A Rapid Point-of-Care Test for the Serodiagnosis of Hepatitis Delta Virus Infection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Recombinant, Pan-Genotypic HDAg
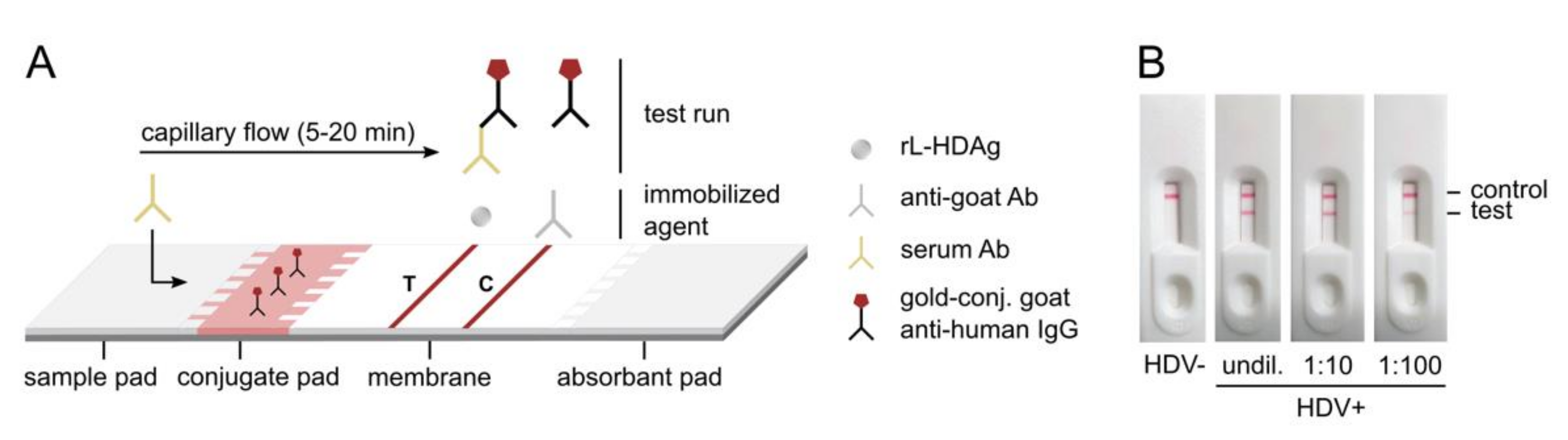
2.2. Preparation of HDV Rapid Test
2.3. Detection of Anti-HDV with the HDV Rapid Test
2.4. Detection of Anti-HDV by Semi-Quantitative ELISA
2.5. Study Population
2.6. Statistics
3. Results
3.1. Development of a Recombinant, Pan-Genotypic HDAg
3.2. Development of the HDV Rapid Test and Proof-of-Principle
3.3. Test Validation
3.4. Pan-Genotypic Activity of the HDV Rapid Test and Multiplexing with HBsAg Detection
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fattovich, G.; Boscaro, S.; Noventa, F.; Pornaro, E.; Stenico, D.; Alberti, A.; Ruol, A.; Realdi, G. Influence of hepatitis delta virus infection on progression to cirrhosis in chronic hepatitis type B. J. Infect. Dis. 1987, 155, 931–935. [Google Scholar] [CrossRef]
- Romeo, R.; Del Ninno, E.; Rumi, M.; Russo, A.; Sangiovanni, A.; De Franchis, R.; Ronchi, G.; Colombo, M. A 28-year study of the course of hepatitis Δ infection: A risk factor for cirrhosis and hepatocellular carcinoma. Gastroenterology 2009, 136, 1629–1638. [Google Scholar] [CrossRef] [PubMed]
- Niro, G.A.; Smedile, A.; Ippolito, A.M.; Ciancio, A.; Fontana, R.; Olivero, A.; Valvano, M.R.; Abate, M.L.; Gioffreda, D.; Caviglia, G.P. Outcome of chronic delta hepatitis in Italy: A long-term cohort study. J. Hepatol. 2010, 53, 834–840. [Google Scholar] [CrossRef] [PubMed]
- Buti, M.; Homs, M.; Rodriguez-Frias, F.; Funalleras, G.; Jardí, R.; Sauleda, S.; Tabernero, D.; Schaper, M.; Esteban, R. Clinical outcome of acute and chronic hepatitis delta over time: A long-term follow-up study. J. Viral. Hepat. 2011, 18, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-J.; Kalpana, G.; Goldberg, J.; Mason, W.; Werner, B.; Gerin, J.; Taylor, J. Structure and replication of the genome of the hepatitis delta virus. Proc. Natl. Acad. Sci. USA 1986, 83, 8774–8778. [Google Scholar] [CrossRef] [Green Version]
- Kos, A.; Dijkema, R.; Arnberg, A.; Van der Meide, P.; Schellekens, H. The hepatitis delta (δ) virus possesses a circular RNA. Nature 1986, 323, 558–560. [Google Scholar] [CrossRef] [PubMed]
- Wang, K.-S.; Choo, Q.-L.; Weiner, A.J.; Ou, J.-H.; Najarian, R.C.; Thayer, R.M.; Mullenbach, G.T.; Denniston, K.J.; Gerin, J.L.; Houghton, M. Structure, sequence and expression of the hepatitis delta (δ) viral genome. Nature 1986, 323, 508–514. [Google Scholar] [CrossRef]
- Kuo, M.; Goldberg, J.; Coates, L.; Mason, W.; Gerin, J.; Taylor, J. Molecular cloning of hepatitis delta virus RNA from an infected woodchuck liver: Sequence, structure, and applications. J. Virol. 1988, 62, 1855–1861. [Google Scholar] [CrossRef] [Green Version]
- Luo, G.; Chao, M.; Hsieh, S.; Sureau, C.; Nishikura, K.; Taylor, J. A specific base transition occurs on replicating hepatitis delta virus RNA. J. Virol. 1990, 64, 1021–1027. [Google Scholar] [CrossRef] [Green Version]
- Rizzetto, M.; Canese, M.G.; Arico, S.; Crivelli, O.; Trepo, C.; Bonino, F.; Verme, G. Immunofluorescence detection of new antigen-antibody system (delta/anti-delta) associated to hepatitis B virus in liver and in serum of HBsAg carriers. Gut 1977, 18, 997–1003. [Google Scholar] [CrossRef] [Green Version]
- Yan, H.; Zhong, G.; Xu, G.; He, W.; Jing, Z.; Gao, Z.; Huang, Y.; Qi, Y.; Peng, B.; Wang, H. Sodium taurocholate cotransporting polypeptide is a functional receptor for human hepatitis B and D virus. elife 2012, 1, e00049. [Google Scholar] [CrossRef] [PubMed]
- Ni, Y.; Lempp, F.A.; Mehrle, S.; Nkongolo, S.; Kaufman, C.; Fälth, M.; Stindt, J.; Königer, C.; Nassal, M.; Kubitz, R. Hepatitis B and D viruses exploit sodium taurocholate co-transporting polypeptide for species-specific entry into hepatocytes. Gastroenterology 2014, 146, 1070–1083. [Google Scholar] [CrossRef] [PubMed]
- Aragona, M.; Caredda, F.; Lavarini, C.; Farci, P.; Macagno, S.; Crivelli, O.; Maran, E.; Purcell, R.; Rizzetto, M. Serological response to the hepatitis delta virus in hepatitis D. Lancet 1987, 329, 478–480. [Google Scholar] [CrossRef]
- Fiedler, M.; Roggendorf, M. Immunology of HDV infection. In Hepatitis Delta Virus; Casey, J.L., Ed.; Springer: Berlin/Heidelberg, Germany, 2006; Volume 3, pp. 187–209. [Google Scholar]
- Wedemeyer, H.; Yurdaydin, C.; Dalekos, G.N.; Erhardt, A.; Cakaloglu, Y.; Degertekin, H.; Gürel, S.; Zeuzem, S.; Zachou, K.; Bozkaya, H.; et al. Peginterferon plus Adefovir versus Either Drug Alone for Hepatitis Delta. N. Engl. J. Med. 2011, 364, 322–331. [Google Scholar] [CrossRef] [Green Version]
- Heidrich, B.; Yurdaydın, C.; Kabaçam, G.; Ratsch, B.A.; Zachou, K.; Bremer, B.; Dalekos, G.N.; Erhardt, A.; Tabak, F.; Yalcin, K.; et al. Late HDV RNA relapse after peginterferon alpha-based therapy of chronic hepatitis delta. Hepatology 2014, 60, 87–97. [Google Scholar] [CrossRef]
- World Health Organization. Global Hepatitis Report; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Terrault, N.A.; Bzowej, N.H.; Chang, K.M.; Hwang, J.P.; Jonas, M.M.; Murad, M.H. AASLD guidelines for treatment of chronic hepatitis B. Hepatology 2016, 63, 261–283. [Google Scholar] [CrossRef] [PubMed]
- EASL. Clinical practice guidelines on the management of hepatitis B virus infection. J. Hepatol. 2017, 67, 370–398. [Google Scholar] [CrossRef] [Green Version]
- Le Gal, F.; Brichler, S.; Sahli, R.; Chevret, S.; Gordien, E. First international external quality assessment for hepatitis delta virus RNA quantification in plasma. Hepatology 2016, 64, 1483–1494. [Google Scholar] [CrossRef]
- Le Gal, F.; Dziri, S.; Gerber, A.; Alloui, C.; Ben Abdesselam, Z.; Roulot, D.; Brichler, S.; Gordien, E. Performance Characteristics of a New Consensus Commercial Kit for Hepatitis D Virus RNA Viral Load Quantification. J. Clin. Microbiol. 2017, 55, 431–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Oidovsambuu, O.; Liu, P.; Grosely, R.; Elazar, M.; Winn, V.D.; Fram, B.; Boa, Z.; Dai, H.; Dashtseren, B.; et al. A novel quantitative microarray antibody capture assay identifies an extremely high hepatitis delta virus prevalence among hepatitis B virus–infected mongolians. Hepatology 2017, 66, 1739–1749. [Google Scholar] [CrossRef] [Green Version]
- Kushner, T.; Serper, M.; Kaplan, D.E. Delta hepatitis within the Veterans Affairs medical system in the United States: Prevalence, risk factors, and outcomes. J. Hepatol. 2015, 63, 586–592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manesis, E.K.; Vourli, G.; Dalekos, G.; Vasiliadis, T.; Manolaki, N.; Hounta, A.; Touloumi, G.; Vafiadis, I.; Nikolopoulou, G.; Giannoulis, G. Prevalence and clinical course of hepatitis delta infection in Greece: A 13-year prospective study. J. Hepatol. 2013, 59, 949–956. [Google Scholar] [CrossRef]
- El Bouzidi, K.; Elamin, W.; Kranzer, K.; Irish, D.N.; Ferns, B.; Kennedy, P.; Rosenberg, W.; Dusheiko, G.; Sabin, C.A.; Smith, B.C. Hepatitis delta virus testing, epidemiology and management: A multicentre cross-sectional study of patients in London. J. Clin. Virol. 2015, 66, 33–37. [Google Scholar] [CrossRef] [Green Version]
- Roggenbach, I.; Chi, X.; Lempp, F.A.; Qu, B.; Walter, L.; Wu, R.; Gao, X.; Schnitzler, P.; Ding, Y.; Urban, S.; et al. HDV Seroprevalence in HBsAg-Positive Patients in China Occurs in Hotspots and Is Not Associated with HCV Mono-Infection. Viruses 2021, 13, 1799. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Shen, D.-T.; Ji, D.-Z.; Han, P.-C.; Zhang, W.-M.; Ma, J.-F.; Chen, W.-S.; Goyal, H.; Pan, S.; Xu, H.-G. Prevalence and burden of hepatitis D virus infection in the global population: A systematic review and meta-analysis. Gut 2019, 68, 512–521. [Google Scholar] [CrossRef]
- Stockdale, A.J.; Kreuels, B.; Henrion, M.Y.; Giorgi, E.; Kyomuhangi, I.; de Martel, C.; Hutin, Y.; Geretti, A.M. The global prevalence of hepatitis D virus infection: Systematic review and meta-analysis. J. Hepatol. 2020, 73, 523–532. [Google Scholar] [CrossRef]
- Bazinet, M.; Pântea, V.; Cebotarescu, V.; Cojuhari, L.; Jimbei, P.; Albrecht, J.; Schmid, P.; Le Gal, F.; Gordien, E.; Krawczyk, A.; et al. Safety and efficacy of REP 2139 and pegylated interferon alfa-2a for treatment-naive patients with chronic hepatitis B virus and hepatitis D virus co-infection (REP 301 and REP 301-LTF): A non-randomised, open-label, phase 2 trial. Lancet Gastroenterol. Hepatol. 2017, 12, 877–889. [Google Scholar] [CrossRef]
- Yurdaydin, C.; Keskin, O.; Kalkan, Ç.; Karakaya, F.; Çalişkan, A.; Karatayli, E.; Karatayli, S.; Bozdayi, A.M.; Koh, C.; Heller, T. Optimizing lonafarnib treatment for the management of chronic delta hepatitis: The LOWR HDV-1 study. Hepatology 2018, 67, 1224–1236. [Google Scholar] [CrossRef] [Green Version]
- Wedemeyer, H.; Bogomolov, P.; Blank, A.; Allweiss, L.; Dandri-Petersen, M.; Bremer, B.; Voronkova, N.; Schöneweis, K.; Pathil, A.; Burhenne, J. Final results of a multicenter, open-label phase 2b clinical trial to assess safety and efficacy of Myrcludex B in combination with Tenofovir in patients with chronic HBV/HDV co-infection. J. Hepatol. 2018, 68, 3. [Google Scholar] [CrossRef]
- Rocco, C.; Bonavolta, R.; Vallefuoco, L.; Braschi, U.; Sorrentino, R.; Terracciano, D.; Portella, G. Comparison of anti–hepatitis D virus (HDV) ETI-AB-DELTAK-2 assay and the novel LIAISON® XL MUREX anti-HDV assay in the diagnosis of HDV infection. Diagn. Microbiol. Infect. Dis. 2019, 95, 114873. [Google Scholar] [CrossRef]
- Casaca, A.; Fardilha, M.; da Cruz e Silva, E.; Cunha, C. In Vivo Interaction of the Hepatitis Delta Virus Small Antigen with the ELAV-Like Protein HuR. Open Virol. J. 2011, 5, 12. [Google Scholar] [CrossRef] [PubMed]
- Alves, C.; Cheng, H.; Roder, H.; Taylor, J. Intrinsic disorder and oligomerization of the hepatitis delta virus antigen. Virology 2010, 407, 333–340. [Google Scholar] [CrossRef] [Green Version]
- Alves, C.; Cheng, H.; Tavanez, J.P.; Casaca, A.; Gudima, S.; Roder, H.; Cunha, C. Structural and nucleic acid binding properties of hepatitis delta virus small antigen. World J. Virol. 2017, 6, 26. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Chang, S.C.; Yu, I.-C.; Tsay, Y.-G.; Chang, M.-F. Large hepatitis delta antigen is a novel clathrin adaptor-like protein. J. Virol. 2007, 81, 5985–5994. [Google Scholar] [CrossRef] [PubMed] [Green Version]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lempp, F.A.; Roggenbach, I.; Nkongolo, S.; Sakin, V.; Schlund, F.; Schnitzler, P.; Wedemeyer, H.; Le Gal, F.; Gordien, E.; Yurdaydin, C.; et al. A Rapid Point-of-Care Test for the Serodiagnosis of Hepatitis Delta Virus Infection. Viruses 2021, 13, 2371. https://doi.org/10.3390/v13122371
Lempp FA, Roggenbach I, Nkongolo S, Sakin V, Schlund F, Schnitzler P, Wedemeyer H, Le Gal F, Gordien E, Yurdaydin C, et al. A Rapid Point-of-Care Test for the Serodiagnosis of Hepatitis Delta Virus Infection. Viruses. 2021; 13(12):2371. https://doi.org/10.3390/v13122371
Chicago/Turabian StyleLempp, Florian A., Imme Roggenbach, Shirin Nkongolo, Volkan Sakin, Franziska Schlund, Paul Schnitzler, Heiner Wedemeyer, Frédéric Le Gal, Emmanuel Gordien, Cihan Yurdaydin, and et al. 2021. "A Rapid Point-of-Care Test for the Serodiagnosis of Hepatitis Delta Virus Infection" Viruses 13, no. 12: 2371. https://doi.org/10.3390/v13122371
APA StyleLempp, F. A., Roggenbach, I., Nkongolo, S., Sakin, V., Schlund, F., Schnitzler, P., Wedemeyer, H., Le Gal, F., Gordien, E., Yurdaydin, C., & Urban, S. (2021). A Rapid Point-of-Care Test for the Serodiagnosis of Hepatitis Delta Virus Infection. Viruses, 13(12), 2371. https://doi.org/10.3390/v13122371






