The Progestin Medroxyprogesterone Acetate Affects HIV-1 Production in Human Lymphoid Tissue Explants in a Dose-Dependent and Glucocorticoid-like Fashion
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tonsillar Tissue Processing for Histoculture
2.2. Viruses
2.3. Compound Reconstitution
2.4. Compound Treatment and Infection of Tissue Explants
2.5. HIV-1 Quantification and Multiplex Cytokine Immunoassay
2.6. Statistical Analysis
3. Results
3.1. Tissue Donors
3.2. MPA Treatment Reduces HIV-1 Production in a Dose-Dependent Fashion
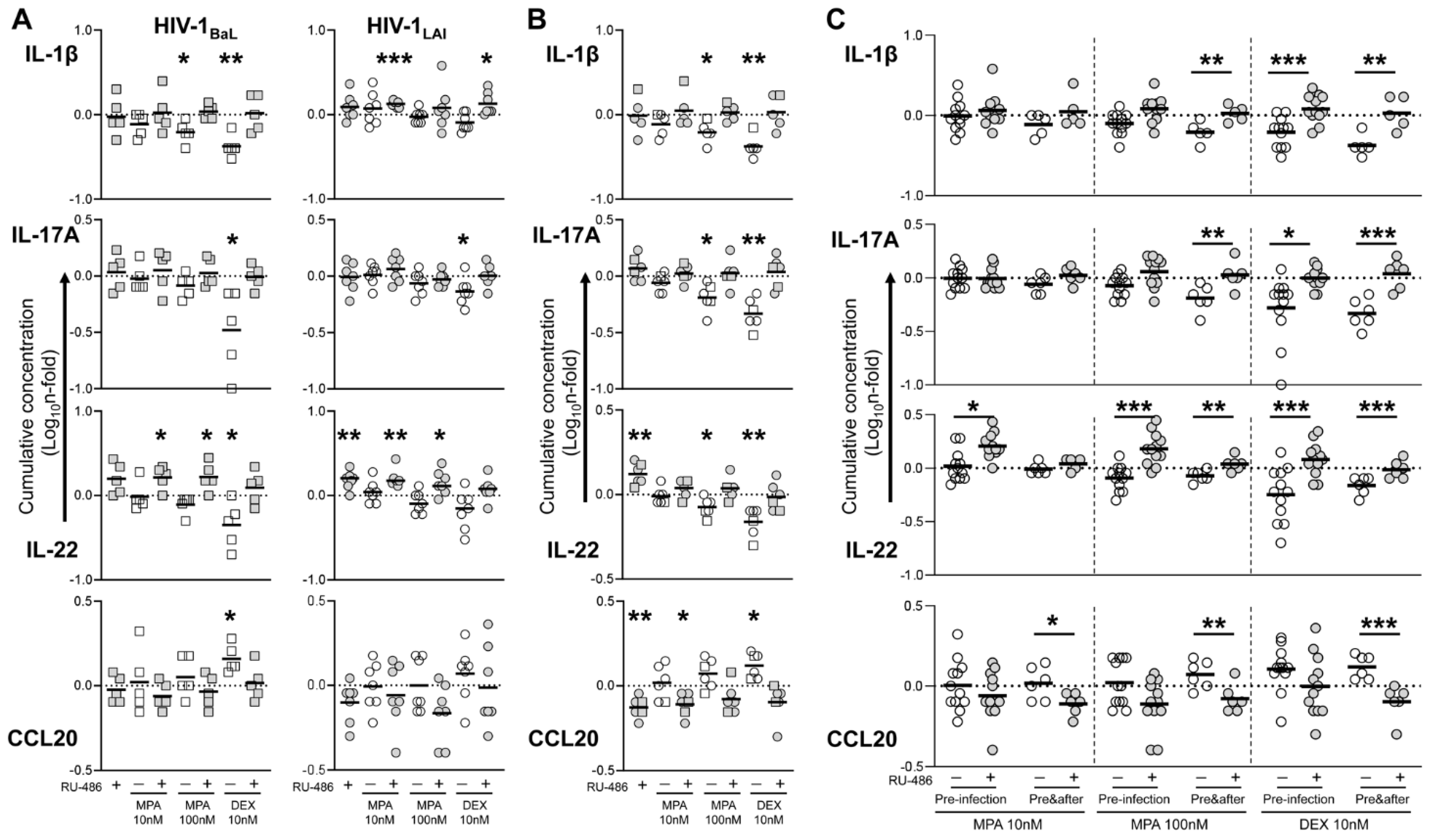
3.3. MPA Treatment Affects Cytokine Production in a Glucocorticoid-Like Fashion
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Population Division, Department of Economic and Social Affairs, United Nations. Contraceptive Use by Method 2019: Data Booklet (ST/ESA/SER.A/435). 2019. Available online: https://www.un.org/en/development/desa/population/publications/pdf/family/ContraceptiveUseByMethodDataBooklet2019.pdf (accessed on 11 November 2021).
- Heffron, R.; Achilles, S.L.; Dorflinger, L.J.; Hapgood, J.P.; Kiarie, J.; Polis, C.B.; Steyn, P.S. Pharmacokinetic, biologic and epidemiologic differences in MPA- and NET-based progestin-only injectable contraceptives relative to the potential impact on HIV acquisition in women. Contraception 2019, 99, 199–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Evidence for Contraceptive Options and HIV Outcomes (ECHO) Trial Consortium. HIV incidence among women using intramuscular depot medroxyprogesterone acetate, a copper intrauterine device, or a levonorgestrel implant for contraception: A randomised, multicentre, open-label trial. Lancet 2019, 394, 303–313. [Google Scholar] [CrossRef] [Green Version]
- Hapgood, J.P. Is the Injectable Contraceptive Depo-Medroxyprogesterone Acetate (DMPA-IM) Associated with an Increased Risk for HIV Acquisition? The Jury Is Still Out. AIDS Res. Hum. Retrovir. 2020, 36, 357–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bick, A.J.; Louw-du Toit, R.; Skosana, S.B.; Africander, D.; Hapgood, J.P. Pharmacokinetics, metabolism and serum concentrations of progestins used in contraception. Pharmacol. Ther. 2021, 222, 107789. [Google Scholar] [CrossRef] [PubMed]
- Kontula, K.; Paavonen, T.; Luukkainen, T.; Andersson, L.C. Binding of progestins to the glucocorticoid receptor. Correlation to their glucocorticoid-like effects on in vitro functions of human mononuclear leukocytes. Biochem. Pharmacol. 1983, 32, 1511–1518. [Google Scholar] [CrossRef]
- Govender, Y.; Avenant, C.; Verhoog, N.J.; Ray, R.M.; Grantham, N.J.; Africander, D.; Hapgood, J.P. The injectable-only contraceptive medroxyprogesterone acetate, unlike norethisterone acetate and progesterone, regulates inflammatory genes in endocervical cells via the glucocorticoid receptor. PLoS ONE 2014, 9, e96497. [Google Scholar] [CrossRef] [Green Version]
- Hapgood, J.P.; Kaushic, C.; Hel, Z. Hormonal Contraception and HIV-1 Acquisition: Biological Mechanisms. Endocr. Rev. 2018, 39, 36–78. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, O.J.; Klein, S.L. Progesterone-based compounds affect immune responses and susceptibility to infections at diverse mucosal sites. Mucosal. Immunol. 2017, 10, 1097–1107. [Google Scholar] [CrossRef] [Green Version]
- Saba, E.; Origoni, M.; Taccagni, G.; Ferrari, D.; Doglioni, C.; Nava, A.; Lisco, A.; Grivel, J.C.; Margolis, L.; Poli, G. Productive HIV-1 infection of human cervical tissue ex vivo is associated with the secretory phase of the menstrual cycle. Mucosal. Immunol. 2013, 6, 1081–1090. [Google Scholar] [CrossRef]
- Ray, R.M.; Maritz, M.F.; Avenant, C.; Tomasicchio, M.; Dlamini, S.; van der Spuy, Z.; Hapgood, J.P. The contraceptive medroxyprogesterone acetate, unlike norethisterone, directly increases R5 HIV-1 infection in human cervical explant tissue at physiologically relevant concentrations. Sci. Rep. 2019, 9, 4334. [Google Scholar] [CrossRef] [Green Version]
- Chun, T.W.; Fauci, A.S. HIV reservoirs: Pathogenesis and obstacles to viral eradication and cure. AIDS 2012, 26, 1261–1268. [Google Scholar] [CrossRef] [PubMed]
- Introini, A.; Vanpouille, C.; Lisco, A.; Grivel, J.C.; Margolis, L. Interleukin-7 facilitates HIV-1 transmission to cervico-vaginal tissue ex vivo. PLoS Pathog. 2013, 9, e1003148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gartner, S.; Markovits, P.; Markovitz, D.M.; Kaplan, M.H.; Gallo, R.C.; Popovic, M. The role of mononuclear phagocytes in HTLV-III/LAV infection. Science 1986, 233, 215–219. [Google Scholar] [CrossRef]
- Barré-Sinoussi, F.; Chermann, J.C.; Rey, F.; Nugeyre, M.T.; Chamaret, S.; Gruest, J.; Dauguet, C.; Axler-Blin, C.; Vézinet-Brun, F.; Rouzioux, C.; et al. Isolation of a T-lymphotropic retrovirus from a patient at risk for acquired immune deficiency syndrome (AIDS). Science 1983, 220, 868–871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Introini, A.; Vanpouille, C.; Fitzgerald, W.; Broliden, K.; Margolis, L. Ex Vivo Infection of Human Lymphoid Tissue and Female Genital Mucosa with Human Immunodeficiency Virus 1 and Histoculture. J. Vis. Exp. 2018, 140, e57013. [Google Scholar] [CrossRef] [PubMed]
- Biancotto, A.; Brichacek, B.; Chen, S.S.; Fitzgerald, W.; Lisco, A.; Vanpouille, C.; Margolis, L.; Grivel, J.C. A highly sensitive and dynamic immunofluorescent cytometric bead assay for the detection of HIV-1 p24. J. Virol. Methods 2009, 157, 98–101. [Google Scholar] [CrossRef] [Green Version]
- Grivel, J.C.; Elliott, J.; Lisco, A.; Biancotto, A.; Condack, C.; Shattock, R.J.; McGowan, I.; Margolis, L.; Anton, P. HIV-1 pathogenesis differs in rectosigmoid and tonsillar tissues infected ex vivo with CCR5- and CXCR4-tropic HIV-1. AIDS 2007, 21, 1263–1272. [Google Scholar] [CrossRef]
- Ayele, H.; Perner, M.; McKinnon, L.R.; Birse, K.; Farr Zuend, C.; Burgener, A. An updated review on the effects of depot medroxyprogesterone acetate on the mucosal biology of the female genital tract. Am. J. Reprod Immunol. 2021, 86, e13455. [Google Scholar] [CrossRef]
- Lederman, M.M.; Margolis, L. The lymph node in HIV pathogenesis. Semin. Immunol. 2008, 20, 187–195. [Google Scholar] [CrossRef] [Green Version]
- Grivel, J.C.; Margolis, L. Use of human tissue explants to study human infectious agents. Nat. Protoc. 2009, 4, 256–269. [Google Scholar] [CrossRef]
- Aksglaede, L.; Juul, A.; Leffers, H.; Skakkebaek, N.E.; Andersson, A.M. The sensitivity of the child to sex steroids: Possible impact of exogenous estrogens. Hum. Reprod Update 2006, 12, 341–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huijbregts, R.P.; Helton, E.S.; Michel, K.G.; Sabbaj, S.; Richter, H.E.; Goepfert, P.A.; Hel, Z. Hormonal contraception and HIV-1 infection: Medroxyprogesterone acetate suppresses innate and adaptive immune mechanisms. Endocrinology 2013, 154, 1282–1295. [Google Scholar] [CrossRef] [Green Version]
- Mariko, H. Medroxyprogesterone Acetate has Minimal Impact on HIV-1 Replication in Ectocervical Tissue Ex Vivo. Master’s Thesis, University of Pittsburgh, Pittsburgh PA, USA, 2017. Available online: http://d-scholarship.pitt.edu/id/eprint/31150 (accessed on 11 November 2021).
- Wessels, J.M.; Nguyen, P.V.; Vitali, D.; Mueller, K.; Vahedi, F.; Felker, A.M.; Dupont, H.A.; Bagri, P.; Verschoor, C.P.; Deshiere, A.; et al. Depot medroxyprogesterone acetate (DMPA) enhances susceptibility and increases the window of vulnerability to HIV-1 in humanized mice. Sci Rep. 2021, 11, 3894. [Google Scholar] [CrossRef] [PubMed]
- Shah, N.M.; Lai, P.F.; Imami, N.; Johnson, M.R. Progesterone-Related Immune Modulation of Pregnancy and Labor. Front. Endocrinol. (Lausanne) 2019, 10, 198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dressing, G.E.; Goldberg, J.E.; Charles, N.J.; Schwertfeger, K.L.; Lange, C.A. Membrane progesterone receptor expression in mammalian tissues: A review of regulation and physiological implications. Steroids 2011, 76, 11–17. [Google Scholar] [CrossRef] [Green Version]
- Klatt, N.R.; Cheu, R.; Birse, K.; Zevin, A.S.; Perner, M.; Noël-Romas, L.; Grobler, A.; Westmacott, G.; Xie, I.Y.; Butler, J.; et al. Vaginal bacteria modify HIV tenofovir microbicide efficacy in African women. Science 2017, 356, 938–945. [Google Scholar] [CrossRef] [Green Version]
- Woods, M.W.; Zahoor, M.A.; Dizzell, S.; Verschoor, C.P.; Kaushic, C. Medroxyprogesterone acetate-treated human, primary endometrial epithelial cells reveal unique gene expression signature linked to innate immunity and HIV-1 susceptibility. Am. J. Reprod. Immunol. 2018, 79, e12781. [Google Scholar] [CrossRef]
- Ghosh, M.; Shen, Z.; Schaefer, T.M.; Fahey, J.V.; Gupta, P.; Wira, C.R. CCL20/MIP3alpha is a novel anti-HIV-1 molecule of the human female reproductive tract. Am. J. Reprod. Immunol. 2009, 62, 60–71. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Estes, J.D.; Schlievert, P.M.; Duan, L.; Brosnahan, A.J.; Southern, P.J.; Reilly, C.S.; Peterson, M.L.; Schultz-Darken, N.; Brunner, K.G.; et al. Glycerol monolaurate prevents mucosal SIV transmission. Nature 2009, 458, 1034–1038. [Google Scholar] [CrossRef]
- Stieh, D.J.; Matias, E.; Xu, H.; Fought, A.J.; Blanchard, J.L.; Marx, P.A.; Veazey, R.S.; Hope, T.J. Th17 Cells Are Preferentially Infected Very Early after Vaginal Transmission of SIV in Macaques. Cell Host Microbe. 2016, 19, 529–540. [Google Scholar] [CrossRef] [Green Version]
- Sabihi, M.; Böttcher, M.; Pelczar, P.; Huber, S. Microbiota-Dependent Effects of IL-22. Cells 2020, 9, 2205. [Google Scholar] [CrossRef]
- Missé, D.; Yssel, H.; Trabattoni, D.; Oblet, C.; Lo Caputo, S.; Mazzotta, F.; Pène, J.; Gonzalez, J.P.; Clerici, M.; Veas, F. IL-22 participates in an innate anti-HIV-1 host-resistance network through acute-phase protein induction. J. Immunol. 2007, 178, 407–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hild-Petito, S.; Veazey, R.S.; Larner, J.M.; Reel, J.R.; Blye, R.P. Effects of two progestin-only contraceptives, Depo-Provera and Norplant-II, on the vaginal epithelium of rhesus monkeys. AIDS Res. Hum. Retrovir. 1998, 14 (Suppl. S1), S125–S130. [Google Scholar] [PubMed]
- Zalenskaya, I.A.; Chandra, N.; Yousefieh, N.; Fang, X.; Adedipe, O.E.; Jackson, S.S.; Anderson, S.M.; Mauck, C.K.; Schwartz, J.L.; Thurman, A.R.; et al. Use of contraceptive depot medroxyprogesterone acetate is associated with impaired cervicovaginal mucosal integrity. J. Clin. Investig. 2018, 128, 4622–4638. [Google Scholar] [CrossRef] [PubMed]
- Edfeldt, G.; Lajoie, J.; Röhl, M.; Oyugi, J.; Åhlberg, A.; Khalilzadeh-Binicy, B.; Bradley, F.; Mack, M.; Kimani, J.; Omollo, K.; et al. Regular use of depot medroxyprogesterone acetate causes thinning of the superficial lining and apical distribution of HIV target cells in the human ectocervix. J. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Banuelos, J.; Shin, S.; Cao, Y.; Bochner, B.S.; Morales-Nebreda, L.; Budinger, G.R.; Zhou, L.; Li, S.; Xin, J.; Lingen, M.W.; et al. BCL-2 protects human and mouse Th17 cells from glucocorticoid-induced apoptosis. Allergy 2016, 71, 640–650. [Google Scholar] [CrossRef] [Green Version]
- Seshadri, S.; Pope, R.L.; Zenewicz, L.A. Glucocorticoids Inhibit Group 3 Innate Lymphocyte IL-22 Production. J. Immunol. 2018, 201, 1267–1274. [Google Scholar] [CrossRef] [Green Version]
- Taves, M.D.; Ashwell, J.D. Glucocorticoids in T cell development, differentiation and function. Nat. Rev. Immunol. 2021, 21, 233–243. [Google Scholar] [CrossRef]
- Tomasicchio, M.; Davids, M.; Pooran, A.; Theron, G.; Smith, L.; Semple, L.; Meldau, R.; Hapgood, J.P.; Dheda, K. The Injectable Contraceptive Medroxyprogesterone Acetate Attenuates Mycobacterium tuberculosis-Specific Host Immunity Through the Glucocorticoid Receptor. J. Infect. Dis. 2019, 219, 1329–1337. [Google Scholar] [CrossRef]
- Klatt, N.R.; Funderburg, N.T.; Brenchley, J.M. Microbial translocation, immune activation, and HIV disease. Trends Microbiol. 2013, 21, 6–13. [Google Scholar] [CrossRef] [Green Version]
- McKinnon, L.R.; Nyanga, B.; Kim, C.J.; Izulla, P.; Kwatampora, J.; Kimani, M.; Shahabi, K.; Mugo, N.; Smith, J.S.; Anzala, A.O.; et al. Early HIV-1 infection is associated with reduced frequencies of cervical Th17 cells. J. Acquir. Immune Defic. Syndr. 2015, 68, 6–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keele, B.F.; Giorgi, E.E.; Salazar-Gonzalez, J.F.; Decker, J.M.; Pham, K.T.; Salazar, M.G.; Sun, C.; Grayson, T.; Wang, S.; Li, H.; et al. Identification and characterization of transmitted and early founder virus envelopes in primary HIV-1 infection. Proc. Natl. Acad. Sci. USA 2008, 105, 7552–7557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omollo, K.; Lajoie, J.; Oyugi, J.; Wessels, J.M.; Mwaengo, D.; Kimani, J.; Kaushic, C.; Fowke, K.R. Differential Elevation of Inflammation and CD4+ T Cell Activation in Kenyan Female Sex Workers and Non-Sex Workers Using Depot-Medroxyprogesterone Acetate. Front. Immunol. 2020, 11, 598307. [Google Scholar] [CrossRef] [PubMed]
- Matubu, A.T.; Hillier, S.L.; Meyn, L.A.; Stoner, K.A.; Mhlanga, F.; Mbizvo, M.; Maramba, A.; Chirenje, Z.M.; Achilles, S.L. Effect of injectable progestin-only contraceptives, depot medroxyprogesterone acetate and norethisterone enanthate, on cytokine production during T-cell activation. Am. J. Reprod. Immunol. 2021, 86, e13405. [Google Scholar] [CrossRef]
- Molatlhegi, R.P.; Liebenberg, L.J.; Leslie, A.; Noel-Romas, L.; Mabhula, A.; Mchunu, N.; Perner, M.; Birse, K.; Ngcapu, S.; Adamson, J.H.; et al. Plasma concentration of injectable contraceptive correlates with reduced cervicovaginal growth factor expression in South African women. Mucosal. Immunol. 2020, 13, 449–459. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vanpouille, C.; Günaydın, G.; Jangard, M.; Clerici, M.; Margolis, L.; Broliden, K.; Introini, A. The Progestin Medroxyprogesterone Acetate Affects HIV-1 Production in Human Lymphoid Tissue Explants in a Dose-Dependent and Glucocorticoid-like Fashion. Viruses 2021, 13, 2303. https://doi.org/10.3390/v13112303
Vanpouille C, Günaydın G, Jangard M, Clerici M, Margolis L, Broliden K, Introini A. The Progestin Medroxyprogesterone Acetate Affects HIV-1 Production in Human Lymphoid Tissue Explants in a Dose-Dependent and Glucocorticoid-like Fashion. Viruses. 2021; 13(11):2303. https://doi.org/10.3390/v13112303
Chicago/Turabian StyleVanpouille, Christophe, Gökçe Günaydın, Mattias Jangard, Mario Clerici, Leonid Margolis, Kristina Broliden, and Andrea Introini. 2021. "The Progestin Medroxyprogesterone Acetate Affects HIV-1 Production in Human Lymphoid Tissue Explants in a Dose-Dependent and Glucocorticoid-like Fashion" Viruses 13, no. 11: 2303. https://doi.org/10.3390/v13112303
APA StyleVanpouille, C., Günaydın, G., Jangard, M., Clerici, M., Margolis, L., Broliden, K., & Introini, A. (2021). The Progestin Medroxyprogesterone Acetate Affects HIV-1 Production in Human Lymphoid Tissue Explants in a Dose-Dependent and Glucocorticoid-like Fashion. Viruses, 13(11), 2303. https://doi.org/10.3390/v13112303







