Mid-Regional Pro-Adrenomedullin, Methemoglobin and Carboxyhemoglobin as Prognosis Biomarkers in Critically Ill Patients with COVID-19: An Observational Prospective Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Measurement of MR-proADM Concentration by Quantitative Enzyme-Linked Immunosorbent Assay
2.3. Measurement of MetHb and COHb Concentration by Co-Oximetry
2.4. Data Collection and Outcomes
2.5. Statistical Analysis
3. Results
3.1. Characteristic of Study Population
3.2. Characteristic of MetHb and COHb in COVID-19 Patients
3.3. Characteristic of MR-proADM in COVID-19 Patients
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Coronavirus COVID-19 Global Cases by the Center for Systems Science and Engineering (CSSE) at Johns Hopkins University. Available online: https://coronavirus.jhu.edu/ (accessed on 6 September 2021).
- Wiersinga, W.J.; Rhodes, A.; Cheng, A.C.; Peacock, S.J.; Prescott, H.C. Pathophysiology, transmission, diagnosis, and treatment of Coronavirus disease 2019 (COVID-19): A review: A review. JAMA 2020, 324, 782–793. [Google Scholar] [CrossRef] [PubMed]
- Guan, W.J.; Ni, Z.Y.; Hu, Y.; Liang, W.H.; Ou, C.Q.; He, J.X. China Medical Treatment Expert Group for Covid-19. Clinical Characteristics of Coronavirus Disease 2019 in China. N. Engl. J. Med. 2020, 382, 1708–1720. [Google Scholar] [CrossRef]
- Sinha, P.; Matthay, M.A.; Calfee, C.S. Is a “Cytokine Storm” Relevant to COVID-19? JAMA Intern Med. 2020, 180, 1152–1154. [Google Scholar] [CrossRef]
- Kox, M.; Waalders, N.J.B.; Kooistra, E.J.; Gerretsen, J.; Pickkers, P. Cytokine Levels in Critically Ill Patients with COVID-19 and Other Conditions. JAMA 2020, 324, 1565–1567. [Google Scholar] [CrossRef] [PubMed]
- Mearelli, F.; Barbati, G.; Casarsa, C.; Giansante, C.; Breglia, A.; Spica, A.; Moras, C.; Olivieri, G.; Occhipinti, A.A.; De Nardo, M.; et al. The Integration of qSOFA with Clinical Variables and Serum Biomarkers Improves the Prognostic Value of qSOFA Alone in Patients with Suspected or Confirmed Sepsis at ED Admission. J. Clin. Med. 2020, 9, 1205. [Google Scholar] [CrossRef]
- Valenzuela-Sánchez, F.; Valenzuela-Méndez, B.; Rodríguez-Gutiérrez, J.F.; Estella, A.; González-García, M. New role of biomarkers: Mid-regional pro-adrenomedullin, the biomarker of organ failure. Ann. Transl. Med. 2016, 4, 329. [Google Scholar] [CrossRef] [Green Version]
- Buendgens, L.; Yagmur, E.; Ginsberg, A.; Weiskirchen, R.; Wirtz, T.; Abu Jhaisha, S.; Eisert, A.; Luedde, T.; Trautwein, C.; Tacke, F.; et al. Midregional Proadrenomedullin (MRproADM) Serum Levels in Critically Ill Patients Are Associated with Short-Term and Overall Mortality during a Two-Year Follow-Up. Mediat. Inflamm. 2020, 2020, 7184803. [Google Scholar] [CrossRef]
- Elke, G.; Bloos, F.; Wilson, D.C.; Brunkhorst, F.M.; Briegel, J.; Reinhart, K. SepNet Critical Care Trials Group. The use of mid-regional proadrenomedullin to identify disease severity and treatment response to sepsis–A secondary analysis of a large randomised controlled trial. Crit. Care 2018, 22, 79. [Google Scholar] [CrossRef] [Green Version]
- Andaluz-Ojeda, D.; Nguyen, H.B.; Meunier-Beillard, N.; Cicuéndez, R.; Quenot, J.-P.; Calvo, D.; Dargent, A.; Zarca, E.; Andrés, C.; Nogales, L.; et al. Superior accuracy of mid-regional proadrenomedullin for mortality prediction in sepsis with varying levels of illness severity. Ann. Intensiv. Care 2017, 7, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gordo-Remartínez, S.; Calderón-Moreno, M.; Fernández-Herranz, J.; Castuera-Gil, A.; Gallego-Alonso-Colmenares, M.; Puertas-López, C.; Nuevo-González, J.A.; Sánchez-Sendín, D.; García-Gámiz, M.; Sevillano-Fernández, J.A.; et al. Usefulness of midregional proadrenomedullin to predict poor outcome in patients with community acquired pneumonia. PLoS ONE 2015, 10, e0125212. [Google Scholar]
- Hare, G.M.; Tsui, A.K.; Crawford, J.H.; Patel, R.P. Is methemoglobin an inert bystander, biomarker or a mediator of oxidative stress--The example of anemia? Redox Biol. 2013, 1, 65–69. [Google Scholar] [CrossRef] [Green Version]
- Ohashi, K.; Yukioka, H.; Hayashi, M.; Asada, A. Elevated methemoglobin in patients with sepsis. Acta Anaesthesiol. Scand. 1998, 42, 713–716. [Google Scholar] [CrossRef] [PubMed]
- Behera, G.C.; Behera, S.K.; Jena, R.K.; Bharati, V.S. Study of Methaemoglobin in Malaria Patients. Indian J. Hematol. Blood Transfus. 2015, 32, 100–103. [Google Scholar] [CrossRef] [Green Version]
- Yeo, T.W.; Lampah, D.A.; Kenangalem, E.; Tjitra, E.; Price, R.N.; Anstey, N.M. Increased carboxyhemoglobin in adult falciparum malaria is associated with disease severity and mortality. J. Infect. Dis. 2013, 208, 813–817. [Google Scholar] [CrossRef] [Green Version]
- Hänscheid, T.; Gresnigt, T.; Löhr, S.; Flamen, A.; Zoller, T.; Melo-Cristino, J.; Grobusch, M.P. Methaemoglobin and COHb in patients with malaria. Malar. J. 2014, 13, 285. [Google Scholar] [CrossRef] [Green Version]
- ARDS Definition Task Force; Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute Respiratory Distress Syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar]
- Vincent, J.L.; Moreno, R.; Takala, J. Working Group on Sepsis-Related Problems of the European Society of Intensive Care Medicine. The SOFA (Sepsis-related Organ Failure Assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996, 22, 707–710. [Google Scholar] [CrossRef]
- Berenguer, J.; Borobia, A.M.; Ryan, P.; Rodríguez-Baño, J.; Bellón, J.M.; Jarrín, I.; Carratalà, J.; Pachón, J.; Carcas, A.J.; Yllescas, M.; et al. Development and validation of a prediction model for 30-day mortality in hospitalised patients with COVID-19: The COVID-19 SEIMC score. Thorax 2021, 76, 920–929. [Google Scholar] [CrossRef] [PubMed]
- Scholkmann, F.; Restin, T.; Ferrari, M.; Quaresima, V. The Role of Methemoglobin and Carboxyhemoglobin in COVID-19: A Review. J. Clin. Med. 2020, 10, 50. [Google Scholar] [CrossRef]
- Paccaud, P.; Castanares-Zapatero, D.; Gerard, L.; Montiel, V.; Wittebole, X.; Collienne, C.; Laterre, P.F.; Hantson, P. Arterial carboxyhemoglobin levels in Covid-19 critically Ill patients. Res. Sq. 2020. [Google Scholar]
- Benedetti, I.; Spinelli, D.; Callegari, T.; Bonometti, R.; Molinaro, E.; Novara, E.; Cassinari, M.; Frino, C. High levels of mid-regional proadrenomedullin in ARDS COVID-19 patients: The experience of a single, Italian Center. Eur. Rev. Med. Pharmacol. Sci. 2021, 25, 1743–1751. [Google Scholar] [PubMed]
- Montrucchio, G.; Sales, G.; Rumbolo, F.; Palmesino, F.; Fanelli, V.; Urbino, R. Effectiveness of mid-regional pro-adrenomedullin (MR-proADM) as prognostic marker in COVID-19 critically ill patients: An observational prospective study. PLoS ONE 2021, 16, e0246771. [Google Scholar] [CrossRef] [PubMed]
- Roedl, K.; Jarczak, D.; Fischer, M.; Haddad, M.; Boenisch, O.; de Heer, G.; Burdelski, C.; Frings, D.; Sensen, B.; Karakas, M.; et al. MR-proAdrenomedullin as a predictor of renal replacement therapy in a cohort of critically ill patients with COVID-19. Biomarkers 2021, 26, 417–424. [Google Scholar] [CrossRef] [PubMed]
- Spoto, S.; Agrò, F.E.; Sambuco, F.; Travaglino, F.; Valeriani, E.; Fogolari, M.; Mangiacapra, F.; Costantino, S.; Ciccozzi, M.; Angeletti, S.; et al. High value of mid-regional proadrenomedullin in COVID-19: A marker of widespread endothelial damage, disease severity, and mortality. J. Med. Virol. 2021, 93, 2820–2827. [Google Scholar] [CrossRef]
- Gregoriano, C.; Koch, D.; Kutz, A.; Haubitz, S.; Conen, A.; Bernasconi, L.; Hammerer-Lercher, A.; Saeed, K.; Mueller, B.; Schuetz, P. The vasoactive peptide MR-pro-adrenomedullin in COVID-19 patients: An observational study. Clin. Chem. Lab. Med. 2020, 59, 995–1004. [Google Scholar] [CrossRef]
- García de Guadiana-Romualdo, L.; Calvo Nieves, M.D.; Rodríguez Mulero, M.D.; Calcerrada Alises, I.; Hernández Olivo, M.; Trapiello Fernández, W.; Morales, M.G.; Jiménez, C.B.; Albaladejo-Otón, M.D.; Ovalle, H.F.; et al. MR-proADM as marker of endotheliitis predicts COVID-19 severity. Eur. J. Clin. Investig. 2021, 51, e13511. [Google Scholar] [CrossRef]
- Sozio, E.; Tascini, C.; Fabris, M.; D’Aurizio, F.; De Carlo, C.; Graziano, E.; Bassi, F.; Sbrana, F.; Ripoli, A.; Pagotto, A.; et al. MR-proADM as prognostic factor of outcome in COVID-19 patients. Sci. Rep. 2021, 11, 5121. [Google Scholar] [CrossRef]
- Zaninotto, M.; Maria Mion, M.; Marchioro, L.; Padoan, A.; Plebani, M. Endothelial dysfunction and Mid-Regional proAdrenomedullin: What role in SARS-CoV-2 infected Patients? Clin. Chim. Acta 2021, 523, 185–190. [Google Scholar] [CrossRef]
- Lo Sasso, B.; Gambino, C.M.; Scichilone, N.; Giglio, R.V.; Bivona, G.; Scazzone, C.; Muratore, R.; Milano, S.; Barbagallo, M.; Agnello, L.; et al. Clinical Utility of Midregional Proadrenomedullin in Patients with COVID-19. Lab. Med. 2021, 52, 493–498. [Google Scholar] [CrossRef]
- Saito, S.; Asai, Y.; Matsunaga, N.; Hayakawa, K.; Terada, M.; Ohtsu, H.; Tsuzuki, S.; Ohmagari, N. First and second COVID-19 waves in Japan: A comparison of disease severity and characteristics. J. Infect. 2020, 82, 84–123. [Google Scholar] [CrossRef]
- Bongiovanni, M.; Arienti, R.; Bini, F.; Bodini, B.D.; Corbetta, E.; Gianturco, L. Differences between the waves in Northern Italy: How the characteristics and the outcome of COVID-19 infected patients admitted to the emergency room have changed. J. Infect. 2021, 83, e32–e33. [Google Scholar] [CrossRef] [PubMed]

| Variable | Total (n = 95) | Survivors (n = 83) | Non-Survivors (n = 12) | p Value | |
|---|---|---|---|---|---|
| Sex male, n (%) | 64 (67.4) | 55 (66.3) | 9 (75) | 0.75 | |
| BMI, n (%) | <25 | 23 (24.2) | 20 (24.1) | 3 (25) | 1.00 |
| 25–30 | 40 (42.1) | 35 (42.2) | 5 (41.7) | 1.00 | |
| ≥30 | 32 (33.7) | 28 (33.7) | 4 (33.3) | 1.00 | |
| Age, years (mean, SD) | 60.3 ± 12.8 | 58.7 ± 12.5 | 71.3 ± 9.1 | 0.001 | |
| BMI, kg/m2 (mean, SD) | 29 ± 5 | 29 ± 4.7 | 29 ± 6.8 | 0.86 | |
| Hemoglobin, mg/dL (median, P25–P75) | 13.3 (12–14.6) | 13.3 (12–14.6) | 13.3 (12.3–13.9) | 0.98 | |
| Anemia (hemoglobin <12 g/dl), n (%) | 26 (27.4) | 23 (27.7) | 3 (25) | 1.00 | |
| Leukocytes, µL−1 (median, P25–P75) | 10,400 (7500–12,800) | 10,200 (7500–12,800) | 11,555 (9200–13,900) | 0.23 | |
| Neutrophils, µL−1 (median, P25–P75) | 9300 (6200–11,700) | 9000 (6100–11,400) | 10,850 (8150–12,850) | 0.12 | |
| Lymphocytes, µL−1 (median, P25–P75) | 600 (400–1000) | 700 (400–1000) | 450 (300–650) | 0.04 | |
| Lymphocytes <1000/µL, n (%) | 68 (71.6) | 57 (68.7) | 11 (91.7) | 0.17 | |
| Neutrophil/lymphocyte ratio, (median, P25–P75) | 13.6 (7.3–23) | 13.4 (6.8–22.2) | 30.9 (12.8–42.3) | 0.02 | |
| Neutrophil/lymphocyte ratio, n (%) | <3.22 | 5 (5.3) | 5 (6) | 0 (0) | 1.00 |
| 3.22–6.53 | 13 (13.7) | 12 (14.5) | 1 (8.3) | 1.00 | |
| >6.53 | 77 (81.1) | 66 (79.5) | 11 (91.7) | 0.45 | |
| Platelets, ×1000∙µL−1 (median, P25–P75) | 241 (194–288) | 242 (196–292) | 212 (147–249) | 0.17 | |
| Platelets ≤ 150,000/µL, n (%) | 14 (14.7) | 10 (12) | 4 (33.3) | 0.07 | |
| INR ≥ 1.25, n (%) | 15 (15.8) | 12 (14.5) | 3 (25) | 0.4 | |
| D-Dimer, ng/mL (median, P25–P75) | 577 (331–1061) | 563 (307–820) | 1124 (438–2710) | 0.046 | |
| D-Dimer, n (%) | ≥600 ng/mL | 45 (47.4) | 38 (45.8) | 7 (58.3) | 0.54 |
| ≥1000 ng/mL | 24 (25.3) | 17 (20.5) | 7 (58.3) | 0.01 | |
| Ferritin ≥ 274 µg/L, n (fraction) | 67/78 (86) | 61/69 (88.4) | 6/9 (66.7) | 0.11 | |
| IL-6 ≥ 4.3 pg/mL, n (fraction) | 76/80 (95) | 65/69 (94.2) | 11/11 (100) | 1.00 | |
| LDH ≥ 225 U/L, n (%) | 91 (95.8) | 79 (95.2) | 12 (100) | 1.00 | |
| Glomerular filtration rate, n (%) | <60 mL/min/1.73 m2 | 14 (14.7) | 10 (12) | 4 (33.3) | 0.07 |
| <30 mL/min/1.73 m2 | 6 (6.3) | 4 (4.8) | 2 (16.7) | 0.16 | |
| Total bilirubin ≥1.2 mg/dL, n (%) | 6 (6.3) | 5 (6) | 1 (8.3) | 0.57 | |
| C-reactive protein, mg/dL (median, P25–P75) | 13.6 (6.3–24.6) | 13.3 (6.3–23.8) | 19.2 (6.4–27.1) | 0.52 | |
| C-reactive protein, n (%) | ≥1 mg/dL | 90 (94.7) | 79 (95.2) | 11 (91.7) | 0.5 |
| ≥5 mg/dL | 75 (78.9) | 65 (78.3) | 10 (83.3) | 1.00 | |
| ≥8 mg/dL | 65 (68.4) | 57 (68.7) | 8 (66.7) | 1.00 | |
| Procalcitonin, µg/L (median, P25–P75) | 0.13 (0.05–0.73) | 0.12 (0.05–0.46) | 0.5 (0.05–2.66) | 0.19 | |
| Procalcitonin ≥0.5 µg/L, n (%) | 25 (26.3) | 19 (22.9) | 6 (50) | 0.07 | |
| MR-proADM, nmol/L (median, P25–P75) | 0.77 (0.61–1.14) | 0.76 (0.6–1.03) | 1.22 (0.84–2.33) | 0.01 | |
| MR-proADM, n (%) | ≥0.75 nmol/L | 53 (55.8) | 43 (51.8) | 10 (83.3) | 0.06 |
| ≥1 nmol/L | 29 (30.5) | 23 (27.7) | 8 (66.7) | 0.02 | |
| MetHb, %Hb total (mean, SD) | 1.09 ± 0.39 | 1.1 ± 0.38 | 1.03 ± 0.56 | 0.34 | |
| MetHb ≥ 1%, n (fraction) | 25/86 (29.1) | 57/76 (75) | 4/10 (40) | 0.06 | |
| COHb, %Hb total (mean, SD) | 1.57 ± 0.52 | 1.57 ± 0.5 | 1.62 ± 0.73 | 0.71 | |
| COHb > 1.3%, n (fraction) | 20/86 (23.3) | 60/76 (78.9) | 6/10 (60) | 0.23 | |
| Arterial pH ≤ 7.35, n (%) | 16 (16.8) | 11 (13.3) | 5 (41.7) | 0.03 | |
| Arterial lactate ≥ 0.8 mmol/L, n (%) | 89 (93.7) | 78 (94) | 11 (91.7) | 0.57 | |
| Duration of symptoms before admission, days (median, P25–75) | 6 (3–8) | 6 (4–8) | 2 (1–6) | 0.02 | |
| Length of stay, days (median, P25–P75) | 12 (6–30) | 12 (6–32) | 14 (4–23) | 0.34 | |
| SOFA score, median (P25–75) | 2 (2–4) | 2 (2–4) | 4 (3–6.5) | 0.003 | |
| SOFA score, n (%) | 1 | 2 (2.1) | 2 (2.4) | 0 (0) | 1.00 |
| 2 | 47 (49.5) | 47 (56.6) | 0 (0) | 0.0002 | |
| 3 | 10 (10.5) | 5 (6) | 5 (41.7) | 0.003 | |
| 4 | 13 (13.7) | 11 (13.3) | 2 (16.7) | 0.67 | |
| 5 | 6 (6.3) | 4 (4.8) | 2 (16.7) | 0.16 | |
| ≥6 | 17 (17.9) | 14 (16.9) | 3 (25) | 0.45 | |
| SEIMC score, n (%) | 3–5 Moderate | 5 (5.3) | 5 (6) | 0 (0) | 1.00 |
| 6–8 High | 29 (30.5) | 29 (34.9) | 0 (0) | 0.02 | |
| ≥9 Very high | 61 (64.2) | 49 (59) | 12 (100) | 0.004 | |
| Variable (n = 95) | n (%) | |
|---|---|---|
| Mortality, n (%) | 12 (12.6) | |
| Time until death, days (median, P25–75) | 18.5 (13.5–25.5) | |
| Place of death, n (%) | ICU | 11/12 (87.5) |
| After ICU discharge | 1/12 (12.5) | |
| Cause of death, n (%) | COVID-19 | 11/12 (87.5) |
| Acute myocardial infarction | 1/12 (12.5) | |
| VTE, n (%) | 8 (8.4) | |
| Time until VTE, days (median, P25–P75) | 14 (9.5–17) | |
| Arterial thrombosis, n (%) | 3 (3.1) | |
| Time until arterial thrombosis, days (median, P25–P75) | 7 (2–17) | |
| OTI, n (%) | 49 (51.6) | |
| Time until OTI, days (median, P25–P75) | 1 (0–2) | |
| Combined event *, n (%) | 54 (56.8) | |
| Variables | OR | 95% CI | p Value | OR | 95% CI | p Value | ||
|---|---|---|---|---|---|---|---|---|
| Mortality | Combined Event | |||||||
| Univariate Logistic Regression Analysis | ||||||||
| Age (years) | 1.14 | 1.03 | 1.26 | 0.011 | 1.02 | 0.99 | 1.05 | 0.244 |
| Oxygen saturation (%) | 0.96 | 0.90 | 1.03 | 0.296 | 0.98 | 0.92 | 1.05 | 0.554 |
| Neutrophils/lymphocytes ratio | 1.03 | 0.99 | 1.05 | 0.07 | 1.01 | 0.98 | 1.05 | 0.375 |
| Glomerular filtration rate (mL/min·1.73 m2) | 0.96 | 0.92 | 1.004 | 0.077 | 0.89 | 0.83 | 0.96 | 0.002 |
| Sex (male) | 0.65 | 0.16 | 2.63 | 0.551 | 0.89 | 0.37 | 2.11 | 0.785 |
| Procalcitonin ≥ 1 ng/mL | 2.69 | 0.70 | 10.34 | 0.149 | 2.06 | 0.66 | 6.43 | 0.215 |
| C-reactive protein ≥ 8 mg/dl | 1.09 | 0.30 | 3.99 | 0.889 | 0.81 | 0.34 | 1.95 | 0.641 |
| MR-proADM ≥ 1 mmol/L | 5.22 | 1.42 | 19.14 | 0.013 | 5.03 | 1.81 | 13.99 | 0.002 |
| COHb ≥ 1.3% | 2.50 | 0.62 | 10.02 | 0.196 | 2.66 | 0.86 | 8.21 | 0.09 |
| MetHb ≥ 1% | 3.75 | 0.96 | 14.68 | 0.058 | 2.92 | 0.95 | 8.96 | 0.062 |
| SOFAscore | 1.28 | 1.08 | 1.52 | 0.005 | 2.4 | 1.52 | 3.78 | 0.000 |
| Multivariate Logistic Regression Analysis | ||||||||
| Age (years) | 1.17 | 1.03 | 1.32 | 0.014 | 1.03 | 0.99 | 1.07 | 0.19 |
| Glomerular filtration rate (ml/min·1.73 m2) | 0.97 | 0.92 | 1.03 | 0.34 | 0.96 | 0.91 | 1.02 | 0.18 |
| MR-proADM ≥ 1 mmol/L | 1.29 | 0.17 | 9.48 | 0.8 | 1.73 | 0.46 | 6.49 | 0.42 |
| SOFAscore | 1.38 | 1.01 | 1.89 | 0.04 | 2.23 | 1.44 | 3.45 | 0.000 |
| Author | n | Age | % ICU Patients | SOFA Score | MR-proADM Levels (nmol/L) | n (%) Deaths | Cut-Off Point for Death (nmol/L) | AUC for 30 Day Mortality | ||
|---|---|---|---|---|---|---|---|---|---|---|
| Total Sample | Survivors | Non-Survivors | ||||||||
| Benedetti I et al. [22] | 21 | 70.9 (54–85) | 23.8% | 3.5 ±2.3 | 2.3 ±2.7 | 1.1 (mean) | 2.3 (mean) | 11 (52.4%) | 1.07 | 0.81 |
| Montrucchio G et al. [23] | 57 | 64 (54–71) | 100% | 7 (4–10) | 2 ± 1.3 | 1.22 ±0.49 | 2.74 ±1.99 | 31 (54.4%) | 1.8 | 0.85 (95%CI 0.78–0.9) |
| Spoto S et al. [25] | 69 | 78 (61–84) | 43.5% | 2 (1–7) | 1.49 (0.67–2.26) | 1.15 (0.57–1.85) | 5.25 (2.67–6.53) | 16 (23.2%) | 2.00 | 0.89 |
| Gregoriano C et al. [26] | 89 | 67 (58–74) | 26% | NR | NR | 0.8 (0.7–0.11) | 1.3 (1.1–2.3) | 17 (19.1%) | 0.93 | 0.78 |
| García de Guadiana-Romualdo L et al. [27] | 99 | 66 ±15 | 16.2% | NR | 0.74 (0.6–1.02) | 0.68 (0.57–0.94) | 1.54 (1.05–2.12) | 14 (14.1%) | 0.88 | 0.91 (95% CI 0.82–0.95) |
| Sozio E et al. [28] ‡ | 111 | 62.3 ± 13.6 | 25.2% * | 2 (1–3) | 0.82 (0.64–1.08) | 0.73 (0.56–0.94) ** | 1.38 (0.94–1.73) ** | 28 (25.2%) ** | 0.9 ** | 0.85 (95% CI 0.77–0.73) ** |
| Zaninotto M et al. [29] ‡ | 135 | 67 (58–77) | 52.6% | NR | 0.93 (0.64–1.46) | NR | NR | 14 (10.4%) | 0.5–1.5 ‡‡ | 0.9 (95% CI 0.827–0.974) |
| Lo Sasso B et al. [30] ‡ | 110 | 62 (52–76) | 1.82% | NR | 0.93 (0.58–1.09) | 0.82 (0.57–1.03) | 2.59 (2.3–2.95) | 14 (12.7%) | 1.73 | 0.95 (95% CI 0.86–0.99 |
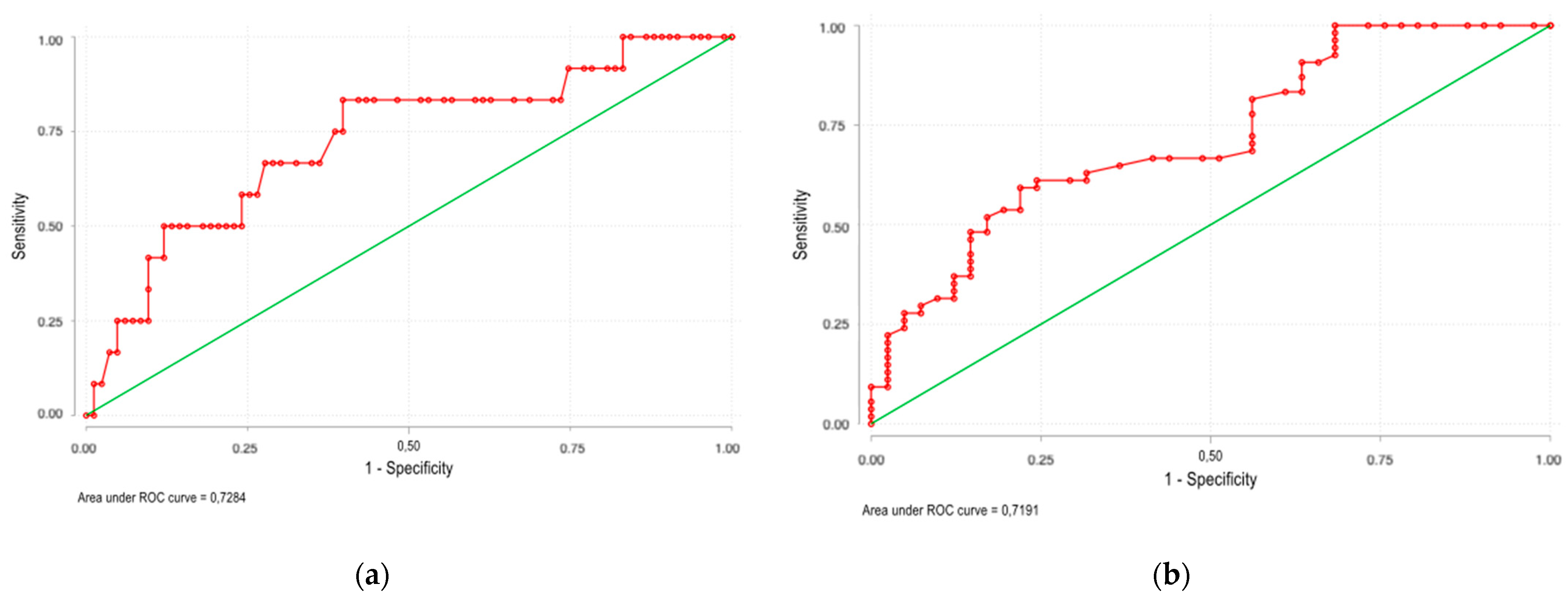
| Present study | 95 | 60.3 ± 12.7 | 100% | 2 (2–4) | 0.77 (0.61–1.14) | 0.76 (0.60–1.03) | 1.21 (0.84–2.33) | 12 (12.6%) | 1 | 0.73 (95% CI 0.63–0.81) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oblitas, C.-M.; Galeano-Valle, F.; Ramírez-Navarro, J.; López-Cano, J.; Monterrubio-Manrique, Á.; García-Gámiz, M.; Sancho-González, M.; Arenal-López, S.; Álvarez-Sala Walther, L.-A.; Demelo-Rodríguez, P. Mid-Regional Pro-Adrenomedullin, Methemoglobin and Carboxyhemoglobin as Prognosis Biomarkers in Critically Ill Patients with COVID-19: An Observational Prospective Study. Viruses 2021, 13, 2445. https://doi.org/10.3390/v13122445
Oblitas C-M, Galeano-Valle F, Ramírez-Navarro J, López-Cano J, Monterrubio-Manrique Á, García-Gámiz M, Sancho-González M, Arenal-López S, Álvarez-Sala Walther L-A, Demelo-Rodríguez P. Mid-Regional Pro-Adrenomedullin, Methemoglobin and Carboxyhemoglobin as Prognosis Biomarkers in Critically Ill Patients with COVID-19: An Observational Prospective Study. Viruses. 2021; 13(12):2445. https://doi.org/10.3390/v13122445
Chicago/Turabian StyleOblitas, Crhistian-Mario, Francisco Galeano-Valle, Jesús Ramírez-Navarro, Jorge López-Cano, Ángel Monterrubio-Manrique, Mercedes García-Gámiz, Milagros Sancho-González, Sara Arenal-López, Luis-Antonio Álvarez-Sala Walther, and Pablo Demelo-Rodríguez. 2021. "Mid-Regional Pro-Adrenomedullin, Methemoglobin and Carboxyhemoglobin as Prognosis Biomarkers in Critically Ill Patients with COVID-19: An Observational Prospective Study" Viruses 13, no. 12: 2445. https://doi.org/10.3390/v13122445
APA StyleOblitas, C.-M., Galeano-Valle, F., Ramírez-Navarro, J., López-Cano, J., Monterrubio-Manrique, Á., García-Gámiz, M., Sancho-González, M., Arenal-López, S., Álvarez-Sala Walther, L.-A., & Demelo-Rodríguez, P. (2021). Mid-Regional Pro-Adrenomedullin, Methemoglobin and Carboxyhemoglobin as Prognosis Biomarkers in Critically Ill Patients with COVID-19: An Observational Prospective Study. Viruses, 13(12), 2445. https://doi.org/10.3390/v13122445







