Past and Future of Phage Therapy and Phage-Derived Proteins in Patients with Bone and Joint Infection
Abstract
:1. Background
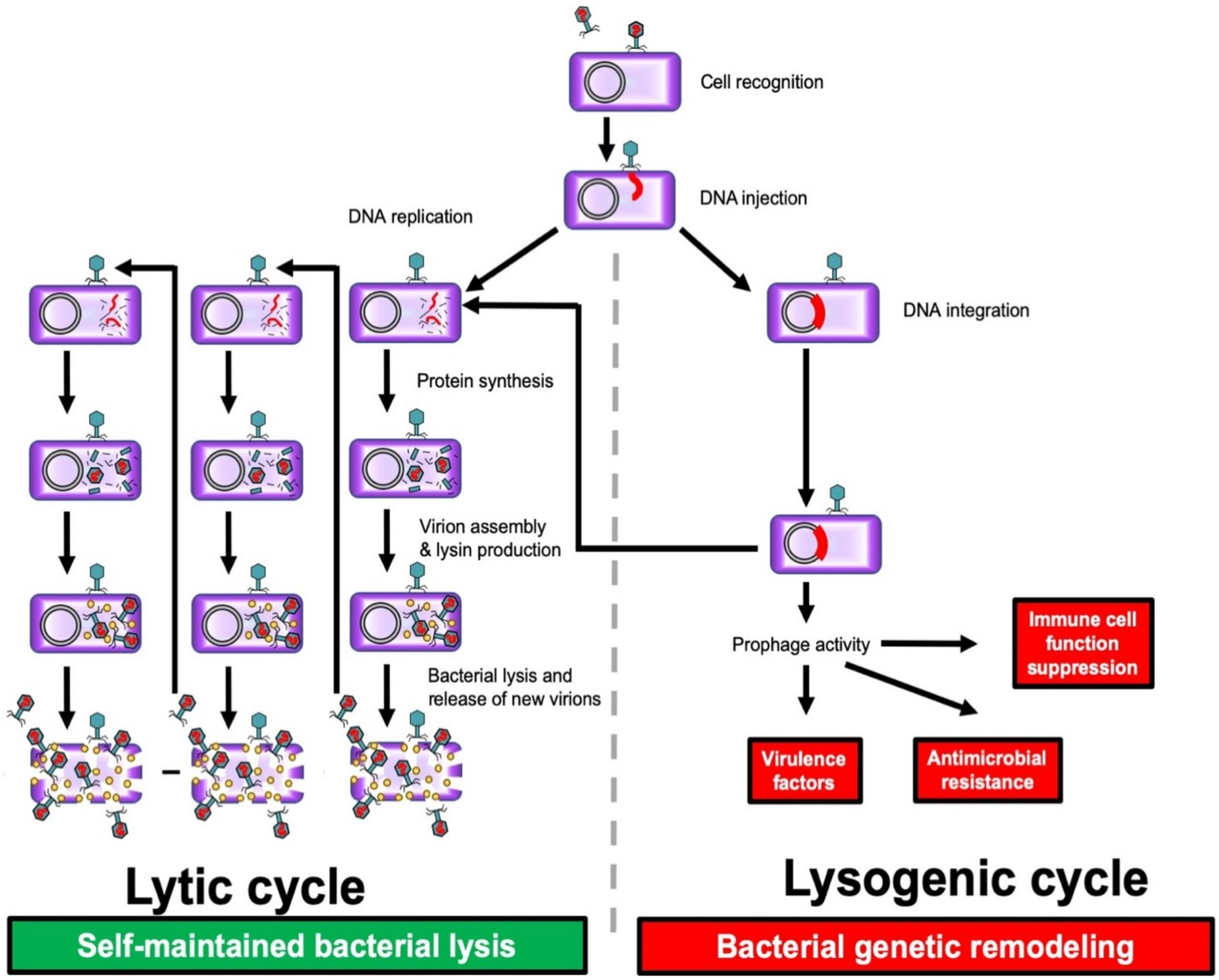
1.1. Bacteriophage Life Circle
1.2. Antibiotics and Phages as Two Different but Synergistic Products That Target Bacteria in the Environment, the Combination of the Two Limiting the Risk of Acquisition of Antimicrobial and Phage Resistance
2. Lessons Learned from the History of Phage Therapy
2.1. F. d’Hérelle
2.2. R.N. Smith and E.W. Schultz
2.3. A. Raiga-Clémenceau
2.4. Lyon Pasteur Institute and the Infectious Diseases Clinic of the Lyon Croix-Rousse Hospital
2.5. Eastern Experience in the Eliava Institute in Georgia, in the USSR and in the Hirszfeld Institute in Poland
2.6. Contemporary Period
2.6.1. Villeneuve Saint Georges Team
2.6.2. Pherecydes Pharma
2.6.3. Compassionate Use of Phages
2.6.4. Creation of a Dedicated Program “PHAGEinLYON” for Phage Therapy in CRIOAc Lyon
2.6.5. Creation of the San Diego (CA) Center for Innovative Phage Applications and Therapeutics (IPATH)
3. Is Bone and Joint Infection a Relevant Indication for Phage Therapy?
4. Is Phage Therapy Feasible in the 2020s in Patients with Bone and Joint Infection?
4.1. Obtaining Pharmaceutical-Quality Phages
4.1.1. Classification
4.1.2. Quality Controls to Be Performed
4.2. Multidisciplinary Clinical Expertise
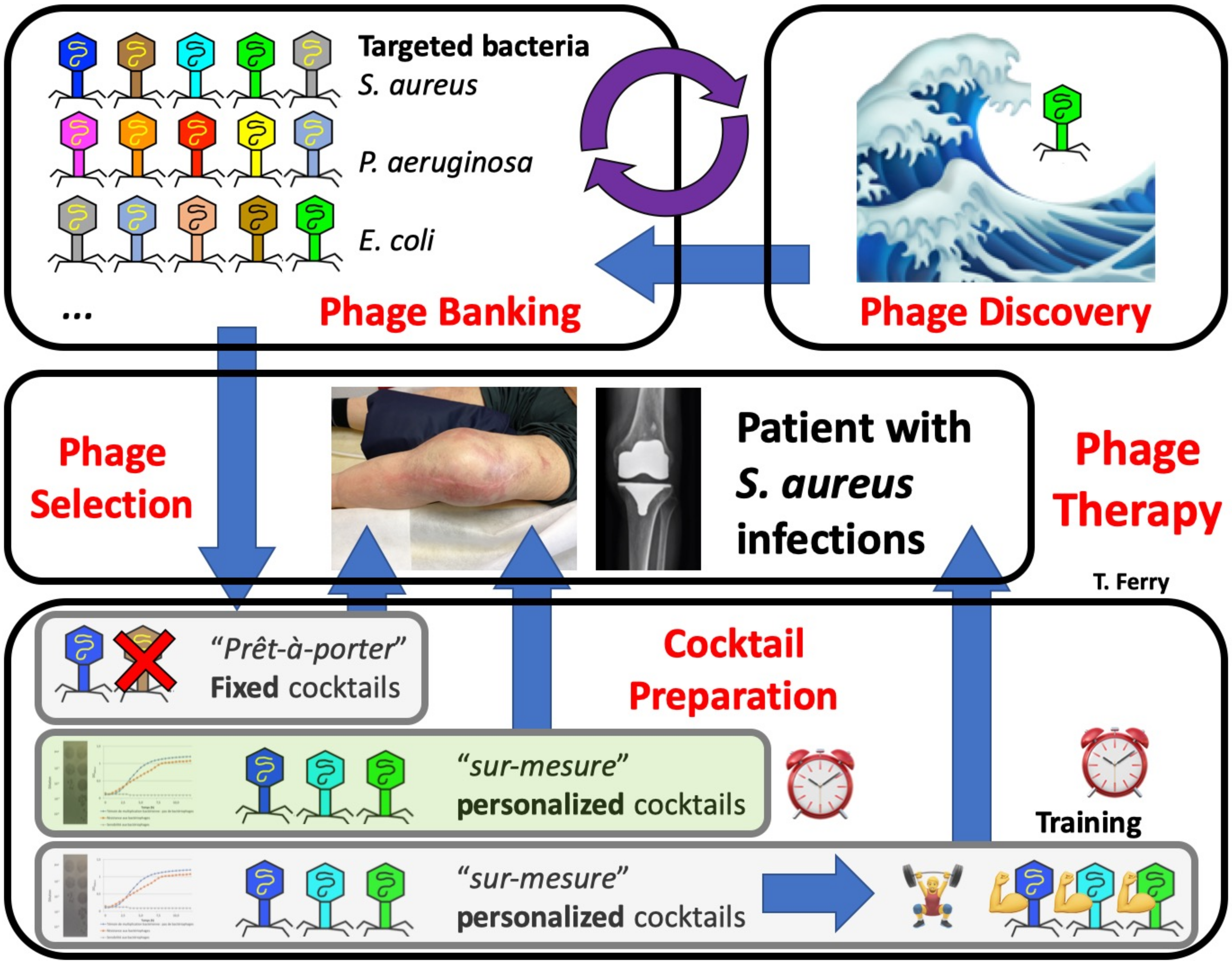
5. What Are the Perspectives for Phage Therapy 2.0?
5.1. Compassionate Cases and Relevant Indications in Bone and Joint Infection
5.2. Availability of Large Industrial or Academic Panels of Purified Pharmaceutical-Grade Therapeutic Phages (i.e., Following Good Manufacturing Practice Guidelines or Being “GMP-like”)
5.3. Performance of Clinical Trials
6. What Are Phage-Derived Proteins?
7. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ferry, T.; Kolenda, C.; Gustave, C.-A.; Lustig, S.; Josse, J.; Batailler, C.; Pirot, F.; Leboucher, G.; Laurent, F. Phagothérapie pour les patients présentant une infection osteoarticulaire: Historique, fondements, faisabilité et perspectives en France. Virologie 2020, 24, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Summers, W.C. Bacteriophage Therapy. Annu. Rev. Microbiol. 2001, 55, 437–451. [Google Scholar] [CrossRef] [Green Version]
- Ackermann, H.-W. 5500 Phages Examined in the Electron Microscope. Arch. Virol. 2007, 152, 227–243. [Google Scholar] [CrossRef] [PubMed]
- Bergh, Ø.; Børsheim, K.Y.; Bratbak, G.; Heldal, M. High Abundance of Viruses Found in Aquatic Environments. Nature 1989, 340, 467–468. [Google Scholar] [CrossRef] [PubMed]
- Jernberg, C.; Löfmark, S.; Edlund, C.; Jansson, J.K. Long-Term Impacts of Antibiotic Exposure on the Human Intestinal Microbiota. Microbiology 2010, 156, 3216–3223. [Google Scholar] [CrossRef] [Green Version]
- Knecht, L.E.; Veljkovic, M.; Fieseler, L. Diversity and Function of Phage Encoded Depolymerases. Front. Microbiol. 2020, 10, 2949. [Google Scholar] [CrossRef]
- Walsh, C. Where Will New Antibiotics Come From? Nat. Rev. Microbiol. 2003, 1, 65–70. [Google Scholar] [CrossRef]
- Magiorakos, A.-P.; Srinivasan, A.; Carey, R.B.; Carmeli, Y.; Falagas, M.E.; Giske, C.G.; Harbarth, S.; Hindler, J.F.; Kahlmeter, G.; Olsson-Liljequist, B.; et al. Multidrug-Resistant, Extensively Drug-Resistant and Pandrug-Resistant Bacteria: An International Expert Proposal for Interim Standard Definitions for Acquired Resistance. Clin. Microbiol. Infect. 2012, 18, 268–281. [Google Scholar] [CrossRef] [Green Version]
- Rostøl, J.T.; Marraffini, L. (Ph)Ighting Phages: How Bacteria Resist Their Parasites. Cell Host Microbe 2019, 25, 184–194. [Google Scholar] [CrossRef] [Green Version]
- Torres-Barceló, C. The Disparate Effects of Bacteriophages on Antibiotic-Resistant Bacteria. Emerg. Microbes Infect. 2018, 7, 168. [Google Scholar] [CrossRef]
- Torres-Barceló, C.; Hochberg, M.E. Evolutionary Rationale for Phages as Complements of Antibiotics. Trends Microbiol. 2016, 24, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Tagliaferri, T.L.; Jansen, M.; Horz, H.-P. Fighting Pathogenic Bacteria on Two Fronts: Phages and Antibiotics as Combined Strategy. Front. Cell. Infect. Microbiol. 2019, 9, 22. [Google Scholar] [CrossRef]
- Gu Liu, C.; Green, S.I.; Min, L.; Clark, J.R.; Salazar, K.C.; Terwilliger, A.L.; Kaplan, H.B.; Trautner, B.W.; Ramig, R.F.; Maresso, A.W. Phage-Antibiotic Synergy Is Driven by a Unique Combination of Antibacterial Mechanism of Action and Stoichiometry. mBio 2020, 11, e01462-20. [Google Scholar] [CrossRef]
- D’Herelle, F. Bacteriophage as a Treatment in Acute Medical and Surgical Infections. Bull. N. Y. Acad. Med. 1931, 7, 329–348. [Google Scholar] [PubMed]
- Straub, M.E. Studies on commercial bacteriophage products. JAMA 1933, 100, 110. [Google Scholar] [CrossRef]
- Eaton, M.D.; Bayne-Jones, S. Bacteriophage therapy: Review of the principles and results of the use of bacteriophage in the treatment of infections. JAMA 1934, 103, 1847. [Google Scholar] [CrossRef]
- Krueger, A.P.; Scribner, E.J. The bacteriophage: Its nature and its therapeutic use. JAMA 1941, 116, 2160. [Google Scholar] [CrossRef]
- Schultz, E.W. Bacteriophage as a Therapeutic Agent in Genito-Urinary Infections. Cal. West Med. 1932, 36, 33–37. [Google Scholar] [PubMed]
- Smith, R.N. Advanced Treatment in Postoperative Ileus. Am. J. Surg. 1933, 19, 272–274. [Google Scholar] [CrossRef]
- Raiga, A. Le Bactériophage et Ses Applications Thérapeutiques; La Science Médicale Pratique: Paris, France, 1932. [Google Scholar]
- Sedallian, P.; Bertoye, A.; Gauthier, J.; Muller, J.M.; Courtieu, A.L. Suppurative meningitis caused by Escherichia coli treated by an intraspinal adapted bacteriophage. Lyon Med. 1958, 90, 509–512. [Google Scholar]
- Bertoye, A.; Gaillard, L.; Courtieu, A.L. Adapted bacteriophages in the treatment of infections caused by antibiotic-resistant microorganisms. J. Med. Lyon 1959, 40, 465–471. [Google Scholar]
- Bertoye, A.; Courtieu, A.L. Treatment of infections caused by pyocyanic bacilli with bacteriophages adapted by selection. J. Med. Lyon 1960, 41, 739–751. [Google Scholar]
- De Monclos, H. Les bactériophages thérapeutiques: De l’empirisme à la biologie moléculaire. Pyrexie 2002, 6, 77–80. [Google Scholar]
- Lang, G.; Kehr, P.; Mathevon, H.; Clavert, J.M.; Séjourne, P.; Pointu, J. Bacteriophage therapy of septic complications of orthopaedic surgery. Rev. Chir. Orthop. Reparatrice Appar. Mot. 1979, 65, 33–37. [Google Scholar] [PubMed]
- Kutateladze, M. Experience of the Eliava Institute in Bacteriophage Therapy. Virol. Sin. 2015, 30, 80–81. [Google Scholar] [CrossRef]
- Myelnikov, D. An Alternative Cure: The Adoption and Survival of Bacteriophage Therapy in the USSR, 1922–1955. J. Hist. Med. Allied Sci. 2018, 73, 385–411. [Google Scholar] [CrossRef] [Green Version]
- Żaczek, M.; Weber-Dąbrowska, B.; Międzybrodzki, R.; Łusiak-Szelachowska, M.; Górski, A. Phage Therapy in Poland—A Centennial Journey to the First Ethically Approved Treatment Facility in Europe. Front. Microbiol. 2020, 11, 1056. [Google Scholar] [CrossRef]
- Patey, O.; McCallin, S.; Mazure, H.; Liddle, M.; Smithyman, A.; Dublanchet, A. Clinical Indications and Compassionate Use of Phage Therapy: Personal Experience and Literature Review with a Focus on Osteoarticular Infections. Viruses 2018, 11, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- EudraLex. Good Manufacturing Practice (GMP) Guidelines; European Union: Brussels, Belgium, 2017. [Google Scholar]
- Jault, P.; Leclerc, T.; Jennes, S.; Pirnay, J.P.; Que, Y.-A.; Resch, G.; Rousseau, A.F.; Ravat, F.; Carsin, H.; Le Floch, R.; et al. Efficacy and Tolerability of a Cocktail of Bacteriophages to Treat Burn Wounds Infected by Pseudomonas Aeruginosa (PhagoBurn): A Randomised, Controlled, Double-Blind Phase 1/2 Trial. Lancet Infect. Dis. 2019, 19, 35–45. [Google Scholar] [CrossRef]
- Albac, S.; Medina, M.; Labrousse, D.; Hayez, D.; Bonnot, D.; Anzala, N.; Laurent, F.; Ferry, T.; Dublanchet, A.; Chavanet, P.; et al. Efficacy of Bacteriophages in a Staphylococcus aureus Nondiabetic or Diabetic Foot Infection Murine Model. Antimicrob. Agents Chemother. 2020, 64, e01870-19. [Google Scholar] [CrossRef] [PubMed]
- Kolenda, C.; Josse, J.; Medina, M.; Fevre, C.; Lustig, S.; Ferry, T.; Laurent, F. Evaluation of the Activity of a Combination of Three Bacteriophages Alone or in Association with Antibiotics on Staphylococcus aureus Embedded in Biofilm or Internalized in Osteoblasts. Antimicrob. Agents Chemother. 2020, 64, e02231-19. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. JAMA 2013, 310, 2191–2194. [Google Scholar] [CrossRef] [Green Version]
- Djebara, S.; Maussen, C.; De Vos, D.; Merabishvili, M.; Damanet, B.; Pang, K.W.; De Leenheer, P.; Strachinaru, I.; Soentjens, P.; Pirnay, J.-P. Processing Phage Therapy Requests in a Brussels Military Hospital: Lessons Identified. Viruses 2019, 11, 265. [Google Scholar] [CrossRef] [Green Version]
- ANSM. Comite Scientifique Specialise Temporaire: Phagothérapie—Retour D’expérience et Perspectives; ANSM: Paris, France, 2019. [Google Scholar]
- Ferry, T.; Boucher, F.; Fevre, C.; Perpoint, T.; Chateau, J.; Petitjean, C.; Josse, J.; Chidiac, C.; L’hostis, G.; Leboucher, G.; et al. Innovations for the Treatment of a Complex Bone and Joint Infection Due to XDR Pseudomonas Aeruginosa Including Local Application of a Selected Cocktail of Bacteriophages. J. Antimicrob. Chemother. 2018, 73, 2901–2903. [Google Scholar] [CrossRef] [Green Version]
- Ferry, T.; Leboucher, G.; Fevre, C.; Herry, Y.; Conrad, A.; Josse, J.; Batailler, C.; Chidiac, C.; Medina, M.; Lustig, S.; et al. Salvage Debridement, Antibiotics and Implant Retention (“DAIR”) with Local Injection of a Selected Cocktail of Bacteriophages: Is It an Option for an Elderly Patient With Relapsing Staphylococcus Aureus Prosthetic-Joint Infection? Open Forum Infect. Dis. 2018, 5, ofy269. [Google Scholar] [CrossRef] [Green Version]
- Ferry, T.; Batailler, C.; Brosset, S.; Kolenda, C.; Goutelle, S.; Sappey-Marinier, E.; Josse, J.; Laurent, F.; Lustig, S.; Lyon BJI Study Group. Medical Innovations to Maintain the Function in Patients with Chronic PJI for Whom Explantation Is Not Desirable: A Pathophysiology-, Multidisciplinary-, and Experience-Based Approach. SICOT J. 2020, 6, 26. [Google Scholar] [CrossRef]
- Anthia, M.; Delattre, R.; Dufour, N.; D’humieres, C.; Pons-Kerjean, N.; Bataille, J. Bacteriophages in Clinical Practice: Follow the Guide! From Provision to Administration. 2021. Available online: https://www.researchgate.net/publication/349988684_Bacteriophages_in_clinical_practice_follow_the_guide_From_provision_to_administration (accessed on 22 November 2021).
- Ferry, T.; Batailler, C.; Petitjean, C.; Chateau, J.; Fevre, C.; Forestier, E.; Brosset, S.; Leboucher, G.; Kolenda, C.; Laurent, F.; et al. The Potential Innovative Use of Bacteriophages within the DAC® Hydrogel to Treat Patients with Knee Megaprosthesis Infection Requiring “Debridement Antibiotics and Implant Retention” and Soft Tissue Coverage as Salvage Therapy. Front. Med. 2020, 7, 342. [Google Scholar] [CrossRef]
- Ferry, T.; Kolenda, C.; Batailler, C.; Gustave, C.-A.; Lustig, S.; Malatray, M.; Fevre, C.; Josse, J.; Petitjean, C.; Chidiac, C.; et al. Phage Therapy as Adjuvant to Conservative Surgery and Antibiotics to Salvage Patients with Relapsing S. aureus Prosthetic Knee Infection. Front. Med. 2020, 7, 570572. [Google Scholar] [CrossRef] [PubMed]
- Ferry, T.; Kolenda, C.; Briot, T.; Lustig, S.; Leboucher, G.; Laurent, F.; Lyon BJI study group; PHAGEinLYON Study Group. Implementation of a Complex Bone and Joint Infection Phage Therapy Centre in France: Lessons to Be Learned after 4 Years’ Experience. Clin. Microbiol. Infect. 2021, S1198-743X(21)00556–5. [Google Scholar] [CrossRef] [PubMed]
- Aslam, S.; Lampley, E.; Wooten, D.; Karris, M.; Benson, C.; Strathdee, S.; Schooley, R.T. Lessons Learned From the First 10 Consecutive Cases of Intravenous Bacteriophage Therapy to Treat Multidrug-Resistant Bacterial Infections at a Single Center in the United States. Open Forum Infect. Dis. 2020, 7, ofaa389. [Google Scholar] [CrossRef] [PubMed]
- Pirnay, J.-P.; Ferry, T.; Resch, G. Recent Progress towards the Implementation of Phage Therapy in Western Medicine. FEMS Microbiol. Rev. 2021, fuab040. [Google Scholar] [CrossRef] [PubMed]
- Josse, J.; Valour, F.; Maali, Y.; Diot, A.; Batailler, C.; Ferry, T.; Laurent, F. Interaction between Staphylococcal Biofilm and Bone: How Does the Presence of Biofilm Promote Prosthesis Loosening? Front. Microbiol. 2019, 10, 1602. [Google Scholar] [CrossRef]
- Osmon, D.R.; Berbari, E.F.; Berendt, A.R.; Lew, D.; Zimmerli, W.; Steckelberg, J.M.; Rao, N.; Hanssen, A.; Wilson, W.R. Infectious Diseases Society of America Diagnosis and Management of Prosthetic Joint Infection: Clinical Practice Guidelines by the Infectious Diseases Society of America. Clin. Infect. Dis. 2013, 56, e1–e25. [Google Scholar] [CrossRef] [Green Version]
- Ariza, J.; Cobo, J.; Baraia-Etxaburu, J.; Benito, N.; Bori, G.; Cabo, J.; Corona, P.; Esteban, J.; Horcajada, J.P.; Lora-Tamayo, J.; et al. Executive Summary of Management of Prosthetic Joint Infections. Clinical Practice Guidelines by the Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC). Enferm. Infecc. Microbiol. Clin. 2017, 35, 189–195. [Google Scholar] [CrossRef]
- Société de Pathologie Infectieuse de Langue Française (SPILF); Collège des Universitaires de Maladies Infectieuses et Tropicales (CMIT); Groupe de Pathologie Infectieuse Pédiatrique (GPIP); Société Française d’Anesthésie et de Réanimation (SFAR); Société Française de Chirurgie Orthopédique et Traumatologique (SOFCOT); Société Française d’Hygiène Hospitalière (SFHH); Société Française de Médecine Nucléaire (SFMN); Société Française de Médecine Physique et de Réadaptation (SOFMER); Société Française de Microbiologie (SFM); Société Française de Radiologie (SFR-Rad); et al. Recommendations for Bone and Joint Prosthetic Device Infections in Clinical Practice (Prosthesis, Implants, Osteosynthesis). Société de Pathologie Infectieuse de Langue Française. Med. Mal. Infect. 2010, 40, 185–211. [Google Scholar] [CrossRef]
- Escudero-Sanchez, R.; Senneville, E.; Digumber, M.; Soriano, A.; del Toro, M.D.; Bahamonde, A.; del Pozo, J.L.; Guio, L.; Murillo, O.; Rico, A.; et al. Suppressive Antibiotic Therapy in Prosthetic Joint Infections: A Multicentre Cohort Study. Clin. Microbiol. Infect. 2019, 26, 499–505. [Google Scholar] [CrossRef] [PubMed]
- Pradier, M.; Robineau, O.; Boucher, A.; Titecat, M.; Blondiaux, N.; Valette, M.; Loïez, C.; Beltrand, E.; Nguyen, S.; Dézeque, H.; et al. Suppressive Antibiotic Therapy with Oral Tetracyclines for Prosthetic Joint Infections: A Retrospective Study of 78 Patients. Infection 2018, 46, 39–47. [Google Scholar] [CrossRef] [PubMed]
- Prendki, V.; Zeller, V.; Passeron, D.; Desplaces, N.; Mamoudy, P.; Stirnemann, J.; Marmor, S.; Ziza, J.-M. Outcome of Patients over 80 Years of Age on Prolonged Suppressive Antibiotic Therapy for at Least 6 Months for Prosthetic Joint Infection. Int. J. Infect. Dis. 2014, 29, 184–189. [Google Scholar] [CrossRef] [Green Version]
- Sandiford, N.A.; Hutt, J.R.; Kendoff, D.O.; Mitchell, P.A.; Citak, M.; Granger, L. Prolonged Suppressive Antibiotic Therapy Is Successful in the Management of Prosthetic Joint Infection. Eur J. Orthop. Surg. Traumatol. 2020, 30, 313–321. [Google Scholar] [CrossRef]
- Segreti, J.; Nelson, J.A.; Trenholme, G.M. Prolonged Suppressive Antibiotic Therapy for Infected Orthopedic Prostheses. Clin. Infect. Dis. 1998, 27, 711–713. [Google Scholar] [CrossRef]
- Wouthuyzen-Bakker, M.; Nijman, J.M.; Kampinga, G.A.; van Assen, S.; Jutte, P.C. Efficacy of Antibiotic Suppressive Therapy in Patients with a Prosthetic Joint Infection. J. Bone Jt. Infect. 2017, 2, 77–83. [Google Scholar] [CrossRef] [Green Version]
- Triffault-Fillit, C.; Ferry, T.; Laurent, F.; Pradat, P.; Dupieux, C.; Conrad, A.; Becker, A.; Lustig, S.; Fessy, M.H.; Chidiac, C.; et al. Microbiologic Epidemiology Depending on Time to Occurrence of Prosthetic Joint Infection: A Prospective Cohort Study. Clin. Microbiol. Infect. 2019, 25, 353–358. [Google Scholar] [CrossRef] [Green Version]
- Papadopoulos, A.; Ribera, A.; Mavrogenis, A.F.; Rodriguez-Pardo, D.; Bonnet, E.; Salles, M.J.; Dolores Del Toro, M.; Nguyen, S.; Blanco-García, A.; Skaliczki, G.; et al. Multidrug-Resistant and Extensively Drug-Resistant Gram-Negative Prosthetic Joint Infections: Role of Surgery and Impact of Colistin Administration. Int. J. Antimicrob. Agents 2019, 53, 294–301. [Google Scholar] [CrossRef] [PubMed]
- Peel, T.N.; Cheng, A.C.; Lorenzo, Y.P.; Kong, D.C.M.; Buising, K.L.; Choong, P.F.M. Factors Influencing the Cost of Prosthetic Joint Infection Treatment. J. Hosp. Infect. 2013, 85, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Grammatico-Guillon, L.; Baron, S.; Gettner, S.; Lecuyer, A.-I.; Gaborit, C.; Rosset, P.; Rusch, E.; Bernard, L. Bone and Joint Infections in Hospitalized Patients in France, 2008: Clinical and Economic Outcomes. J. Hosp. Infect. 2012, 82, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Ferry, T.; Seng, P.; Mainard, D.; Jenny, J.-Y.; Laurent, F.; Senneville, E.; Grare, M.; Jolivet-Gougeon, A.; Bernard, L.; Marmor, S.; et al. The CRIOAc Healthcare Network in France: A Nationwide Health Ministry Program to Improve the Management of Bone and Joint Infection. Orthop. Traumatol. Surg. Res. 2019, 105, 185–190. [Google Scholar] [CrossRef]
- Debarbieux, L.; Pirnay, J.-P.; Verbeken, G.; De Vos, D.; Merabishvili, M.; Huys, I.; Patey, O.; Schoonjans, D.; Vaneechoutte, M.; Zizi, M.; et al. A Bacteriophage Journey at the European Medicines Agency. FEMS Microbiol. Lett. 2016, 363, fnv225. [Google Scholar] [CrossRef] [Green Version]
- European Medicines Agency. Workshop on the Therapeutic Use of Bacteriophages; European Medicines Agency: Amsterdam, The Netherlands, 2015; Available online: https://www.ema.europa.eu/en/events/workshop-therapeutic-use-bacteriophages (accessed on 22 November 2021).
- European Medicines Agency. Committee for Medicinal Products for Human Use. Available online: https://www.ema.europa.eu/en/committees/committee-medicinal-products-human-use-chmp (accessed on 22 November 2021).
- Pirnay, J.-P.; Blasdel, B.G.; Bretaudeau, L.; Buckling, A.; Chanishvili, N.; Clark, J.R.; Corte-Real, S.; Debarbieux, L.; Dublanchet, A.; De Vos, D.; et al. Quality and Safety Requirements for Sustainable Phage Therapy Products. Pharm. Res. 2015, 32, 2173–2179. [Google Scholar] [CrossRef] [Green Version]
- Naureen, Z.; Malacarne, D.; Anpilogov, K.; Dautaj, A.; Camilleri, G.; Cecchin, S.; Bressan, S.; Casadei, A.; Albion, E.; Sorrentino, E.; et al. Comparison between American and European Legislation in the Therapeutical and Alimentary Bacteriophage Usage. Acta Biomed. 2020, 91, e2020023. [Google Scholar] [CrossRef]
- Froissart, R.; Brives, C. Evolutionary Biology and Development Model of Medicines: A Necessary “pas de Deux” for Future Successful Bacteriophage Therapy. J. Evol. Biol. 2021, 1–12. [Google Scholar] [CrossRef]
- Adnan, M.; Ali Shah, M.R.; Jamal, M.; Jalil, F.; Andleeb, S.; Nawaz, M.A.; Pervez, S.; Hussain, T.; Shah, I.; Imran, M.; et al. Isolation and Characterization of Bacteriophage to Control Multidrug-Resistant Pseudomonas Aeruginosa Planktonic Cells and Biofilm. Biologicals 2020, 63, 89–96. [Google Scholar] [CrossRef]
- Merabishvili, M.; Pirnay, J.-P.; Verbeken, G.; Chanishvili, N.; Tediashvili, M.; Lashkhi, N.; Glonti, T.; Krylov, V.; Mast, J.; Van Parys, L.; et al. Quality-Controlled Small-Scale Production of a Well-Defined Bacteriophage Cocktail for Use in Human Clinical Trials. PLoS ONE 2009, 4, e4944. [Google Scholar] [CrossRef]
- Zheng, X.-F.; Yang, Z.-Q.; Zhang, H.; Jin, W.-X.; Xu, C.-W.; Gao, L.; Rao, S.-Q.; Jiao, X.-A. Isolation of Virulent Phages Infecting Dominant Mesophilic Aerobic Bacteria in Cucumber Pickle Fermentation. Food Microbiol. 2020, 86, 103330. [Google Scholar] [CrossRef] [PubMed]
- Bhetwal, A.; Maharjan, A.; Shakya, S.; Satyal, D.; Ghimire, S.; Khanal, P.R.; Parajuli, N.P. Isolation of Potential Phages against Multidrug-Resistant Bacterial Isolates: Promising Agents in the Rivers of Kathmandu, Nepal. Biomed. Res. Int. 2017, 2017, 3723254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lukman, C.; Yonathan, C.; Magdalena, S.; Waturangi, D.E. Isolation and Characterization of Pathogenic Escherichia Coli Bacteriophages from Chicken and Beef Offal. BMC Res. Notes 2020, 13, 8. [Google Scholar] [CrossRef]
- Van Belleghem, J.D.; Merabishvili, M.; Vergauwen, B.; Lavigne, R.; Vaneechoutte, M. A Comparative Study of Different Strategies for Removal of Endotoxins from Bacteriophage Preparations. J. Microbiol. Methods 2017, 132, 153–159. [Google Scholar] [CrossRef]
- Adriaenssens, E.M.; Lehman, S.M.; Vandersteegen, K.; Vandenheuvel, D.; Philippe, D.L.; Cornelissen, A.; Clokie, M.R.J.; García, A.J.; De Proft, M.; Maes, M.; et al. CIM® Monolithic Anion-Exchange Chromatography as a Useful Alternative to CsCl Gradient Purification of Bacteriophage Particles. Virology 2012, 434, 265–270. [Google Scholar] [CrossRef] [Green Version]
- Pirnay, J.-P.; Verbeken, G.; Ceyssens, P.-J.; Huys, I.; De Vos, D.; Ameloot, C.; Fauconnier, A. The Magistral Phage. Viruses 2018, 10, 64. [Google Scholar] [CrossRef] [Green Version]
- Metsemakers, W.-J.; Morgenstern, M.; Senneville, E.; Borens, O.; Govaert, G.A.M.; Onsea, J.; Depypere, M.; Richards, R.G.; Trampuz, A.; Verhofstad, M.H.J.; et al. General Treatment Principles for Fracture-Related Infection: Recommendations from an International Expert Group. Arch. Orthop. Trauma Surg. 2019, 140, 1013–1027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Onsea, J.; Soentjens, P.; Djebara, S.; Merabishvili, M.; Depypere, M.; Spriet, I.; De Munter, P.; Debaveye, Y.; Nijs, S.; Vanderschot, P.; et al. Bacteriophage Application for Difficult-to-Treat Musculoskeletal Infections: Development of a Standardized Multidisciplinary Treatment Protocol. Viruses 2019, 11, 891. [Google Scholar] [CrossRef] [Green Version]
- Tkhilaishvili, T.; Winkler, T.; Müller, M.; Perka, C.; Trampuz, A. Bacteriophages as Adjuvant to Antibiotics for the Treatment of Periprosthetic Joint Infection Caused by Multidrug-Resistant Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 2019, 64, e00924-19. [Google Scholar] [CrossRef] [Green Version]
- Ferry, T.; Kolenda, C.; Batailler, C.; Gaillard, R.; Gustave, C.-A.; Lustig, S.; Fevre, C.; Petitjean, C.; Leboucher, G.; Laurent, F.; et al. Case Report: Arthroscopic “Debridement Antibiotics and Implant Retention” with Local Injection of Personalized Phage Therapy to Salvage a Relapsing Pseudomonas aeruginosa Prosthetic Knee Infection. Front. Med. 2021, 8, 569159. [Google Scholar] [CrossRef]
- Dąbrowska, K.; Abedon, S.T. Pharmacologically Aware Phage Therapy: Pharmacodynamic and Pharmacokinetic Obstacles to Phage Antibacterial Action in Animal and Human Bodies. Microbiol. Mol. Biol. Rev. 2019, 83, e00012-19. [Google Scholar] [CrossRef]
- Amankwah, S.; Abdusemed, K.; Kassa, T. Bacterial Biofilm Destruction: A Focused Review On The Recent Use of Phage-Based Strategies with Other Antibiofilm Agents. NSA 2021, 14, 161–177. [Google Scholar] [CrossRef]
- Fischetti, V.A. Development of Phage Lysins as Novel Therapeutics: A Historical Perspective. Viruses 2018, 10, 310. [Google Scholar] [CrossRef] [Green Version]
- Schuch, R.; Khan, B.K.; Raz, A.; Rotolo, J.A.; Wittekind, M. Bacteriophage Lysin CF-301, a Potent Antistaphylococcal Biofilm Agent. Antimicrob. Agents Chemother. 2017, 61, e02666-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watson, A.; Oh, J.T.; Sauve, K.; Bradford, P.A.; Cassino, C.; Schuch, R. Antimicrobial Activity of Exebacase (Lysin CF-301) against the Most Common Causes of Infective Endocarditis. Antimicrob. Agents Chemother. 2019, 63, e01078-19. [Google Scholar] [CrossRef]
- Fowler, V.G.; Das, A.F.; Lipka-Diamond, J.; Schuch, R.; Pomerantz, R.; Jáuregui-Peredo, L.; Bressler, A.; Evans, D.; Moran, G.J.; Rupp, M.E.; et al. Exebacase for Patients with Staphylococcus Aureus Bloodstream Infection and Endocarditis. J. Clin. Investig. 2020, 130, 3750–3760. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, N.-H.; Park, W.B.; Cho, J.E.; Choi, Y.J.; Choi, S.J.; Jun, S.Y.; Kang, C.K.; Song, K.-H.; Choe, P.G.; Bang, J.-H.; et al. Effects of Phage Endolysin SAL200 Combined with Antibiotics on Staphylococcus aureus Infection. Antimicrob. Agents Chemother. 2018, 62, e00731-18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karau, M.J.; Schmidt-Malan, S.M.; Yan, Q.; Greenwood-Quaintance, K.E.; Mandrekar, J.; Lehoux, D.; Schuch, R.; Cassino, C.; Patel, R. Exebacase in Addition to Daptomycin Is More Active than Daptomycin or Exebacase Alone in Methicillin-Resistant Staphylococcus aureus Osteomyelitis in Rats. Antimicrob. Agents Chemother. 2019, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferry, T.; Batailler, C.; Souche, A.; Cassino, C.; Chidiac, C.; Perpoint, T.; Le Corvaisier, C.; Josse, J.; Gaillard, R.; Roger, J.; et al. Arthroscopic “Debridement And Implant Retention” (DAIR) with Local Administration of Exebacase (Lysin CF-301) (LysinDAIR) Followed by Suppressive Tedizolid as Salvage Therapy in Elderly Patients for Relapsing Multidrug-Resistant (MDR) S. Epidermidis Prosthetic Knee Infection LysinDAIR for Prosthetic Knee Infection. Front. Med. 2021, 8, 549, In Press. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferry, T.; Kolenda, C.; Briot, T.; Souche, A.; Lustig, S.; Josse, J.; Batailler, C.; Pirot, F.; Medina, M.; Leboucher, G.; et al. Past and Future of Phage Therapy and Phage-Derived Proteins in Patients with Bone and Joint Infection. Viruses 2021, 13, 2414. https://doi.org/10.3390/v13122414
Ferry T, Kolenda C, Briot T, Souche A, Lustig S, Josse J, Batailler C, Pirot F, Medina M, Leboucher G, et al. Past and Future of Phage Therapy and Phage-Derived Proteins in Patients with Bone and Joint Infection. Viruses. 2021; 13(12):2414. https://doi.org/10.3390/v13122414
Chicago/Turabian StyleFerry, Tristan, Camille Kolenda, Thomas Briot, Aubin Souche, Sébastien Lustig, Jérôme Josse, Cécile Batailler, Fabrice Pirot, Mathieu Medina, Gilles Leboucher, and et al. 2021. "Past and Future of Phage Therapy and Phage-Derived Proteins in Patients with Bone and Joint Infection" Viruses 13, no. 12: 2414. https://doi.org/10.3390/v13122414
APA StyleFerry, T., Kolenda, C., Briot, T., Souche, A., Lustig, S., Josse, J., Batailler, C., Pirot, F., Medina, M., Leboucher, G., Laurent, F., on behalf of the Lyon BJI Study Group, & on behalf of the PHAGEinLYON Study Group. (2021). Past and Future of Phage Therapy and Phage-Derived Proteins in Patients with Bone and Joint Infection. Viruses, 13(12), 2414. https://doi.org/10.3390/v13122414





