Possible Arbovirus Found in Virome of Melophagus ovinus
Abstract
1. Introduction
2. Materials and Methods
2.1. Collection and Pooling of Melophagus ovinus
2.2. Sample Preparation and High-Throughtput Sequencing
2.3. Sanger Sequencing
2.4. Assembly and Analysis
2.5. Phylogenetics and Visualization
2.6. Virus Passages in Pig Embryo Kidney Cell Line
3. Results
3.1. High-Throughput Sequencing and Detection of Virus-like Contigs
3.2. Iflaviridae—Related Contigs
3.3. Solemoviridae—Related Contigs
3.4. Sigmavirus—Related Contigs
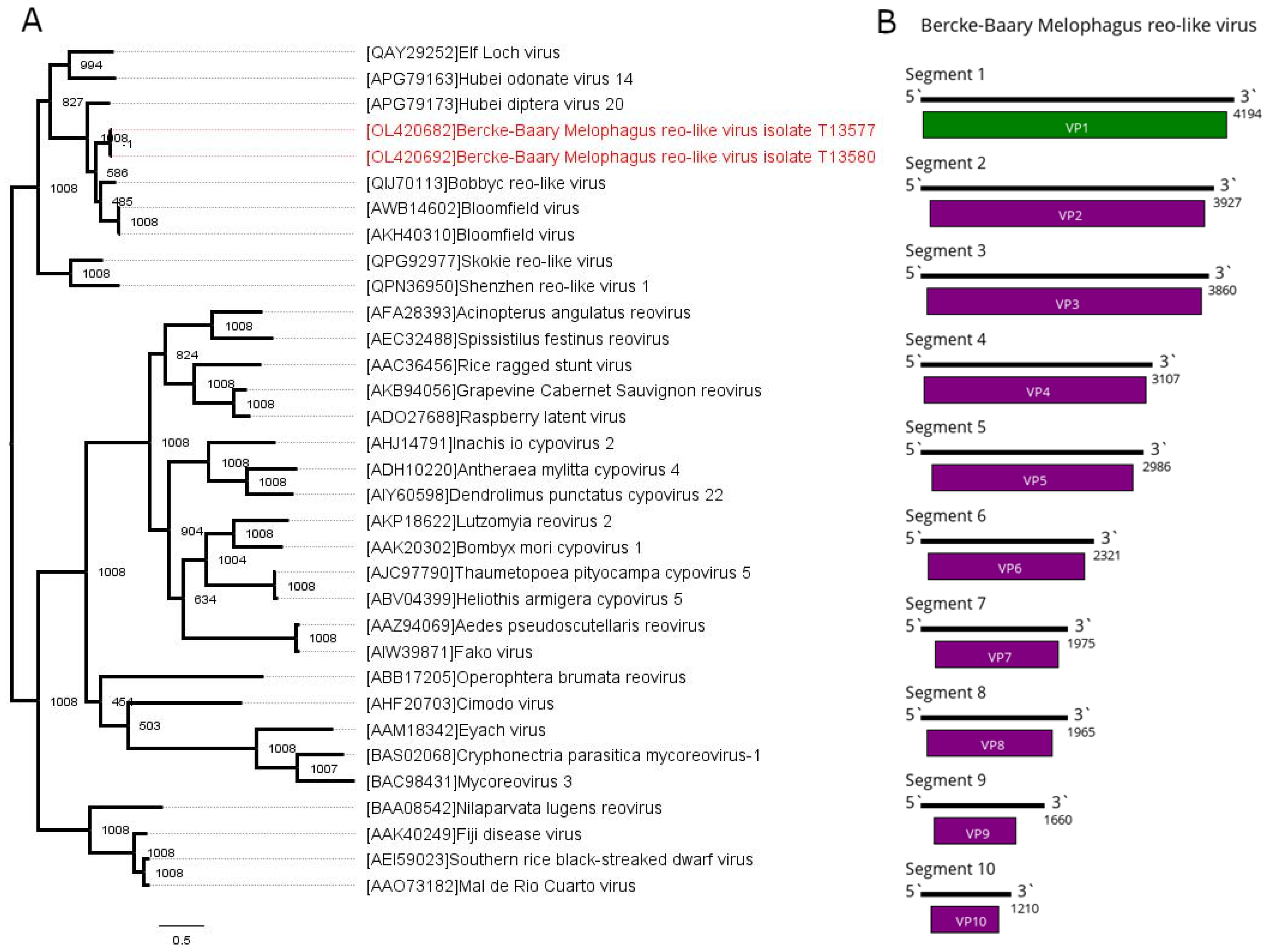
3.5. Reoviridae—Related Contigs
3.6. Multiplication of Viruses in Mammalian Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, C.; Shi, M.; Tian, J.; Lin, X.; Kang, Y.; Chen, L.; Qin, X.; Xu, J.; Holmes, E.C.; Zhang, Y. Unprecedented genomic diversity of RNA viruses in arthropods reveals the ancestry of negative-sense RNA viruses. Elife 2015, 4, e05378. [Google Scholar] [CrossRef]
- Shi, M.; Lin, X.; Tian, J.; Chen, L.; Chen, X.; Li, C.; Qin, X.; Li, J.; Cao, J.; Eden, J.; et al. Redefining the invertebrate RNA virosphere. Nature 2016, 540, 539–543. [Google Scholar] [CrossRef]
- Paraskevopoulou, S.; Ka, S.; Zirkel, F.; Donath, A.; Petersen, M.; Liu, S.; Zhou, X.; Drosten, C.; Misof, B.; Junglen, S. Viromics of extant insect orders unveil the evolution of the flavi-like superfamily. Virus 2021, 7, veab030. [Google Scholar] [CrossRef]
- Dolja, V.V.; Koonin, E.V. Metagenomics reshapes the concepts of RNA virus evolution by revealing extensive horizontal virus transfer. Virus Res. 2018, 244, 36–52. [Google Scholar] [CrossRef]
- Young, P.R. Arboviruses: A Family on the Move. In Dengue and Zika: Control and Antiviral Treatment Strategies; Hilgenfield, R., Vasudevan, S., Eds.; Springer: Singapore, 2018; pp. 1–10. [Google Scholar]
- Marseille, R.; Nebbak, A.; Monteil-bouchard, S.; Berenger, J.; Almeras, L.; Parola, P.; Desnues, C. Virome Diversity among Mosquito Populations in a Sub-Urban Region of Marseille, France. Viruses 2021, 13, 768. [Google Scholar]
- Atoni, E.; Wang, Y.; Karungu, S.; Waruhiu, C.; Zohaib, A.; Obanda, V.; Agwanda, B.; Mutua, M.; Xia, H.; Yuan, Z. Metagenomic Virome Analysis of Culex Mosquitoes from Kenya and China. Viruses 2018, 10, 30. [Google Scholar] [CrossRef] [PubMed]
- Hameed, M.; Wahaab, A.; Shan, T.; Wang, X.; Khan, S.; Di, D.; Xiqian, L.; Zhang, J.-J.; Anwar, M.N.; Nawaz, M.; et al. A Metagenomic Analysis of Mosquito Virome Collected From Different Animal Farms at Yunnan—Myanmar Border of China. Front. Microbiol. 2021, 11, 11. [Google Scholar] [CrossRef] [PubMed]
- Harvey, E.; Rose, K.; Eden, J.; Lo, N.; Abeyasuriya, T.; Shi, M.; Doggett, S.L.; Holmes, E.C. Extensive Diversity of RNA Viruses in Australian Ticks. J. Virol. 2019, 93, e01358-18. [Google Scholar] [CrossRef] [PubMed]
- Pettersson, J.H.; Shi, M.; Bohlin, J.; Eldhol, V.; Brynildsrud, O.B.; Paulsen, K.M.; Andreassen, Å.; Holmes, E.C. Characterizing the virome of Ixodes ricinus ticks from northern Europe. Sci. Rep. 2017, 7, 10870. [Google Scholar] [CrossRef] [PubMed]
- Tetley, J.H. The sheep ked, Melophagus ovinus L. I. Dissemination potential. Parasitology 1958, 48, 353–363. [Google Scholar] [CrossRef]
- Karbowiak, G.; Demiaszkiewicz, A.W.; Pyziel, A.M.; Wita, I.; Moskwa, B.; Werszko, J.; Bień, J.; Goździk, K.; Lachowicz, J.; Cabaj, W. The parasitic fauna of the European bison (Bison bonasus) (Linnaeus, 1758) and their impact on the conservation. Part 1 The summarising list of parasites noted. Acta Parasitol. 2014, 59, 363–371. [Google Scholar] [CrossRef] [PubMed]
- Sertse, T.; Wossene, A. Effect of ectoparasites on quality of pickled skins and their impact on the tanning industries in Amhara regional state, Ethiopia. Small Rumin. Res. 2007, 69, 55–61. [Google Scholar] [CrossRef]
- Jiang, B.-G.; Qiu, E.-C.; Zhang, F.; Zuo, S.-Q.; Yang, H.; Liu, W.; Cao, W.-C. Borrelia burgdorferi sensu lato in sheep keds (Melophagus ovinus), Tibet, China. Vet. Microbiol. 2011, 149, 526–529. [Google Scholar] [CrossRef]
- Zhang, Q.; Wang, Y.; Li, Y.; Han, S.; Wang, B.; Yuan, G.; Zhang, P.; Yang, Z.; Wang, S.; Chen, J.; et al. Vector-Borne Pathogens with Veterinary and Public Health Significance in Melophagus ovinus (Sheep Ked) from the Qinghai-Tibet Plateau. Pathogens 2021, 10, 249. [Google Scholar] [CrossRef]
- Zhao, L.; He, B.; Li, K.; Li, F.; Zhang, L.; Li, X.; Liu, Y. First report of Anaplasma ovis in pupal and adult Melophagus ovinus (sheep ked) collected in South Xinjiang, China. Parasit. Vectors 2018, 11, 258. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; He, B.; Li, F.; Li, K.; Zhang, L.; Li, X.; Zhao, L. Molecular Identification of Bartonella melophagi and Wolbachia Supergroup F from Sheep Keds in Xinjiang, China. Korean J. Parasitol. 2018, 56, 365–370. [Google Scholar] [CrossRef]
- Hao, L.; Yuan, D.; Li, S.; Jia, T.; Guo, L.; Hou, W.; Lu, Z.; Mo, X.; Yin, J.; Yang, A. Detection of Theileria spp. in ticks, sheep keds (Melophagus ovinus), and livestock in the eastern Tibetan Plateau, China. Parasitol. Res. 2020, 119, 2641–2648. [Google Scholar] [CrossRef]
- Martinkovic, F.; Matanovic, K.; Rodrigues, A.C.; Garcia, H.A.; Teixeira, M.M.G. Trypanosoma (Megatrypanum) melophagium in the Sheep Ked Melophagus ovinus from Organic Farms in Croatia: Phylogenetic Inferences Support Restriction to Sheep and Sheep Keds and Close Relationship with Trypanosomes from Other Ruminant Species. J. Eukaryot. Microbiol. 2012, 59, 134–144. [Google Scholar] [CrossRef]
- Liu, D.; Wang, Y.; Zhang, H.; Liu, Z.; Wureli, H.; Wang, S.; Tu, C.; Chen, C. First report of Rickettsia raoultii and R. slovaca in Melophagus ovinus, the sheep ked. Parasit. Vectors 2016, 9, 600. [Google Scholar] [CrossRef][Green Version]
- Duan, D.Y.; Zhou, H.M.; Cheng, T.Y. Comparative analysis of microbial community in the whole body and midgut from fully engorged and unfed female adult Melophagus ovinus. Vet. Entomol. 2019, 34, 215–224. [Google Scholar] [CrossRef]
- Liu, Y.-H.; He, B.; Li, K.; Li, F.; Zhang, L.; Li, X.; Zhao, L. First report of border disease virus in Melophagus ovinus (sheep ked) collected in Xinjiang, China. PLoS ONE 2019, 14, e0221435. [Google Scholar] [CrossRef] [PubMed]
- Luedke, A.J.; Jochim, M.M.; Bowne, J.G. Preliminary Bluetongue Transmission with the Sheep Ked Melophagus Ovinus. Can. J. Comp. Med. Vet. Sci. 1965, 29, 9–11. [Google Scholar]
- Setién, Á.A.; Baltazar, A.G.; Leyva, I.O.; Rojas, M.S.; Koldenkova, V.P.; Garcia, M.P.-P.; Ceballos, N.A.; Romero, G.G.; Villegas, E.O.L.; Malacara, J.B.M.; et al. Ectoparasitic hematophagous dipters: Potential reservoirs of dengue virus? Gac. Med. Mex. 2017, 153, 2–10. [Google Scholar]
- Ramirez-Martinez, M.; Bennett, A.J.; Dunn, C.D.; Yuill, T.M.; Goldberg, T.L. Bat Flies of the Family Streblidae (Diptera: Hippoboscoidea) Host Relatives of Medically and Agriculturally Important. Viruses 2021, 13, 860. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular Evolutionary Genetics Analysis across Computing Platforms. Mol. Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef]
- Okonechnikov, K.; Golosova, O.; Fursov, M.; The UGENE Team. Unipro UGENE: A unified bioinformatics toolkit. Bioinformatics 2012, 28, 1166–1167. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2013, 9, 357–359. [Google Scholar] [CrossRef]
- Kazutaka, K.; Standley, D.M. Standley MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar]
- Capella-Gutierrez, S.; Silla-Martinez, J.M.; Gabaldon, T. trimAl: A tool for automated alignment trimming in large-scale phylogenetic analyses. Bioinformatics 2009, 25, 1972–1973. [Google Scholar] [CrossRef] [PubMed]
- Guindon, S.; Gascuel, O. PhyML: “A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood”. Syst. Biol. 2003, 52, 696–704. [Google Scholar] [CrossRef] [PubMed]
- Valles, S.M.; Chen, Y.; Firth, A.E.; Gu, D.M.A.; Hashimoto, Y.; Herrero, S.; De Miranda, J.R.; Ryabov, E. ICTV Virus Taxonomy Profile: Iflaviridae. J. Gen. Virol. 2017, 527–528. [Google Scholar] [CrossRef]
- Calla, B.; Hall, B.; Hou, S.; Geib, S.M. A genomic perspective to assessing quality of mass-reared SIT flies used in Mediterranean fruit fly (Ceratitis capitata) eradication in California. BMC Genom. 2014, 15, 98. [Google Scholar] [CrossRef] [PubMed]
- Webster, C.L.; Longdon, B.; Lewis, S.H.; Obbard, D.J. Twenty-Five New Viruses Associated with the Drosophilidae (Diptera). Evol. Bioinform. 2016, 12, 13–25. [Google Scholar] [CrossRef]
- Sharpe, S.R.; Morrow, J.L.; Brettell, L.E.; Shearman, D.C.; Gilchrist, S.; Cook, J.M.; Riegler, M. Tephritid fruit flies have a large diversity of co-occurring RNA viruses. J. Invertebr. Pathol. 2021, 107569. [Google Scholar] [CrossRef] [PubMed]
- Remnant, E.J.; Baty, J.W.; Bulgarella, M.; Dobelmann, J.; Quinn, O.; Gruber, M.A.M.; Lester, P.J. A Diverse Viral Community from Predatory Wasps in Their Native and Invaded Range, with a New Virus Infectious to Honey Bees. Viruses 2021, 13, 1431. [Google Scholar] [CrossRef]
- Ju, H.; Lim, H.; Domier, L.L. Soybean Thrips (Thysanoptera: Thripidae) Harbor Highly Diverse Populations of Arthropod, Fungal and Plant Viruses. Viruses 2020, 12, 1376. [Google Scholar]
- Webster, C.L.; Waldron, F.M.; Robertson, S.; Crowson, D.; Ferrari, G.; Quintana, J.F.; Brouqui, J.M.; Bayne, E.H.; Longdon, B.; Buck, A.H.; et al. The discovery, distribution, and evolution of viruses associated with Drosophila melanogaster. PLoS Biol. 2015, 13, e1002210. [Google Scholar] [CrossRef]
- Medd, N.C.; Fellous, S.; Waldron, F.M.; Xue, A.; Nakai, M.; Cross, J.V.; Obbard, D.J. The virome of Drosophila suzukii, an invasive pest of soft fruit. Virus Evol. 2018, 4, vey009. [Google Scholar] [CrossRef]
- Mahar, J.E.; Shi, M.; Hall, R.N.; Strive, T.; Holmesa, E.C. Comparative Analysis of RNA Virome Composition in Rabbits and Associated Ectoparasites. J. Virol. 2020, 94, e02119-19. [Google Scholar] [CrossRef]
- Dalmon, A.; Gayral, P.; Decante, D.; Klopp, C.; Bigot, D.; Thomasson, M.; Herniou, E.A.; Alaux, C.; Conte, Y. Le Viruses in the Invasive Hornet Vespa velutina. Viruses 2019, 11, 1041. [Google Scholar] [CrossRef] [PubMed]
- Longdon, B.; Murray, G.G.R.; Palmer, W.J.; Day, J.P.; Parker, D.J.; Welch, J.J.; Obbard, D.J.; Jiggins, F.M. The evolution, diversity, and host associations of rhabdoviruses. Virus Evol. 2015, 1, vev014. [Google Scholar] [CrossRef] [PubMed]
- King, A.M.Q.; Adams, M.J.; Carstens, E.B.; Lefkowitz, E.J. Family Reoviridae. In Virus Taxonomy: Ninth Report of the International Committee on Taxonomy of Viruses; Elsevier: San Diego, CA, USA, 2011; pp. 541–637. ISBN 9780123846846. [Google Scholar]
- Dedkov, V.G.; Dolgova, A.S.; Safonova, M.V.; Samoilov, A.E.; Belova, O.A.; Kholodilov, I.S.; Matsvay, A.D.; Speranskaya, A.S.; Khafizov, K.; Karganova, G.G. Isolation and characterization of Wad Medani virus obtained in the tuva Republic of Russia. Ticks Tick. Borne. Dis. 2021, 12, 101612. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.-C.; Shi, M.; Tian, J.-H.; Lin, X.-D.; Gao, D.-Y.; He, J.-R.; Wang, J.-B.; Li, C.-X.; Kang, Y.-J.; Yu, B.; et al. A tick-borne segmented RNA virus contains genome segments derived from unsegmented viral ancestors. Proc. Natl. Acad. Sci. USA 2014, 111, 6744–6749. [Google Scholar] [CrossRef] [PubMed]
- Jia, N.; Liu, H.B.; Ni, X.B.; Bell-Sakyi, L.; Zheng, Y.C.; Song, J.L.; Li, J.; Jiang, B.G.; Wang, Q.; Sun, Y.; et al. Emergence of human infection with Jingmen tick virus in China: A retrospective study. EBioMedicine 2019, 43, 317–324. [Google Scholar] [CrossRef] [PubMed]
- Kholodilov, I.S.; Belova, O.A.; Morozkin, E.S.; Litov, A.G.; Ivannikova, A.Y.; Makenov, M.T.; Shchetinin, A.M.; Aibulatov, S.V.; Bazarova, G.K.; Bell-sakyi, L.; et al. Geographical and Tick-Dependent Distribution of Flavi-Like Alongshan and Yanggou Tick Viruses in Russia. Viruses 2021, 13, 458. [Google Scholar] [CrossRef]
- Kholodilov, I.S.; Litov, A.G.; Klimentov, A.S.; Belova, O.A.; Polienko, A.E.; Nikitin, N.A.; Shchetinin, A.M.; Ivannikova, A.Y.; Bell-sakyi, L.; Yakovlev, A.S.; et al. Isolation and Characterisation of Alongshan Virus in Russia. Viruses 2020, 12, 362. [Google Scholar] [CrossRef]
- Moureau, G.; Cook, S.; Lemey, P.; Nougairede, A.; Forrester, L.; Khasnatinov, M.; Charrel, R.N.; Firth, A.E.; Gould, E.A.; de Lamballerie, X. New Insights into Flavivirus Evolution, Taxonomy and Biogeographic History, Extended by Analysis of Canonical and Alternative Coding Sequences. PLoS ONE 2015, 10, e0117849. [Google Scholar] [CrossRef]
- Karabatos, N. International Catalogue of Arboviruses; American Society of Tropical Medicine and Hygiene: San Antonio, TX, USA, 1985. [Google Scholar]
- Longdon, B.; Wilfert, L.; Obbard, D.J.; Jiggins, F.M. Rhabdoviruses in Two Species of Drosophila: Vertical Transmission and a Recent Sweep. Gebetics 2011, 188, 141–150. [Google Scholar] [CrossRef]
- Aznar-lopez, C.; Vazquez-moron, S.; Marston, D.A.; Juste, J.; Iba, C.; Berciano, J.M.; Salsamendi, E.; Aihartza, J.; Banyard, A.C.; Mcelhinney, L.; et al. Detection of rhabdovirus viral RNA in oropharyngeal swabs and ectoparasites of Spanish bats. J. Gen. Virol. 2013, 94, 69–75. [Google Scholar] [CrossRef] [PubMed]



| Pool Number | Sheep Number | Specimen Number in the Pool | Location | Collection Date |
|---|---|---|---|---|
| 20 | 1 | 5 | 50.73376, 92.26536 | 2010 |
| 21 | 1 | 6 | 50.73376, 92.26536 | 2010 |
| 22 | 2 | 5 | 50.73376, 92.26536 | 2010 |
| 23 | 3 | 3 | 50.73727, 92.26536 | 2012 |
| 24 | 3 | 2 | 50.73727, 92.26536 | 2012 |
| Pool Number | Number of Reads after Filtering | KMIV 1 | UMSV 2 | BKUMSV 3 | ADMSV 4 | BBMRV 5 | Virus Reads Total |
|---|---|---|---|---|---|---|---|
| 20 | 7 852 210 | 0.14% | 1.17% | 1.03% | 0.15% | 2.49% | |
| 21 | 8 033 216 | 0.02% | 0.77% | 0.41% | 2.42% | 3.62% | |
| 22 | 6 958 480 | 0.07% | 0.63% | 0.43% | 1.97% | 3.10% | |
| 23 | 7 527 561 | 0.09% | 0.97% | 0.46% | 4.76% | 19.36% | 25.64% |
| 24 | 4 622 179 | 0.12% | 0.47% | 1.07% | 2.35% | 54.33% | 58.34% |
| Ked Suspension | Pool Number | Viruses Detected in the 1st Passage | Viruses Detected in the 2nd Passage | Viruses Detected in the 3rd Passage 2 | |
|---|---|---|---|---|---|
| Number | Viruses Detected 1 | ||||
| 7456 | no | 20 | no | KMIV | no |
| 7457 | KMIV | no | no | nd | |
| 7458 | UMSV | no | no | nd | |
| 7459 | UMSV | no | no | nd | |
| 7460 | UMSV | no | no | nd | |
| 7461 | no | 21 | no | no | nd |
| 7462 | no | no | no | nd | |
| 7463 | no | no | no | nd | |
| 7464 | ADMSV/UMSV | no | no | nd | |
| 7465 | no | no | no | nd | |
| 7466 | nd | no | no | nd | |
| 7473 | no | 22 | no | ADMSV | no |
| 7474 | ADMSV | ADMSV | ADMSV | ADMSV | |
| 7475 | no | no | no | nd | |
| 7476 | UMSV/BKUMSV | no | no | nd | |
| 7477 | ADMSV/UMSV/BKUMSV | ADMSV | ADMSV | no | |
| 13577 | no | 23 | no | no | nd |
| 13578 | BBMRV/ADMSV/KMIV/UMSV | BBMRV/ADMSV | no | no | |
| 13579 | BBMRV/ADMSV/KMIV/UMSV | ADMSV | ADMSV/KMIV | no | |
| 13580 | nd | 24 | all | KMIV | nd |
| 13581 | no | ADMSV/KMIV | no | nd | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Litov, A.G.; Belova, O.A.; Kholodilov, I.S.; Gadzhikurbanov, M.N.; Gmyl, L.V.; Oorzhak, N.D.; Saryglar, A.A.; Ishmukhametov, A.A.; Karganova, G.G. Possible Arbovirus Found in Virome of Melophagus ovinus. Viruses 2021, 13, 2375. https://doi.org/10.3390/v13122375
Litov AG, Belova OA, Kholodilov IS, Gadzhikurbanov MN, Gmyl LV, Oorzhak ND, Saryglar AA, Ishmukhametov AA, Karganova GG. Possible Arbovirus Found in Virome of Melophagus ovinus. Viruses. 2021; 13(12):2375. https://doi.org/10.3390/v13122375
Chicago/Turabian StyleLitov, Alexander G., Oxana A. Belova, Ivan S. Kholodilov, Magomed N. Gadzhikurbanov, Larissa V. Gmyl, Natalia D. Oorzhak, Anna A. Saryglar, Aydar A. Ishmukhametov, and Galina G. Karganova. 2021. "Possible Arbovirus Found in Virome of Melophagus ovinus" Viruses 13, no. 12: 2375. https://doi.org/10.3390/v13122375
APA StyleLitov, A. G., Belova, O. A., Kholodilov, I. S., Gadzhikurbanov, M. N., Gmyl, L. V., Oorzhak, N. D., Saryglar, A. A., Ishmukhametov, A. A., & Karganova, G. G. (2021). Possible Arbovirus Found in Virome of Melophagus ovinus. Viruses, 13(12), 2375. https://doi.org/10.3390/v13122375






