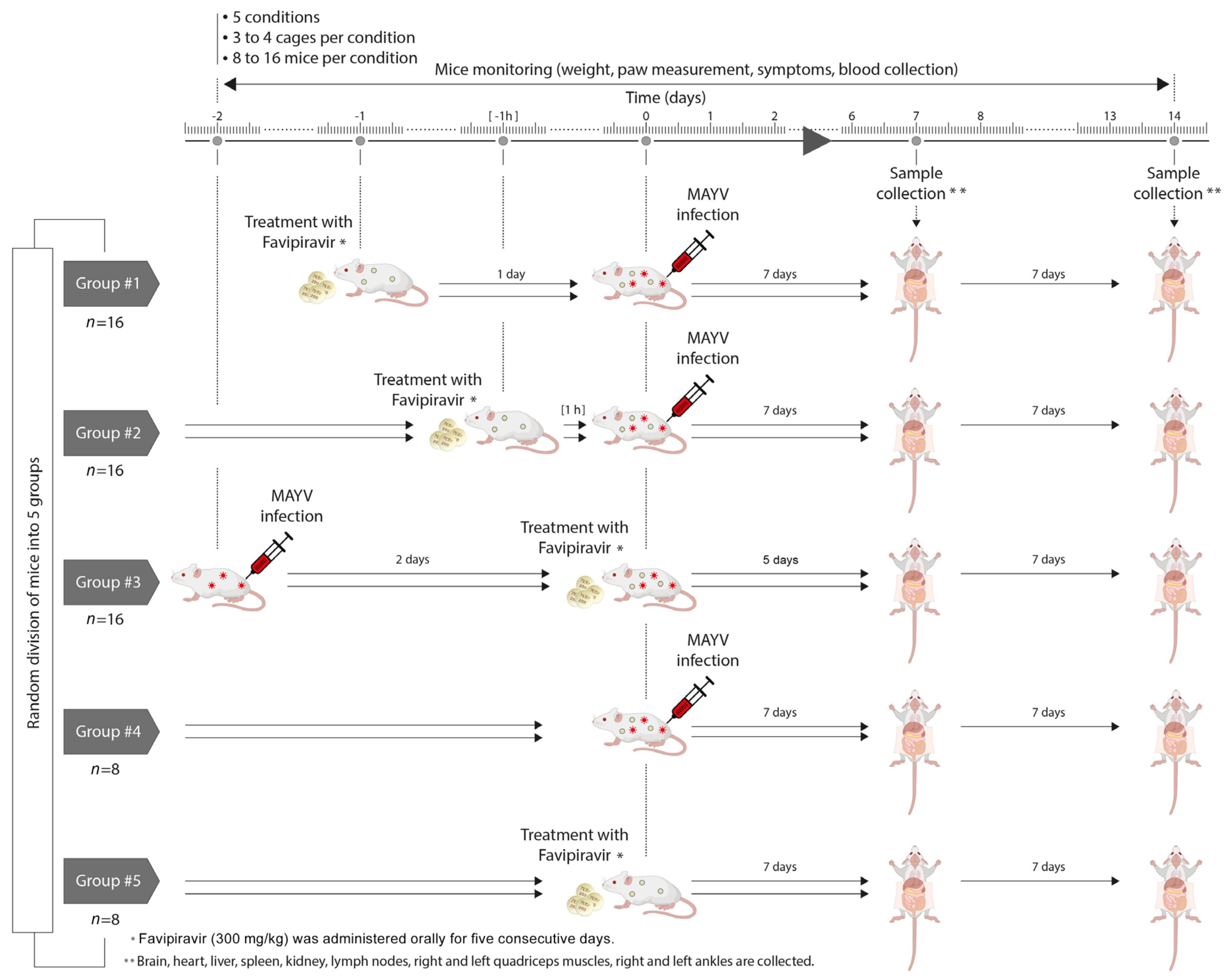
2.3. Mouse Experiment
2.4. In Vitro Activity of Favipiravir
2.5. In Vivo Activity of Favipiravir
2.6. Tissue Collection
2.7. Determination of Viral Spread in Organs and Blood
2.8. Detection of Infectious Viral Particles
2.9. Measurement of ASAT and ALAT Serum Levels
2.10. Statistical Analyses
3. Results
3.1. Favipiravir Exerts Antiviral Activity against MAYV In Vitro
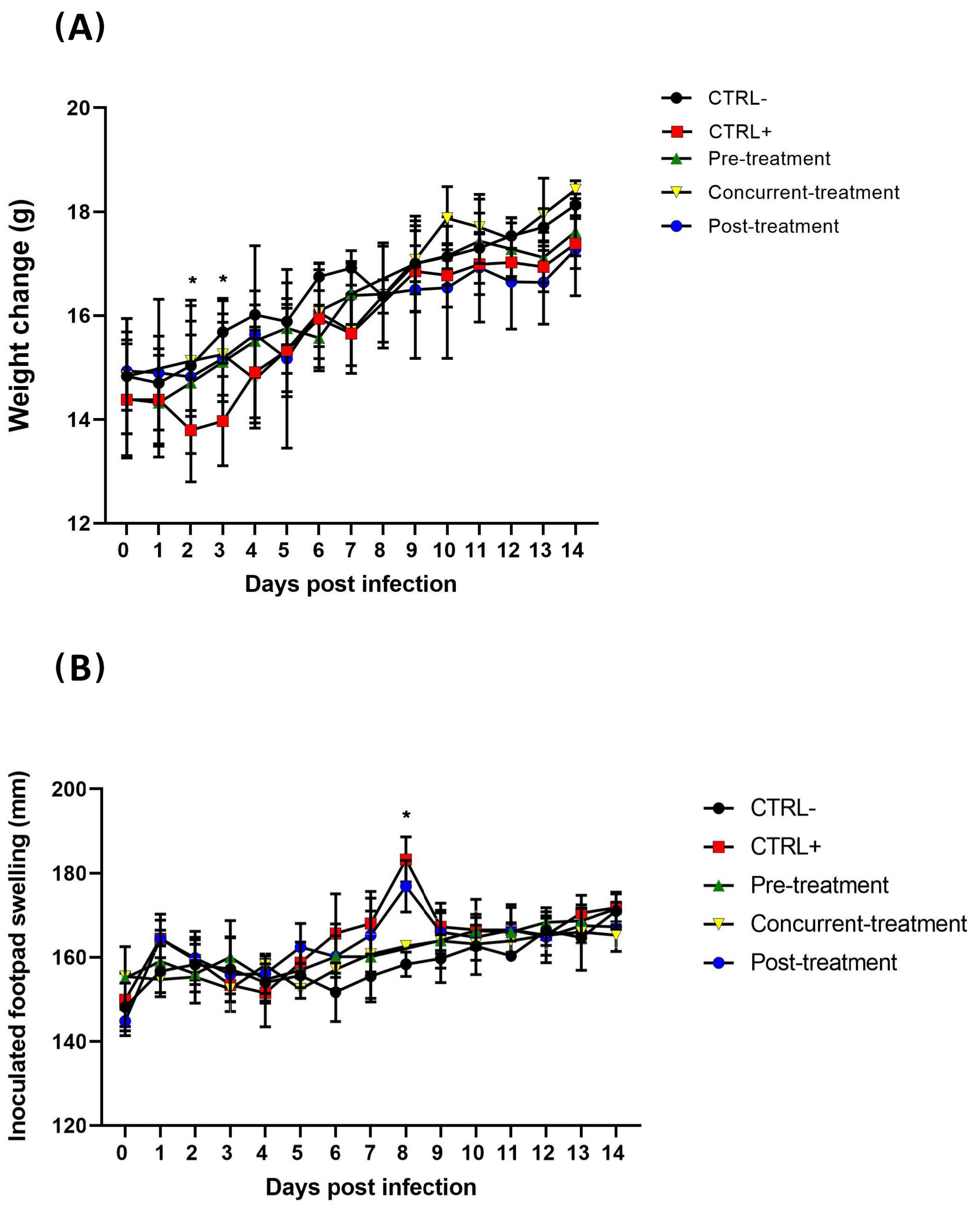
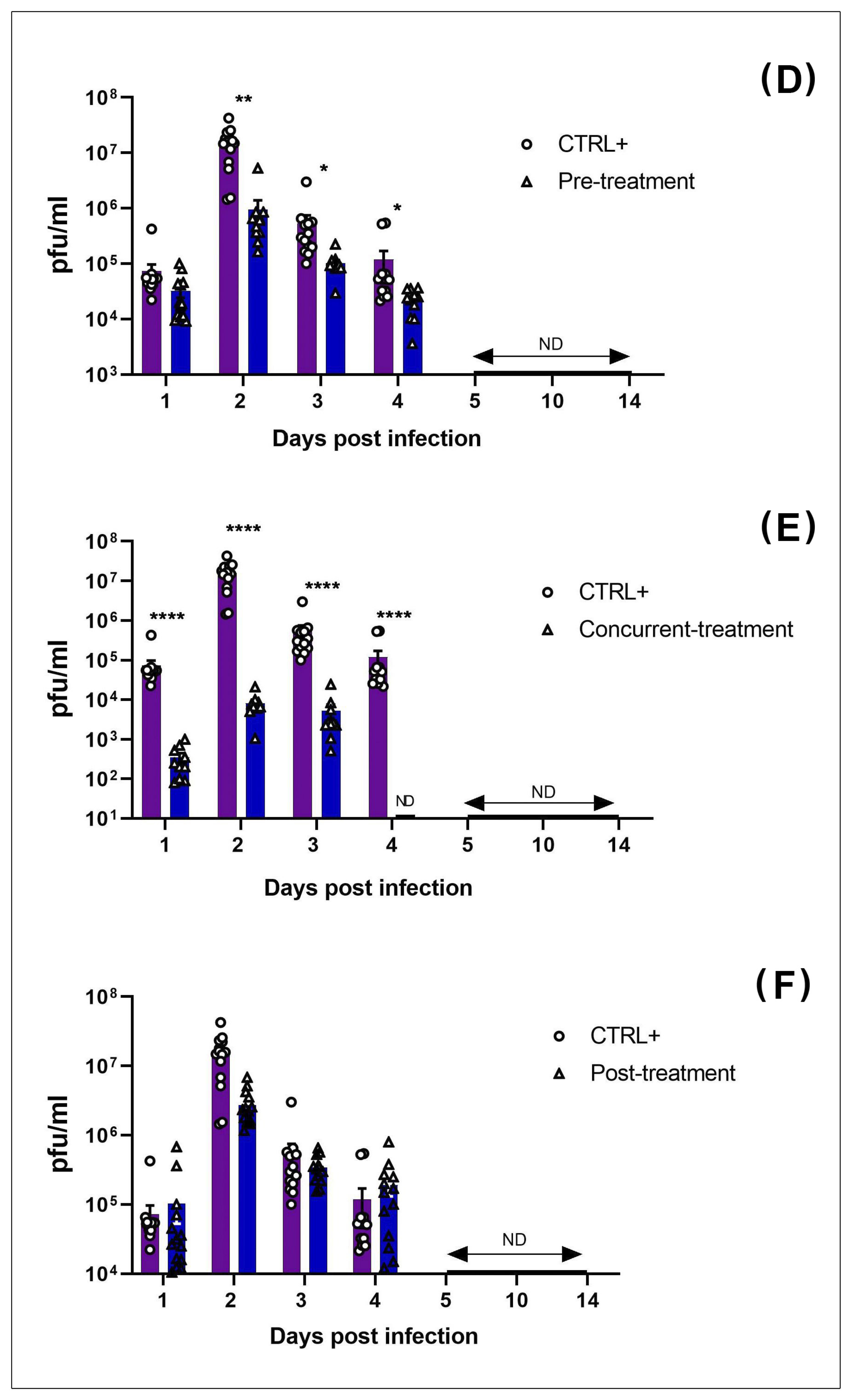
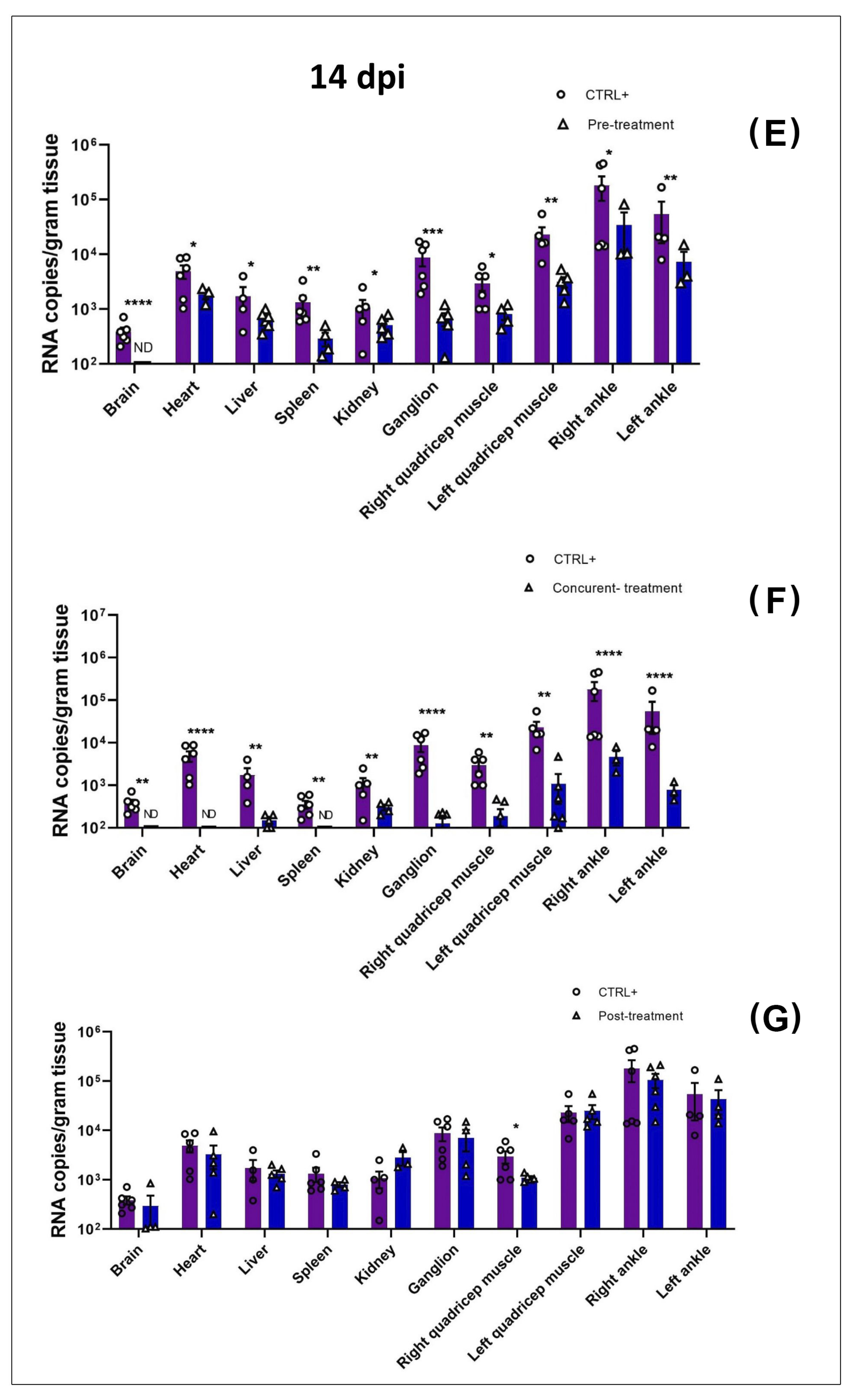
3.2. MAYV Is Susceptible to Favipiravir In Vivo
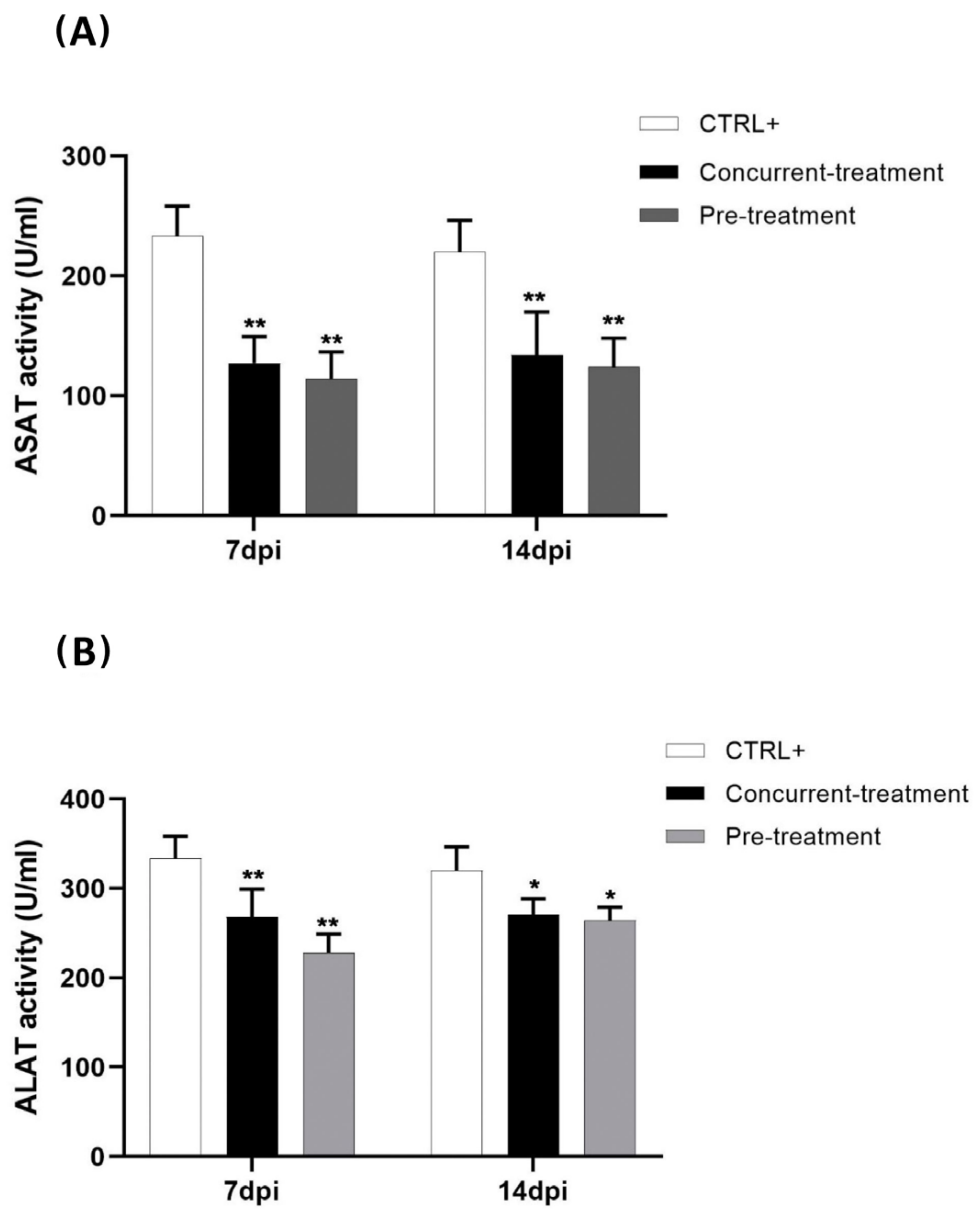
3.3. Favipiravir Decreases MAYV-Induced Transaminases
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lorenz, C.; Freitas Ribeiro, A.; Chiaravalloti-Neto, F. Mayaro Virus Distribution in South America. Acta Trop. 2019, 198, 105093. [Google Scholar] [CrossRef] [PubMed]
- Tesh, R.B.; Watts, D.M.; Russell, K.L.; Damodaran, C.; Calampa, C.; Cabezas, C.; Ramirez, G.; Vasquez, B.; Hayes, C.G.; Rossi, C.A.; et al. Mayaro Virus Disease: An Emerging Mosquito-Borne Zoonosis in Tropical South America. Clin. Infect. Dis. 1999, 28, 67–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anderson, C.R.; Downs, W.G.; Wattley, G.H.; Ahin, N.W.; Reese, A.A. Mayaro Virus: A New Human Disease Agent. II. Isolation from Blood of Patients in Trinidad, B.W.I. Am. J. Trop. Med. Hyg. 1957, 6, 1012–1016. [Google Scholar] [CrossRef] [PubMed]
- Lednicky, J.; De Rochars, V.M.B.; Elbadry, M.; Loeb, J.; Telisma, T.; Chavannes, S.; Anilis, G.; Cella, E.; Ciccozzi, M.; Okech, B.; et al. Mayaro Virus in Child with Acute Febrile Illness, Haiti, 2015. Emerg. Infect. Dis. 2016, 22, 2000–2002. [Google Scholar] [CrossRef]
- Hozé, N.; Salje, H.; Rousset, D.; Fritzell, C.; Vanhomwegen, J.; Bailly, S.; Najm, M.; Enfissi, A.; Manuguerra, J.-C.; Flamand, C.; et al. Reconstructing Mayaro Virus Circulation in French Guiana Shows Frequent Spillovers. Nat. Commun. 2020, 11, 2842. [Google Scholar] [CrossRef]
- Leung, J.Y.-S.; Ng, M.M.-L.; Chu, J.J.H. Replication of Alphaviruses: A Review on the Entry Process of Alphaviruses into Cells. Available online: https://www.hindawi.com/journals/av/2011/249640/ (accessed on 20 April 2020).
- Rupp, J.C.; Sokoloski, K.J.; Gebhart, N.N.; Hardy, R.W. Alphavirus RNA Synthesis and Non-Structural Protein Functions. J. Gen. Virol. 2015, 96, 2483–2500. [Google Scholar] [CrossRef]
- Suhrbier, A.; Jaffar-Bandjee, M.-C.; Gasque, P. Arthritogenic Alphaviruses—An Overview. Nat. Rev. Rheumatol. 2012, 8, 420–429. [Google Scholar] [CrossRef]
- Halsey, E.S.; Siles, C.; Guevara, C.; Vilcarromero, S.; Jhonston, E.J.; Ramal, C.; Aguilar, P.V.; Ampuero, J.S. Mayaro Virus Infection, Amazon Basin Region, Peru, 2010–2013. Emerg. Infect. Dis. 2013, 19, 1839–1842. [Google Scholar] [CrossRef]
- Friedrich-Jänicke, B.; Emmerich, P.; Tappe, D.; Günther, S.; Cadar, D.; Schmidt-Chanasit, J. Genome Analysis of Mayaro Virus Imported to Germany from French Guiana. Emerg. Infect. Dis. 2014, 20, 1255–1257. [Google Scholar] [CrossRef]
- Galatas, B.; Ly, S.; Duong, V.; Baisley, K.; Nguon, K.; Chan, S.; Huy, R.; Ly, S.; Sorn, S.; Som, L.; et al. Long-Lasting Immune Protection and Other Epidemiological Findings after Chikungunya Emergence in a Cambodian Rural Community, April 2012. PLoS Negl. Trop. Dis. 2016, 10, e0004281. [Google Scholar] [CrossRef]
- Kiwanuka, N.; Sanders, E.J.; Rwaguma, E.B.; Kawamata, J.; Ssengooba, F.P.; Najjemba, R.; Were, W.A.; Lamunu, M.; Bagambisa, G.; Burkot, T.R.; et al. O’nyong-Nyong Fever in South-Central Uganda, 1996–1997: Clinical Features and Validation of a Clinical Case Definition for Surveillance Purposes. Clin. Infect. Dis. 1999, 29, 1243–1250. [Google Scholar] [CrossRef]
- Harley, D.; Bossingham, D.; Purdie, D.M.; Pandeya, N.; Sleigh, A.C. Ross River Virus Disease in Tropical Queensland: Evolution of Rheumatic Manifestations in an Inception Cohort Followed for Six Months. Med. J. Aust. 2002, 177, 352–355. [Google Scholar] [CrossRef]
- Slegers, C.A.D.; Keuter, M.; Günther, S.; Schmidt-Chanasit, J.; van der Ven, A.J.; De Mast, Q. Persisting Arthralgia Due to Mayaro Virus Infection in a Traveler from Brazil: Is There a Risk for Attendants to the 2014 FIFA World Cup? J. Clin. Virol. 2014, 60, 317–319. [Google Scholar] [CrossRef]
- Taylor, S.F.; Patel, P.R.; Herold, T.J.S. Recurrent Arthralgias in a Patient with Previous Mayaro Fever Infection. South. Med. J. 2005, 98, 484–485. [Google Scholar] [CrossRef]
- McGill, P.E. Viral Infections: Alpha-Viral Arthropathy. Baillieres Clin. Rheumatol. 1995, 9, 145–150. [Google Scholar] [CrossRef]
- Diagne, C.T.; Bengue, M.; Choumet, V.; Hamel, R.; Pompon, J.; Missé, D. Mayaro Virus Pathogenesis and Transmission Mechanisms. Pathogens 2020, 9, 738. [Google Scholar] [CrossRef]
- Mourão, M.P.G.; Bastos, M.D.S.; De Figueiredo, R.P.; Gimaque, J.B.L.; Galusso, E.D.S.; Kramer, V.M.; De Oliveira, C.M.C.; Naveca, F.; Figueiredo, L.T.M. Mayaro Fever in the City of Manaus, Brazil, 2007–2008. Vector-Borne Zoonotic Dis. 2012, 12, 42–46. [Google Scholar] [CrossRef]
- Figueiredo, C.M.; Neris, R.L.D.S.; Gavino-Leopoldino, D.; Da Silva, M.O.L.; Almeida, J.S.; dos Santos, J.S.; Figueiredo, C.P.; Bellio, M.; Bozza, M.T.; Assunção-Miranda, I. Mayaro Virus Replication Restriction and Induction of Muscular Inflammation in Mice Are Dependent on Age, Type-I Interferon Response, and Adaptive Immunity. Front. Microbiol. 2019, 10, 2246. [Google Scholar] [CrossRef]
- Murillo-Zamora, E.; Mendoza-Cano, O.; Trujillo-Hernández, B.; Guzmán-Esquivel, J.; Higareda-Almaraz, E.; Higareda-Almaraz, M.A.; Sánchez-Piña, R.A.; Lugo-Radillo, A. Persistent Arthralgia and Related Risks Factors: A Cohort Study at 12 Months from Laboratory-Confirmed Chikungunya Infection. Arch. Med. Res. 2018, 49, 65–73. [Google Scholar] [CrossRef]
- Santiago, F.W.; Halsey, E.S.; Siles, C.; Vilcarromero, S.; Guevara, C.; Silvas, J.A.; Ramal, C.; Ampuero, J.S.; Aguilar, P.V. Long-Term Arthralgia after Mayaro Virus Infection Correlates with Sustained Pro-Inflammatory Cytokine Response. PLoS Negl. Trop. Dis. 2015, 9, e0004104. [Google Scholar] [CrossRef] [Green Version]
- Santos, F.M.; Dias, R.S.; De Oliveira, M.D.; Costa, I.C.T.A.; Fernandes, L.D.S.; Pessoa, C.R.; Da Matta, S.L.P.; Costa, V.V.; Souza, D.G.; Da Silva, C.C.; et al. Animal Model of Arthritis and Myositis Induced by the Mayaro Virus. PLoS Negl. Trop. Dis. 2019, 13, e0007375. [Google Scholar] [CrossRef]
- Camini, F.C.; da Silva Caetano, C.C.; Almeida, L.T.; da Costa Guerra, J.F.; de Mello Silva, B.; de Queiroz Silva, S.; de Magalhães, J.C.; de Brito Magalhães, C.L. Oxidative Stress in Mayaro Virus Infection. Virus Res. 2017, 236, 1–8. [Google Scholar] [CrossRef]
- Joubert, P.-E.; Werneke, S.; de la Calle, C.; Guivel-Benhassine, F.; Giodini, A.; Peduto, L.; Levine, B.; Schwartz, O.; Lenschow, D.; Albert, M.L. Chikungunya-Induced Cell Death Is Limited by ER and Oxidative Stress-Induced Autophagy. Autophagy 2012, 8, 1261–1263. [Google Scholar] [CrossRef] [Green Version]
- Remenyi, R.; Gao, Y.; Hughes, R.E.; Curd, A.; Zothner, C.; Peckham, M.; Merits, A.; Harris, M. Persistent Replication of a Chikungunya Virus Replicon in Human Cells Is Associated with Presence of Stable Cytoplasmic Granules Containing Nonstructural Protein 3. J. Virol. 2018, 92, e00477-18. [Google Scholar] [CrossRef] [Green Version]
- Rodriguez, J.L.; Steel, J.J. SINV Induces Oxidative Stress in Baby Hamster Kidney Host Cells. El Río: A Stud. Res. J. 2018, 1. [Google Scholar]
- Acosta-Ampudia, Y.; Monsalve, D.M.; Rodríguez, Y.; Pacheco, Y.; Anaya, J.-M.; Ramírez-Santana, C. Mayaro: An Emerging Viral Threat? Emerg. Microbes Infect. 2018, 7, 1–11. [Google Scholar] [CrossRef]
- Da Silva Caetano, C.C.; Camini, F.C.; Almeida, L.T.; Ferraz, A.C.; da Silva, T.F.; Lima, R.L.S.; de Freitas Carvalho, M.M.; de Freitas Castro, T.; Carneiro, C.M.; de Mello Silva, B.; et al. Mayaro Virus Induction of Oxidative Stress Is Associated With Liver Pathology in a Non-Lethal Mouse Model. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef]
- Moriles, K.E.; Azer, S.A. Alanine Amino Transferase. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. [Google Scholar]
- Abdelnabi, R.; Jochmans, D.; Verbeken, E.; Neyts, J.; Delang, L. Antiviral Treatment Efficiently Inhibits Chikungunya Virus Infection in the Joints of Mice during the Acute but Not during the Chronic Phase of the Infection. Antivir. Res. 2018, 149, 113–117. [Google Scholar] [CrossRef]
- De Mello, M.V.; Domingos, T.F.; Ferreira, D.; Ribeiro, M.; Ribeiro, T.; Souza, A.; Rodrigues, C. Antiviral Drug Discovery and Development for Mayaro Fever—What Do We Have so Far? Mini Rev. Med. Chem. 2020, 20, 921–928. [Google Scholar] [CrossRef]
- Abu Bakar, F.; Ng, L.F.P. Nonstructural Proteins of Alphavirus—Potential Targets for Drug Development. Viruses 2018, 10, 71. [Google Scholar] [CrossRef] [Green Version]
- Delogu, I.; Pastorino, B.; Baronti, C.; Nougairède, A.; Bonnet, E.; de Lamballerie, X. In Vitro Antiviral Activity of Arbidol against Chikungunya Virus and Characteristics of a Selected Resistant Mutant. Antivir. Res. 2011, 90, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Amorim, R.; de Meneses, M.D.F.; Borges, J.C.; da Silva Pinheiro, L.C.; Caldas, L.A.; Cirne-Santos, C.C.; de Mello, M.V.P.; de Souza, A.M.T.; Castro, H.C.; de Palmer Paixão, I.C.N.; et al. Thieno[2,3-b]Pyridine Derivatives: A New Class of Antiviral Drugs against Mayaro Virus. Arch. Virol. 2017, 162, 1577–1587. [Google Scholar] [CrossRef] [PubMed]
- Da Costa, L.J.; Rebello, M.A. Effect of Brefeldin A on Mayaro Virus Replication in Aedes Albopictus and Vero Cells. Acta Virol. 1999, 43, 357–360. [Google Scholar] [PubMed]
- Carvalho, C.A.M.; Sousa, I.P.; Silva, J.L.; Oliveira, A.C.; Gonçalves, R.B.; Gomes, A.M.O. Inhibition of Mayaro Virus Infection by Bovine Lactoferrin. Virology 2014, 452–453, 297–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langendries, L.; Abdelnabi, R.; Neyts, J.; Delang, L. Repurposing Drugs for Mayaro Virus: Identification of EIDD-1931, Favipiravir and Suramin as Mayaro Virus Inhibitors. Microorganisms 2021, 9, 734. [Google Scholar] [CrossRef] [PubMed]
- Delang, L.; Segura Guerrero, N.; Tas, A.; Quérat, G.; Pastorino, B.; Froeyen, M.; Dallmeier, K.; Jochmans, D.; Herdewijn, P.; Bello, F.; et al. Mutations in the Chikungunya Virus Non-Structural Proteins Cause Resistance to Favipiravir (T-705), a Broad-Spectrum Antiviral. J. Antimicrob. Chemother. 2014, 69, 2770–2784. [Google Scholar] [CrossRef]
- Julander, J.G.; Smee, D.F.; Morrey, J.D.; Furuta, Y. Effect of T-705 Treatment on Western Equine Encephalitis in a Mouse Model. Antivir. Res. 2009, 82, 169–171. [Google Scholar] [CrossRef] [Green Version]
- Roques, P.; Thiberville, S.-D.; Dupuis-Maguiraga, L.; Lum, F.-M.; Labadie, K.; Martinon, F.; Gras, G.; Lebon, P.; Ng, L.F.P.; De Lamballerie, X.; et al. Paradoxical Effect of Chloroquine Treatment in Enhancing Chikungunya Virus Infection. Viruses 2018, 10, 268. [Google Scholar] [CrossRef] [Green Version]
- Belarbi, E.; Legros, V.; Basset, J.; Desprès, P.; Roques, P.; Choumet, V. Bioluminescent Ross River Virus Allows Live Monitoring of Acute and Long-Term Alphaviral Infection by In Vivo Imaging. Viruses 2019, 11, 584. [Google Scholar] [CrossRef] [Green Version]
- Kirillov, L.V. Method of quantitative determination of vaccine activity against botulism of the mink. Dev. Biol. Stand. 1976, 32, 203–209. [Google Scholar]
- Bengue, M.; Ferraris, P.; Baronti, C.; Diagne, C.T.; Talignani, L.; Wichit, S.; Liegeois, F.; Bisbal, C.; Nougairède, A.; Missé, D. Mayaro Virus Infects Human Chondrocytes and Induces the Expression of Arthritis-Related Genes Associated with Joint Degradation. Viruses 2019, 11, 797. [Google Scholar] [CrossRef] [Green Version]
- Diop, F.; Alout, H.; Diagne, C.T.; Bengue, M.; Baronti, C.; Hamel, R.; Talignani, L.; Liegeois, F.; Pompon, J.; Vargas, R.E.M.; et al. Differential Susceptibility and Innate Immune Response of Aedes Aegypti and Aedes Albopictus to the Haitian Strain of the Mayaro Virus. Viruses 2019, 11, 924. [Google Scholar] [CrossRef] [Green Version]
- Suchowiecki, K.; Reid, S.P.; Simon, G.L.; Firestein, G.S.; Chang, A. Persistent Joint Pain Following Arthropod Virus Infections. Curr. Rheumatol. Rep. 2021, 23, 1–12. [Google Scholar] [CrossRef]
- Dupuis-Maguiraga, L.; Noret, M.; Brun, S.; Le Grand, R.; Gras, G.; Roques, P. Chikungunya Disease: Infection-Associated Markers from the Acute to the Chronic Phase of Arbovirus-Induced Arthralgia. PLoS Negl. Trop. Dis. 2012, 6, e1446. [Google Scholar] [CrossRef] [Green Version]
- Furuta, Y.; Gowen, B.B.; Takahashi, K.; Shiraki, K.; Smee, D.F.; Barnard, D.L. Favipiravir (T-705), a Novel Viral RNA Polymerase Inhibitor. Antivir. Res. 2013, 100, 446–454. [Google Scholar] [CrossRef] [Green Version]
- Driouich, J.-S.; Cochin, M.; Lingas, G.; Moureau, G.; Touret, F.; Petit, P.-R.; Piorkowski, G.; Barthélémy, K.; Laprie, C.; Coutard, B.; et al. Favipiravir Antiviral Efficacy against SARS-CoV-2 in a Hamster Model. Nat. Commun. 2021, 12, 1735. [Google Scholar] [CrossRef]
- Borrego, B.; de Ávila, A.I.; Domingo, E.; Brun, A. Lethal Mutagenesis of Rift Valley Fever Virus Induced by Favipiravir. Antimicrob. Agents Chemother. 2019, 63, e00669-19. [Google Scholar] [CrossRef] [Green Version]
- Arias, A.; Thorne, L.; Goodfellow, I. Favipiravir Elicits Antiviral Mutagenesis during Virus Replication in Vivo. eLife 2014, 3, e03679. [Google Scholar] [CrossRef]
- Assunção-Miranda, I.; Cruz-Oliveira, C.; Da Poian, A.T. Molecular Mechanisms Involved in the Pathogenesis of Alphavirus-Induced Arthritis. BioMed Res. Int. 2013. [Google Scholar] [CrossRef] [Green Version]
- Camini, F.C.; da Silva, T.F.; da Silva Caetano, C.C.; Almeida, L.T.; Ferraz, A.C.; Alves Vitoreti, V.M.; de Mello Silva, B.; de Queiroz Silva, S.; de Magalhães, J.C.; de Brito Magalhães, C.L. Antiviral Activity of Silymarin against Mayaro Virus and Protective Effect in Virus-Induced Oxidative Stress. Antivir. Res. 2018, 158, 8–12. [Google Scholar] [CrossRef]
- Gardner, J.; Anraku, I.; Le, T.T.; Larcher, T.; Major, L.; Roques, P.; Schroder, W.A.; Higgs, S.; Suhrbier, A. Chikungunya Virus Arthritis in Adult Wild-Type Mice. J. Virol. 2010, 84, 8021–8032. [Google Scholar] [CrossRef] [Green Version]
- Labadie, K.; Larcher, T.; Joubert, C.; Mannioui, A.; Delache, B.; Brochard, P.; Guigand, L.; Dubreil, L.; Lebon, P.; Verrier, B.; et al. Chikungunya Disease in Nonhuman Primates Involves Long-Term Viral Persistence in Macrophages. J. Clin. Investig. 2010, 120, 894–906. [Google Scholar] [CrossRef] [Green Version]
- Nunes, M.L.; Carlini, C.R.; Marinowic, D.; Kalil, F.; Fiori, H.H.; Scotta, M.C.; Zanella, P.L.Á; Soder, R.B.; da Costa, J.C. Microcephaly and Zika Virus: A Clinical and Epidemiological Analysis of the Current Outbreak in Brazil. Jornal de Pediatria 2016, 92, 230–240. [Google Scholar] [CrossRef] [Green Version]
- Varghese, F.S.; Thaa, B.; Amrun, S.N.; Simarmata, D.; Rausalu, K.; Nyman, T.A.; Merits, A.; McInerney, G.M.; Ng, L.F.P.; Ahola, T. The Antiviral Alkaloid Berberine Reduces Chikungunya Virus-Induced Mitogen-Activated Protein Kinase Signaling. J. Virol. 2016, 90, 9743–9757. [Google Scholar] [CrossRef] [Green Version]
- Couderc, T.; Chrétien, F.; Schilte, C.; Disson, O.; Brigitte, M.; Guivel-Benhassine, F.; Touret, Y.; Barau, G.; Cayet, N.; Schuffenecker, I.; et al. A Mouse Model for Chikungunya: Young Age and Inefficient Type-I Interferon Signaling Are Risk Factors for Severe Disease. PLoS Pathog. 2008, 4, e29. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bengue, M.; Pintong, A.-r.; Liegeois, F.; Nougairède, A.; Hamel, R.; Pompon, J.; de Lamballerie, X.; Roques, P.; Choumet, V.; Missé, D. Favipiravir Inhibits Mayaro Virus Infection in Mice. Viruses 2021, 13, 2213. https://doi.org/10.3390/v13112213
Bengue M, Pintong A-r, Liegeois F, Nougairède A, Hamel R, Pompon J, de Lamballerie X, Roques P, Choumet V, Missé D. Favipiravir Inhibits Mayaro Virus Infection in Mice. Viruses. 2021; 13(11):2213. https://doi.org/10.3390/v13112213
Chicago/Turabian StyleBengue, Michèle, Ai-rada Pintong, Florian Liegeois, Antoine Nougairède, Rodolphe Hamel, Julien Pompon, Xavier de Lamballerie, Pierre Roques, Valérie Choumet, and Dorothée Missé. 2021. "Favipiravir Inhibits Mayaro Virus Infection in Mice" Viruses 13, no. 11: 2213. https://doi.org/10.3390/v13112213
APA StyleBengue, M., Pintong, A.-r., Liegeois, F., Nougairède, A., Hamel, R., Pompon, J., de Lamballerie, X., Roques, P., Choumet, V., & Missé, D. (2021). Favipiravir Inhibits Mayaro Virus Infection in Mice. Viruses, 13(11), 2213. https://doi.org/10.3390/v13112213









