The Nucleocapsid of Paramyxoviruses: Structure and Function of an Encapsidated Template
Abstract
:1. Introduction
2. Gene Expression and Genome Replication
2.1. The Viral Genome
2.2. Components of the RNA Synthesis Machinery
2.3. Transcription and Replication
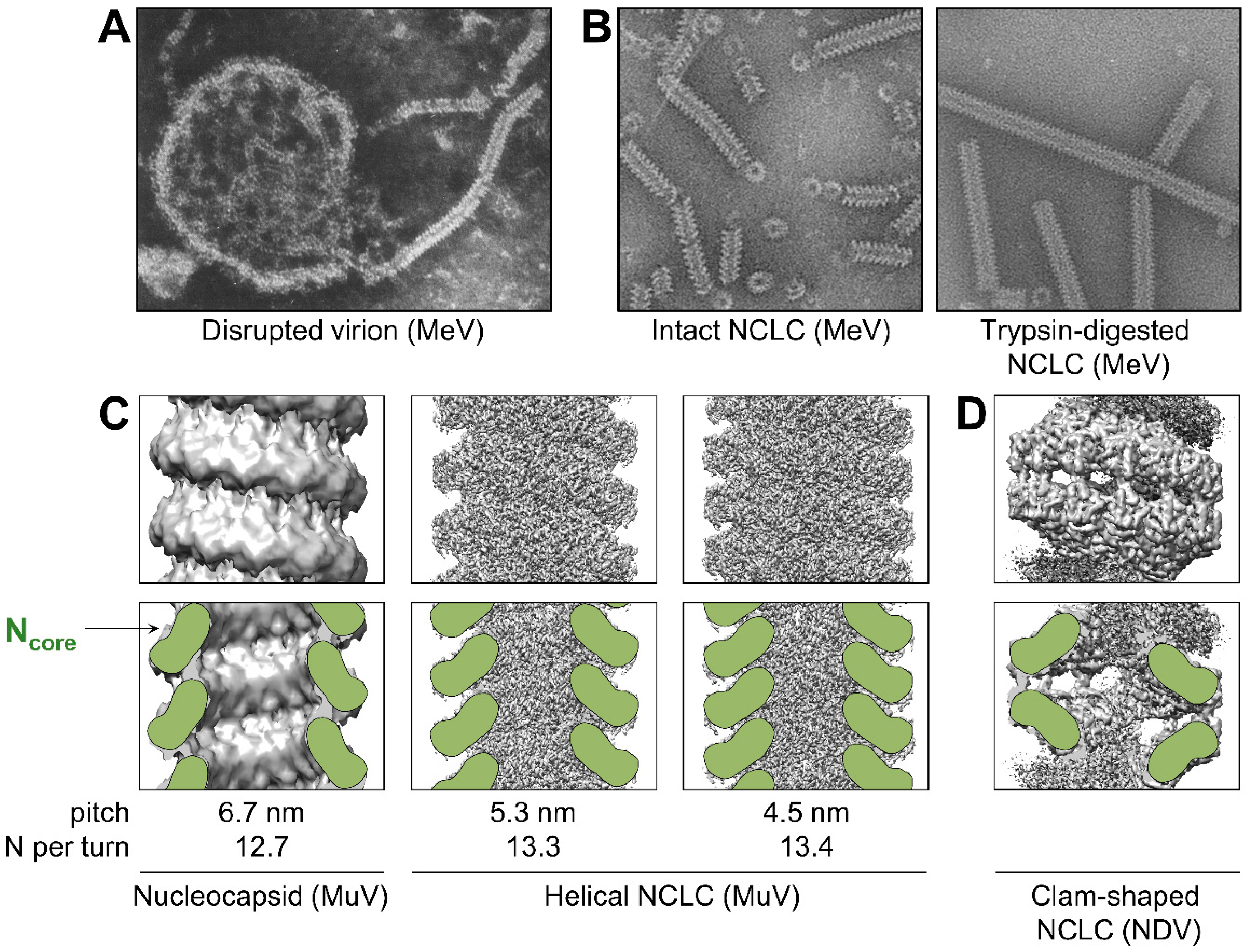
3. Structure of the Nucleocapsid
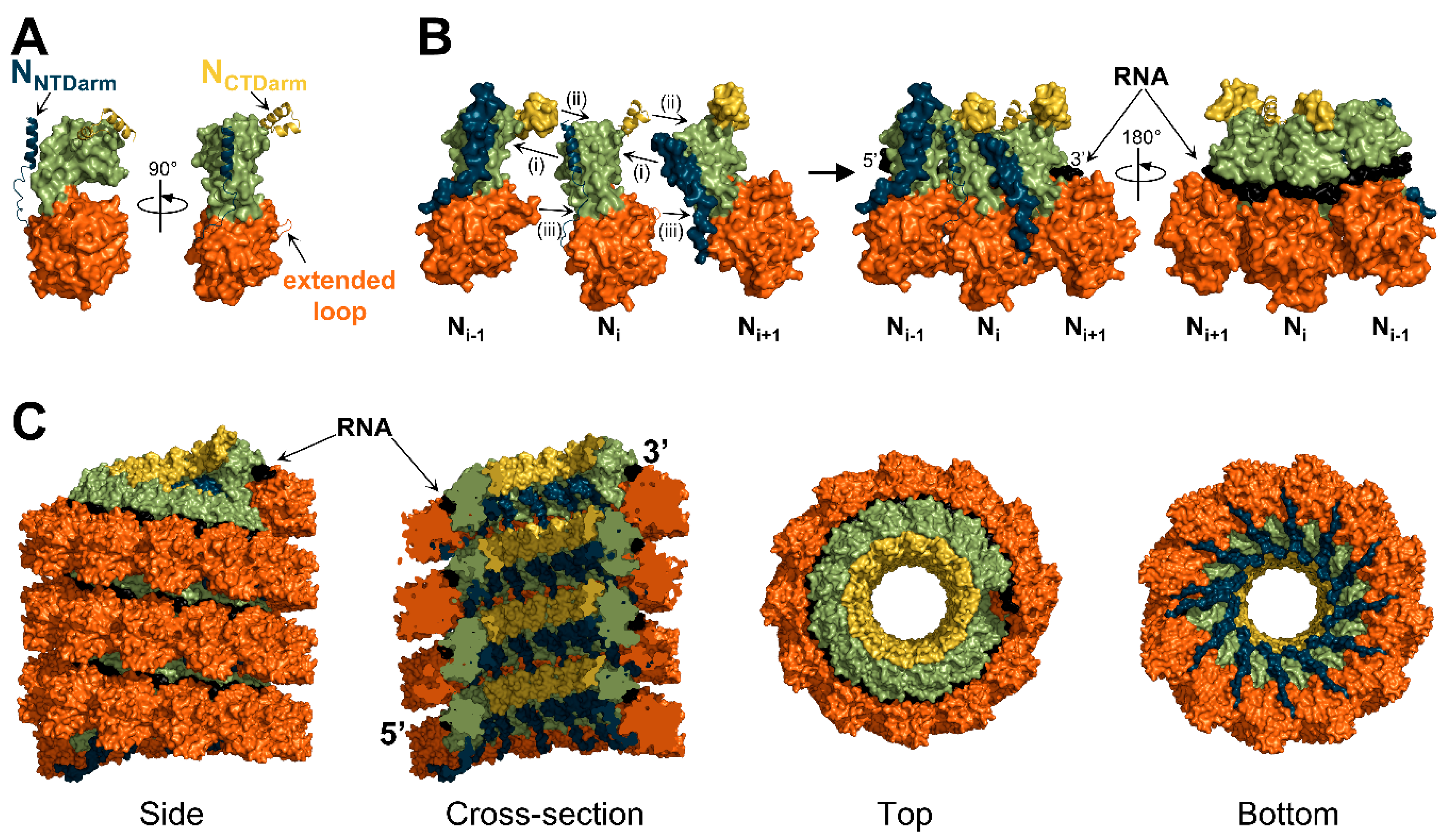
3.1. Overall Architecture
3.2. Structure of the Nucleoprotein
3.3. Interactions between Protomers
3.4. Interactions with RNA
3.5. Position and Influence of Ntail within the Nucleocapsid
4. Formation of the Nucleocapsid
4.1. Structure of the N0-P Complex
4.2. Encapsidation of the RNA
5. The Nucleocapsid as a Template
5.1. Recruitment and Progression of the Polymerase Complex
5.2. Access to the RNA
6. Model of Transcription and Replication
6.1. Transcription
6.2. Replication
7. Conclusions and Perspectives
Funding
Acknowledgments
Conflicts of Interest
References
- Rima, B.; Balkema-Buschmann, A.; Dundon, W.G.; Duprex, P.; Easton, A.; Fouchier, R.; Kurath, G.; Lamb, R.; Lee, B.; Rota, P.; et al. ICTV Virus Taxonomy Profile: Paramyxoviridae. J. Gen. Virol. 2019, 100, 1593–1594. [Google Scholar] [CrossRef] [PubMed]
- Cox, R.; Plemper, R.K. The paramyxovirus polymerase complex as a target for next-generation anti-paramyxovirus therapeutics. Front. Microbiol. 2015, 6, 459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aggarwal, M.; Plemper, R.K. Structural Insight into Paramyxovirus and Pneumovirus Entry Inhibition. Viruses 2020, 12, 342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emerson, S.U.; Wagner, R.R. Dissociation and reconstitution of the transcriptase and template activities of vesicular stomatitis B and T virions. J. Virol. 1972, 10, 297–309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emerson, S.U.; Yu, Y. Both NS and L proteins are required for in vitro RNA synthesis by vesicular stomatitis virus. J. Virol. 1975, 15, 1348–1356. [Google Scholar] [CrossRef] [Green Version]
- Hamaguchi, M.; Yoshida, T.; Nishikawa, K.; Naruse, H.; Nagai, Y. Transcriptive complex of Newcastle disease virus. I. Both L and P proteins are required to constitute an active complex. Virology 1983, 128, 105–117. [Google Scholar] [CrossRef]
- Horikami, S.M.; Curran, J.; Kolakofsky, D.; Moyer, S.A. Complexes of Sendai virus NP-P and P-L proteins are required for defective interfering particle genome replication in vitro. J. Virol. 1992, 66, 4901–4908. [Google Scholar] [CrossRef] [Green Version]
- Stone, H.O.; Kingsbury, D.W.; Darlington, R.W. Sendai virus-induced transcriptase from infected cells: Polypeptides in the transcriptive complex. J. Virol. 1972, 10, 1037–1043. [Google Scholar] [CrossRef] [Green Version]
- Colonno, R.J.; Stone, H.O. Isolation of a transcriptive complex from Newcastle disease virions. J. Virol. 1976, 19, 1080–1089. [Google Scholar] [CrossRef] [Green Version]
- Portner, A.; Murti, K.G.; Morgan, E.M.; Kingsbury, D.W. Antibodies against Sendai virus L protein: Distribution of the protein in nucleocapsids revealed by immunoelectron microscopy. Virology 1988, 163, 236–239. [Google Scholar] [CrossRef]
- Mura, M.; Combredet, C.; Najburg, V.; David, R.Y.S.; Tangy, F.; Komarova, A.V. Nonencapsidated 5′ Copy-Back Defective Interfering Genomes Produced by Recombinant Measles Viruses Are Recognized by RIG-I and LGP2 but Not MDA5. J. Virol. 2017, 91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nilsson-Payant, B.E.; Blanco-Melo, D.; Uhl, S.; Escudero-Pérez, B.; Olschewski, S.; Thibault, P.; Panis, M.; Rosenthal, M.; Muñoz-Fontela, C.; Lee, B.; et al. Reduced Nucleoprotein Availability Impairs Negative-Sense RNA Virus Replication and Promotes Host Recognition. J. Virol. 2021, 95, e02274-20. [Google Scholar] [CrossRef] [PubMed]
- Compans, R.W.; Choppin, P.W. The nucleic acid of the parainfluenza virus SV5. Virology 1968, 35, 289–296. [Google Scholar] [CrossRef]
- Lynch, S.; Kolakofsky, D. Ends of the RNA within Sendai virus defective interfering nucleocapsids are not free. J. Virol. 1978, 28, 584–589. [Google Scholar] [CrossRef] [Green Version]
- Bitko, V.; Barik, S. Phenotypic silencing of cytoplasmic genes using sequence-specific double-stranded short interfering RNA and its application in the reverse genetics of wild type negative-strand RNA viruses. BMC Microbiol. 2001, 1, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mottet-Osman, G.; Iseni, F.; Pelet, T.; Wiznerowicz, M.; Garcin, D.; Roux, L. Suppression of the Sendai Virus M Protein through a Novel Short Interfering RNA Approach Inhibits Viral Particle Production but Does Not Affect Viral RNA Synthesis. J. Virol. 2007, 81, 2861–2868. [Google Scholar] [CrossRef] [Green Version]
- Alayyoubi, M.; Leser, G.P.; Kors, C.A.; Lamb, R.A. Structure of the paramyxovirus parainfluenza virus 5 nucleoprotein-RNA complex. Proc. Natl. Acad. Sci. USA 2015, 112, E1792–E1799. [Google Scholar] [CrossRef] [Green Version]
- Gutsche, I.; Desfosses, A.; Effantin, G.; Ling, W.L.; Haupt, M.; Ruigrok, R.W.H.; Sachse, C.; Schoehn, G. Near-atomic cryo-EM structure of the helical measles virus nucleocapsid. Science 2015, 348, 704–707. [Google Scholar] [CrossRef]
- Song, X.; Shan, H.; Zhu, Y.; Hu, S.; Xue, L.; Chen, Y.; Ding, W.; Niu, T.; Gu, J.; Ouyang, S.; et al. Self-capping of nucleoprotein filaments protects the Newcastle disease virus genome. eLife 2019, 8. [Google Scholar] [CrossRef]
- Desfosses, A.; Milles, S.; Jensen, M.R.; Guseva, S.; Colletier, J.-P.; Maurin, D.; Schoehn, G.; Gutsche, I.; Ruigrok, R.W.H.; Blackledge, M. Assembly and cryo-EM structures of RNA-specific measles virus nucleocapsids provide mechanistic insight into paramyxoviral replication. Proc. Natl. Acad. Sci. USA 2019, 116, 4256–4264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, N.; Shan, H.; Liu, M.; Li, T.; Luo, R.; Yang, L.; Qi, L.; Chu, X.; Su, X.; Wang, R.; et al. Structure and assembly of double-headed Sendai virus nucleocapsids. Commun. Biol. 2021, 4, 494. [Google Scholar] [CrossRef]
- Ker, D.-S.; Jenkins, H.T.; Greive, S.J.; Antson, A.A. CryoEM structure of the Nipah virus nucleocapsid assembly. PLoS Pathog. 2021, 17, e1009740. [Google Scholar] [CrossRef]
- Zinzula, L.; Beck, F.; Klumpe, S.; Bohn, S.; Pfeifer, G.; Bollschweiler, D.; Nagy, I.; Plitzko, J.M.; Baumeister, W. Cryo-EM structure of the cetacean morbillivirus nucleoprotein-RNA complex. J. Struct. Biol. 2021, 213, 107750. [Google Scholar] [CrossRef] [PubMed]
- Yabukarski, F.; Lawrence, P.; Tarbouriech, N.; Bourhis, J.-M.; Delaforge, E.; Jensen, M.R.; Ruigrok, R.W.H.; Blackledge, M.; Volchkov, V.; Jamin, M. Structure of Nipah virus unassembled nucleoprotein in complex with its viral chaperone. Nat. Struct. Mol. Biol. 2014, 21, 754–759. [Google Scholar] [CrossRef]
- Guryanov, S.G.; Liljeroos, L.; Kasaragod, P.; Kajander, T.; Butcher, S.J. Crystal Structure of the Measles Virus Nucleoprotein Core in Complex with an N-Terminal Region of Phosphoprotein. J. Virol. 2016, 90, 2849–2857. [Google Scholar] [CrossRef] [Green Version]
- Aggarwal, M.; Leser, G.P.; Kors, C.A.; Lamb, R.A. Structure of the Paramyxovirus Parainfluenza Virus 5 Nucleoprotein in Complex with an Amino-Terminal Peptide of the Phosphoprotein. J. Virol. 2018, 92, e01304-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, X.; Wang, X.; Xie, M.; Wu, W.; Chen, Z. Structural Basis of Human Parainfluenza Virus 3 Unassembled Nucleoprotein in Complex with Its Viral ChaperoneHPIV3 Nucleoprotein-Phosphoprotein Structure. J. Virol. 2021, JVI0164821. [Google Scholar] [CrossRef]
- Abdella, R.; Aggarwal, M.; Okura, T.; Lamb, R.A.; He, Y. Structure of a paramyxovirus polymerase complex reveals a unique methyltransferase-CTD conformation. Proc. Natl. Acad. Sci. USA 2020, 117, 4931–4941. [Google Scholar] [CrossRef]
- Glazier, K.; Raghow, R.; Kingsbury, D.W. Regulation of Sendai virus transcription: Evidence for a single promoter in vivo. J. Virol. 1977, 21, 863–871. [Google Scholar] [CrossRef] [Green Version]
- Le Mercier, P.; Kolakofsky, D. Bipartite promoters and RNA editing of paramyxoviruses and filoviruses. RNA 2019, 25, 279–285. [Google Scholar] [CrossRef] [PubMed]
- Plemper, R.K.; Lamb, R.A. Paramyxoviridae: The viruses and their replication. In Fields Virology: Emerging Viruses, 7th ed.; Howley, P.M., Knipe, D.M., Eds.; Lippincott Williams and Wilkins, Wolters Kluwer: Philadelphia, PA, USA, 2020; pp. 504–558. [Google Scholar]
- Calain, P.; Roux, L. The rule of six, a basic feature for efficient replication of Sendai virus defective interfering RNA. J. Virol. 1993, 67, 4822–4830. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolakofsky, D.; Pelet, T.; Garcin, D.; Hausmann, S.; Curran, J.; Roux, L. Paramyxovirus RNA synthesis and the requirement for hexamer genome length: The rule of six revisited. J. Virol. 1998, 72, 891–899. [Google Scholar] [CrossRef] [Green Version]
- Longhi, S.; Receveur-Brechot, V.; Karlin, D.; Johansson, K.; Darbon, H.; Bhella, D.; Yeo, R.; Finet, S.; Canard, B. The C-terminal Domain of the Measles Virus Nucleoprotein Is Intrinsically Disordered and Folds upon Binding to the C-terminal Moiety of the Phosphoprotein. J. Biol. Chem. 2003, 278, 18638–18648. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mountcastle, W.E.; Compans, R.W.; Lackland, H.; Choppin, P.W. Proteolytic cleavage of subunits of the nucleocapsid of the paramyxovirus simian virus 5. J. Virol. 1974, 14, 1253–1261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buchholz, C.J.; Spehner, D.; Drillien, R.; Neubert, W.J.; Homann, H.E. The conserved N-terminal region of Sendai virus nucleocapsid protein NP is required for nucleocapsid assembly. J. Virol. 1993, 67, 5803–5812. [Google Scholar] [CrossRef] [Green Version]
- Curran, J.; Homann, H.; Buchholz, C.; Rochat, S.; Neubert, W.; Kolakofsky, D. The hypervariable C-terminal tail of the Sendai paramyxovirus nucleocapsid protein is required for template function but not for RNA encapsidation. J. Virol. 1993, 67, 4358–4364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bankamp, B.; Horikami, S.M.; Thompson, P.; Huber, M.; Billeter, M.; Moyer, S.A. Domains of the Measles Virus N Protein Required for Binding to P Protein and Self-Assembly. Virology 1996, 216, 272–277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Buchholz, C.J.; Retzler, C.; Homann, H.E.; Neubert, W.J. The Carboxy-terminal Domain of Sendai Virus Nucleocapsid Protein Is Involved in Complex Formation between Phosphoprotein and Nucleocapsid-like Particles. Virology 1994, 204, 770–776. [Google Scholar] [CrossRef] [PubMed]
- Liston, P.; Batal, R.; Diflumeri, C.; Briedis, D.J. Protein interaction domains of the measles virus nucleocapsid protein (NP). Arch. Virol. 1997, 142, 305–321. [Google Scholar] [CrossRef]
- Rahmeh, A.A.; Schenk, A.; Danek, E.I.; Kranzusch, P.J.; Liang, B.; Walz, T.; Whelan, S.P.J. Molecular architecture of the vesicular stomatitis virus RNA polymerase. Proc. Natl. Acad. Sci. USA 2010, 107, 20075–20080. [Google Scholar] [CrossRef] [Green Version]
- Liang, B. Structures of the Mononegavirales Polymerases. J. Virol. 2020, 94, e00175-20. [Google Scholar] [CrossRef]
- Pyle, J.D.; Whelan, S.P.; Bloyet, L.-M. Structure and function of negative-strand RNA virus polymerase complexes. Enzymes 2021, 50, 21–78. [Google Scholar] [CrossRef]
- Abraham, G.; Rhodes, D.P.; Banerjee, A.K. Novel initiation of RNA synthesis in vitro by vesicular stomatitis virus. Nature 1975, 255, 37–40. [Google Scholar] [CrossRef] [PubMed]
- Ogino, T.; Banerjee, A.K. Unconventional Mechanism of mRNA Capping by the RNA-Dependent RNA Polymerase of Vesicular Stomatitis Virus. Mol. Cell 2007, 25, 85–97. [Google Scholar] [CrossRef]
- Hercyk, N.; Horikami, S.M.; Moyer, S.A. The vesicular stomatitis virus L protein possesses the mRNA methyltransferase activities. Virology 1988, 163, 222–225. [Google Scholar] [CrossRef]
- Ogino, T.; Kobayashi, M.; Iwama, M.; Mizumoto, K. Sendai Virus RNA-dependent RNA Polymerase L Protein Catalyzes Cap Methylation of Virus-specific mRNA. J. Biol. Chem. 2005, 280, 4429–4435. [Google Scholar] [CrossRef] [Green Version]
- Hunt, D.M.; Mehta, R.; Hutchinson, K.L. The L Protein of Vesicular Stomatitis Virus Modulates the Response of the Polyadenylic Acid Polymerase to S-Adenosylhomocysteine. J. Gen. Virol. 1988, 69, 2555–2561. [Google Scholar] [CrossRef]
- Jordan, P.C.; Liu, C.; Raynaud, P.; Lo, M.; Spiropoulou, C.F.; Symons, J.A.; Beigelman, L.; Deval, J. Initiation, extension, and termination of RNA synthesis by a paramyxovirus polymerase. PLoS Pathog. 2018, 14, e1006889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smallwood, S.; Ryan, K.W.; Moyer, S.A. Deletion Analysis Defines a Carboxyl-Proximal Region of Sendai Virus P Protein That Binds to the Polymerase L Protein. Virology 1994, 202, 154–163. [Google Scholar] [CrossRef] [PubMed]
- Canter, D.; Perrault, J. Stabilization of Vesicular Stomatitis Virus L Polymerase Protein by P Protein Binding: A Small Deletion in the C-Terminal Domain of L Abrogates Binding. Virology 1996, 219, 376–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bloyet, L.-M.; Welsch, J.; Enchery, F.; Mathieu, C.; de Breyne, S.; Horvat, B.; Grigorov, B.; Gerlier, D. HSP90 Chaperoning in Addition to Phosphoprotein Required for Folding but Not for Supporting Enzymatic Activities of Measles and Nipah Virus L Polymerases. J. Virol. 2016, 90, 6642–6656. [Google Scholar] [CrossRef] [Green Version]
- Mellon, M.G.; Emerson, S.U. Rebinding of transcriptase components (L and NS proteins) to the nucleocapsid template of vesicular stomatitis virus. J. Virol. 1978, 27, 560–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masters, P.S.; Banerjee, A.K. Complex formation with vesicular stomatitis virus phosphoprotein NS prevents binding of nucleocapsid protein N to nonspecific RNA. J. Virol. 1988, 62, 2658–2664. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curran, J.; Marq, J.B.; Kolakofsky, D. An N-terminal domain of the Sendai paramyxovirus P protein acts as a chaperone for the NP protein during the nascent chain assembly step of genome replication. J. Virol. 1995, 69, 849–855. [Google Scholar] [CrossRef] [Green Version]
- Spehner, D.; Drillien, R.; Howley, P.M. The Assembly of the Measles Virus Nucleoprotein into Nucleocapsid-like Particles Is Modulated by the Phosphoprotein. Virology 1997, 232, 260–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karlin, D.; Ferron, F.; Canard, B.; Longhi, S. Structural disorder and modular organization in Paramyxovirinae N and P. J. Gen. Virol. 2003, 84, 3239–3252. [Google Scholar] [CrossRef]
- Gerard, F.C.; Ribeiro, E.D.A.; Leyrat, C.; Ivanov, I.; Blondel, D.; Longhi, S.; Ruigrok, R.W.; Jamin, M. Modular Organization of Rabies Virus Phosphoprotein. J. Mol. Biol. 2009, 388, 978–996. [Google Scholar] [CrossRef] [PubMed]
- Habchi, J.; Mamelli, L.; Darbon, H.; Longhi, S. Structural Disorder within Henipavirus Nucleoprotein and Phosphoprotein: From Predictions to Experimental Assessment. PLoS ONE 2010, 5, e11684. [Google Scholar] [CrossRef]
- Pereira, N.; Cardone, C.; Lassoued, S.; Galloux, M.; Fix, J.; Assrir, N.; Lescop, E.; Bontems, F.; Eléouët, J.-F.; Sizun, C. New Insights into Structural Disorder in Human Respiratory Syncytial Virus Phosphoprotein and Implications for Binding of Protein Partners. J. Biol. Chem. 2017, 292, 2120–2131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Longhi, S.; Bloyet, L.-M.; Gianni, S.; Gerlier, D. How order and disorder within paramyxoviral nucleoproteins and phosphoproteins orchestrate the molecular interplay of transcription and replication. Cell. Mol. Life Sci. 2017, 74, 3091–3118. [Google Scholar] [CrossRef]
- Jensen, M.R.; Yabukarski, F.; Communie, G.; Condamine, E.; Mas, C.; Volchkova, V.; Tarbouriech, N.; Bourhis, J.-M.; Volchkov, V.; Blackledge, M.; et al. Structural Description of the Nipah Virus Phosphoprotein and Its Interaction with STAT1. Biophys. J. 2020, 118, 2470–2488. [Google Scholar] [CrossRef]
- Tarbouriech, N.; Curran, J.; Ruigrok, R.W.; Burmeister, W.P. Tetrameric coiled coil domain of Sendai virus phosphoprotein. Nat. Genet. 2000, 7, 777–781. [Google Scholar] [CrossRef]
- Communie, G.; Crepin, T.; Maurin, D.; Jensen, M.R.; Blackledge, M.; Ruigrok, R.W.H. Structure of the Tetramerization Domain of Measles Virus Phosphoprotein. J. Virol. 2013, 87, 7166–7169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blocquel, D.; Habchi, J.; Durand, E.; Sevajol, M.; Ferron, F.; Erales, J.; Papageorgiou, N.; Longhi, S. Coiled-coil deformations in crystal structures: The measles virus phosphoprotein multimerization domain as an illustrative example. Acta Crystallogr. D Biol. Crystallogr. 2014, 70, 1589–1603. [Google Scholar] [CrossRef] [PubMed]
- Bruhn, J.F.; Barnett, K.; Bibby, J.; Thomas, J.M.H.; Keegan, R.; Rigden, D.J.; Bornholdt, Z.A.; Saphire, E.O. Crystal Structure of the Nipah Virus Phosphoprotein Tetramerization Domain. J. Virol. 2013, 88, 758–762. [Google Scholar] [CrossRef] [Green Version]
- Galvan, J.R.; Donner, B.; Veseley, C.H.; Reardon, P.; Forsythe, H.M.; Howe, J.; Fujimura, G.; Barbar, E. Human Parainfluenza Virus 3 Phosphoprotein Is a Tetramer and Shares Structural and Interaction Features with Ebola Phosphoprotein VP35. Biomolecules 2021, 11, 1603. [Google Scholar] [CrossRef] [PubMed]
- Cox, R.; Green, T.J.; Purushotham, S.; Deivanayagam, C.; Bedwell, G.J.; Prevelige, P.E.; Luo, M. Structural and Functional Characterization of the Mumps Virus Phosphoprotein. J. Virol. 2013, 87, 7558–7568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pickar, A.; Elson, A.; Yang, Y.; Xu, P.; Luo, M.; He, B. Oligomerization of Mumps Virus Phosphoprotein. J. Virol. 2015, 89, 11002–11010. [Google Scholar] [CrossRef] [Green Version]
- Ryan, K.W.; Kingsbury, D.W. Carboxyl-terminal region of sendai virus P protein is required for binding to viral nucleocapsids. Virology 1988, 167, 106–112. [Google Scholar] [CrossRef]
- Johansson, K.; Bourhis, J.-M.; Campanacci, V.; Cambillau, C.; Canard, B.; Longhi, S. Crystal Structure of the Measles Virus Phosphoprotein Domain Responsible for the Induced Folding of the C-terminal Domain of the Nucleoprotein. J. Biol. Chem. 2003, 278, 44567–44573. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blanchard, L.; Tarbouriech, N.; Blackledge, M.; Timmins, P.; Burmeister, W.P.; Ruigrok, R.W.; Marion, D. Structure and dynamics of the nucleocapsid-binding domain of the Sendai virus phosphoprotein in solution. Virology 2004, 319, 201–211. [Google Scholar] [CrossRef] [Green Version]
- Kingston, R.L.; Hamel, D.J.; Gay, L.S.; Dahlquist, F.W.; Matthews, B.W. Structural basis for the attachment of a paramyxoviral polymerase to its template. Proc. Natl. Acad. Sci. USA 2004, 101, 8301–8306. [Google Scholar] [CrossRef] [Green Version]
- Habchi, J.; Blangy, S.; Mamelli, L.; Jensen, M.R.; Blackledge, M.; Darbon, H.; Oglesbee, M.; Shu, Y.; Longhi, S. Characterization of the Interactions between the Nucleoprotein and the Phosphoprotein of Henipavirus. J. Biol. Chem. 2011, 286, 13583–13602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Communie, G.; Habchi, J.; Yabukarski, F.; Blocquel, D.; Schneider, R.; Tarbouriech, N.; Papageorgiou, N.; Ruigrok, R.W.H.; Jamin, M.; Jensen, M.R.; et al. Atomic Resolution Description of the Interaction between the Nucleoprotein and Phosphoprotein of Hendra Virus. PLoS Pathog. 2013, 9, e1003631. [Google Scholar] [CrossRef] [PubMed]
- Blumberg, B.M.; Leppert, M.; Kolakofsky, D. Interaction of VSV leader RNA and nucleocapsid protein may control VSV genome replication. Cell 1981, 23, 837–845. [Google Scholar] [CrossRef]
- Plumet, S.; Duprex, W.P.; Gerlier, D. Dynamics of Viral RNA Synthesis during Measles Virus Infection. J. Virol. 2005, 79, 6900–6908. [Google Scholar] [CrossRef] [Green Version]
- Ranadheera, C.; Proulx, R.; Chaiyakul, M.; Jones, S.; Grolla, A.; Leung, A.; Rutherford, J.; Kobasa, D.; Carpenter, M.; Czub, M. The interaction between the Nipah virus nucleocapsid protein and phosphoprotein regulates virus replication. Sci. Rep. 2018, 8, 15994. [Google Scholar] [CrossRef] [PubMed]
- Gubbay, O.; Curran, J.; Kolakofsky, D. Sendai virus genome synthesis and assembly are coupled: A possible mechanism to promote viral RNA polymerase processivity. J. Gen. Virol. 2001, 82, 2895–2903. [Google Scholar] [CrossRef] [PubMed]
- Guseva, S.; Milles, S.; Jensen, M.R.; Schoehn, G.; Ruigrok, R.W.; Blackledge, M. Structure, dynamics and phase separation of measles virus RNA replication machinery. Curr. Opin. Virol. 2020, 41, 59–67. [Google Scholar] [CrossRef]
- Nikolic, J.; Lagaudrière-Gesbert, C.; Scrima, N.; Blondel, D.; Gaudin, Y. Structure and Function of Negri Bodies. Adv. Exp. Med. Biol. 2019, 1215, 111–127. [Google Scholar] [CrossRef] [PubMed]
- Brocca, S.; Grandori, R.; Longhi, S.; Uversky, V. Liquid-Liquid Phase Separation by Intrinsically Disordered Protein Regions of Viruses: Roles in Viral Life Cycle and Control of Virus-Host Interactions. Int. J. Mol. Sci. 2020, 21, 9045. [Google Scholar] [CrossRef] [PubMed]
- Hagiwara, K.; Sato, H.; Inoue, Y.; Watanabe, A.; Yoneda, M.; Ikeda, F.; Fujita, K.; Fukuda, H.; Takamura, C.; Kozuka-Hata, H.; et al. Phosphorylation of measles virus nucleoprotein upregulates the transcriptional activity of minigenomic RNA. Proteomics 2008, 8, 1871–1879. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Sato, H.; Hagiwara, K.; Watanabe, A.; Sugai, A.; Ikeda, F.; Kozuka-Hata, H.; Oyama, M.; Yoneda, M.; Kai, C. Determination of a phosphorylation site in Nipah virus nucleoprotein and its involvement in virus transcription. J. Gen. Virol. 2011, 92, 2133–2141. [Google Scholar] [CrossRef] [PubMed]
- Zengel, J.; Pickar, A.; Xu, P.; Lin, A.; He, B. Roles of Phosphorylation of the Nucleocapsid Protein of Mumps Virus in Regulating Viral RNA Transcription and Replication. J. Virol. 2015, 89, 7338–7347. [Google Scholar] [CrossRef] [Green Version]
- Vidal, S.; Curran, J.; Orvell, C.; Kolakofsky, D. Mapping of monoclonal antibodies to the Sendai virus P protein and the location of its phosphates. J. Virol. 1988, 62, 2200–2203. [Google Scholar] [CrossRef] [Green Version]
- Das, T.; Schuster, A.; Schneider-Schaulies, S.; Banerjee, A.K. Involvement of Cellular Casein Kinase II in the Phosphorylation of Measles Virus P Protein: Identification of Phosphorylation Sites. Virology 1995, 211, 218–226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huntley, C.C.; De, B.P.; Murray, N.R.; Fields, A.P.; Banerjee, A.K. Human parainfluenza virus type 3 phosphoprotein: Identification of serine 333 as the major site for PKC zeta phosphorylation. Virology 1995, 211, 561–567. [Google Scholar] [CrossRef] [Green Version]
- Byrappa, S.; Pan, Y.-B.; Gupta, K.C. Sendai Virus P Protein Is Constitutively Phosphorylated at Serine249: High Phosphorylation Potential of the P Protein. Virology 1996, 216, 228–234. [Google Scholar] [CrossRef] [Green Version]
- Timani, K.A.; Sun, D.; Sun, M.; Keim, C.; Lin, Y.; Schmitt, P.T.; Schmitt, A.; He, B. A Single Amino Acid Residue Change in the P Protein of Parainfluenza Virus 5 Elevates Viral Gene Expression. J. Virol. 2008, 82, 9123–9133. [Google Scholar] [CrossRef] [Green Version]
- Sun, D.; Luthra, P.; Xu, P.; Yoon, H.; He, B. Identification of a Phosphorylation Site within the P Protein Important for mRNA Transcription and Growth of Parainfluenza Virus 5. J. Virol. 2011, 85, 8376–8385. [Google Scholar] [CrossRef] [Green Version]
- Sugai, A.; Sato, H.; Yoneda, M.; Kai, C. Phosphorylation of measles virus phosphoprotein at S86 and/or S151 downregulates viral transcriptional activity. FEBS Lett. 2012, 586, 3900–3907. [Google Scholar] [CrossRef] [PubMed]
- Pickar, A.; Xu, P.; Elson, A.; Li, Z.; Zengel, J.; He, B. Roles of Serine and Threonine Residues of Mumps Virus P Protein in Viral Transcription and Replication. J. Virol. 2014, 88, 4414–4422. [Google Scholar] [CrossRef] [Green Version]
- Qiu, X.; Zhan, Y.; Meng, C.; Wang, J.; Dong, L.; Sun, Y.; Tan, L.; Song, C.; Yu, S.; Ding, C. Identification and functional analysis of phosphorylation in Newcastle disease virus phosphoprotein. Arch. Virol. 2016, 161, 2103–2116. [Google Scholar] [CrossRef]
- De, B.P.; Gupta, S.; Banerjee, A.K. Cellular protein kinase C isoform zeta regulates human parainfluenza virus type 3 replication. Proc. Natl. Acad. Sci. USA 1995, 92, 5204–5208. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Huntley, C.C.; De, B.P.; Das, T.; Banerjee, A.K.; Oglesbee, M.J. Phosphorylation of canine distemper virus P protein by protein kinase C-zeta and casein kinase II. Virology 1997, 232, 198–206. [Google Scholar] [CrossRef] [Green Version]
- Huntley, C.C.; De, B.P.; Banerjee, A.K. Phosphorylation of Sendai virus phosphoprotein by cellular protein kinase C zeta. J. Biol. Chem. 1997, 272, 16578–16584. [Google Scholar] [CrossRef] [Green Version]
- Kaushik, R.; Shaila, M.S. Cellular casein kinase II-mediated phosphorylation of rinderpest virus P protein is a prerequisite for its role in replication/transcription of the genome. J. Gen. Virol. 2004, 85, 687–691. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Fuentes, S.M.; Timani, K.; Sun, D.; Murphy, C.; Lin, Y.; August, A.; Teng, M.N.; He, B. Akt Plays a Critical Role in Replication of Nonsegmented Negative-Stranded RNA Viruses. J. Virol. 2008, 82, 105–114. [Google Scholar] [CrossRef] [Green Version]
- Sun, D.; Luthra, P.; Li, Z.; He, B. PLK1 Down-Regulates Parainfluenza Virus 5 Gene Expression. PLoS Pathog. 2009, 5, e1000525. [Google Scholar] [CrossRef] [Green Version]
- Sugai, A.; Sato, H.; Yoneda, M.; Kai, C. PIM 3 kinase, a proto-oncogene product, regulates phosphorylation of the measles virus nucleoprotein tail domain at Ser 479 and Ser 510. Biochem. Biophys. Res. Commun. 2020, 531. [Google Scholar] [CrossRef]
- Young, D.F.; Wignall-Fleming, E.B.; Busse, D.C.; Pickin, M.J.; Hankinson, J.; Randall, E.M.; Tavendale, A.; Davison, A.J.; Lamont, D.; Tregoning, J.S.; et al. The switch between acute and persistent paramyxovirus infection caused by single amino acid substitutions in the RNA polymerase P subunit. PLoS Pathog. 2019, 15, e1007561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saikia, P.; Gopinath, M.; Shaila, M.S. Phosphorylation status of the phosphoprotein P of rinderpest virus modulates transcription and replication of the genome. Arch. Virol. 2008, 153, 615–626. [Google Scholar] [CrossRef] [PubMed]
- Sugai, A.; Sato, H.; Yoneda, M.; Kai, C. Phosphorylation of Measles Virus Nucleoprotein Affects Viral Growth by Changing Gene Expression and Genomic RNA Stability. J. Virol. 2013, 87, 11684–11692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugai, A.; Sato, H.; Hagiwara, K.; Kozuka-Hata, H.; Oyama, M.; Yoneda, M.; Kai, C. Newly Identified Minor Phosphorylation Site Threonine-279 of Measles Virus Nucleoprotein Is a Prerequisite for Nucleocapsid Formation. J. Virol. 2013, 88, 1140–1149. [Google Scholar] [CrossRef] [Green Version]
- Zhou, Y.; Su, J.M.; Samuel, C.E.; Ma, D. Measles Virus Forms Inclusion Bodies with Properties of Liquid Organelles. J. Virol. 2019, 93, e00948-19. [Google Scholar] [CrossRef]
- Horne, R.W.; Waterson, A.P.; Wildy, P.; Farnham, A.E. The structure and composition of the myxoviruses. I. Electron microscope studies of the structure of myxovirus particles by negative staining techniques. Virology 1960, 11, 79–98. [Google Scholar] [CrossRef]
- Compans, R.; Choppin, P.W. Isolation and properties of the helical nucleocapsid of the parainfluenza virus SV5. Proc. Natl. Acad. Sci. USA 1967, 57, 949–956. [Google Scholar] [CrossRef] [Green Version]
- Finch, J.T.; Gibbs, A.J. Observations on the Structure of the Nucleocapsids of some Paramyxoviruses. J. Gen. Virol. 1970, 6, 141–150. [Google Scholar] [CrossRef]
- Egelman, E.H.; Wu, S.S.; Amrein, M.; Portner, A.; Murti, G. The Sendai virus nucleocapsid exists in at least four different helical states. J. Virol. 1989, 63, 2233–2243. [Google Scholar] [CrossRef] [Green Version]
- Heggeness, M.H.; Scheid, A.; Choppin, P.W. Conformation of the helical nucleocapsids of paramyxoviruses and vesicular stomatitis virus: Reversible coiling and uncoiling induced by changes in salt concentration. Proc. Natl. Acad. Sci. USA 1980, 77, 2631–2635. [Google Scholar] [CrossRef] [Green Version]
- Bhella, D.; Ralph, A.; Murphy, L.B.; Yeo, R.P. Significant differences in nucleocapsid morphology within the Paramyxoviridae. J. Gen. Virol. 2002, 83, 1831–1839. [Google Scholar] [CrossRef]
- Bhella, D.; Ralph, A.; Yeo, R.P. Conformational Flexibility in Recombinant Measles Virus Nucleocapsids Visualised by Cryo-negative Stain Electron Microscopy and Real-space Helical Reconstruction. J. Mol. Biol. 2004, 340, 319–331. [Google Scholar] [CrossRef]
- Cox, R.; Pickar, A.; Qiu, S.; Tsao, J.; Rodenburg, C.; Dokland, T.; Elson, A.; He, B.; Luo, M. Structural studies on the authentic mumps virus nucleocapsid showing uncoiling by the phosphoprotein. Proc. Natl. Acad. Sci. USA 2014, 111, 15208–15213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ke, Z.; Strauss, J.D.; Hampton, C.M.; Brindley, M.A.; Dillard, R.S.; Leon, F.; Lamb, K.M.; Plemper, R.K.; Wright, E.R. Promotion of virus assembly and organization by the measles virus matrix protein. Nat. Commun. 2018, 9, 1736. [Google Scholar] [CrossRef]
- Nakai, M.; Imagawa, D.T. Electron microscopy of measles virus replication. J. Virol. 1969, 3, 187–197. [Google Scholar] [CrossRef] [Green Version]
- Desfosses, A.; Goret, G.; Estrozi, L.F.; Ruigrok, R.W.H.; Gutsche, I. Nucleoprotein-RNA Orientation in the Measles Virus Nucleocapsid by Three-Dimensional Electron Microscopy. J. Virol. 2011, 85, 1391–1395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spehner, D.; Kirn, A.; Drillien, R. Assembly of nucleocapsidlike structures in animal cells infected with a vaccinia virus recombinant encoding the measles virus nucleoprotein. J. Virol. 1991, 65, 6296–6300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fooks, A.R.; Stephenson, J.R.; Warnes, A.; Dowsett, A.B.; Rima, B.K.; Wilkinson, G.W.G. Measles virus nucleocapsid protein expressed in insect cells assembles into nucleocapsid-like structures. J. Gen. Virol. 1993, 74, 1439–1444. [Google Scholar] [CrossRef] [PubMed]
- Warnes, A.; Fooks, A.R.; Dowsett, A.; Wilkinson, G.W.; Stephenson, J.R. Expression of the measles virus nucleoprotein gene in Escherichia coli and assembly of nucleocapsid-like structures. Gene 1995, 160, 173–178. [Google Scholar] [CrossRef]
- Schoehn, G.; Mavrakis, M.; Albertini, A.; Wade, R.; Hoenger, A.; Ruigrok, R.W. The 12 Å Structure of Trypsin-treated Measles Virus N-RNA. J. Mol. Biol. 2004, 339, 301–312. [Google Scholar] [CrossRef] [PubMed]
- Cox, R.; Green, T.J.; Qiu, S.; Kang, J.; Tsao, J.; Prevelige, P.E.; He, B.; Luo, M. Characterization of a Mumps Virus Nucleocapsidlike Particle. J. Virol. 2009, 83, 11402–11406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rager, M.; Vongpunsawad, S.; Duprex, W.P.; Cattaneo, R. Polyploid measles virus with hexameric genome length. EMBO J. 2002, 21, 2364–2372. [Google Scholar] [CrossRef]
- Loney, C.; Mottet-Osman, G.; Roux, L.; Bhella, D. Paramyxovirus Ultrastructure and Genome Packaging: Cryo-Electron Tomography of Sendai Virus. J. Virol. 2009, 83, 8191–8197. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goff, P.H.; Gao, Q.; Palese, P. A Majority of Infectious Newcastle Disease Virus Particles Contain a Single Genome, while a Minority Contain Multiple Genomes. J. Virol. 2012, 86, 10852–10856. [Google Scholar] [CrossRef] [Green Version]
- Shan, H.; Su, X.; Li, T.; Qin, Y.; Zhang, N.; Yang, L.; Ma, L.; Bai, Y.; Qi, L.; Liu, Y.; et al. Structural plasticity of mumps virus nucleocapsids with cryo-EM structures. Commun. Biol. 2021, 4, 833. [Google Scholar] [CrossRef]
- Baronti, L.; Erales, J.; Habchi, J.; Felli, I.C.; Pierattelli, R.; Longhi, S. Dynamics of the Intrinsically Disordered C-Terminal Domain of the Nipah Virus Nucleoprotein and Interaction with the X Domain of the Phosphoprotein as Unveiled by NMR Spectroscopy. ChemBioChem 2014, 16, 268–276. [Google Scholar] [CrossRef]
- Houben, K.; Marion, D.; Tarbouriech, N.; Ruigrok, R.W.H.; Blanchard, L. Interaction of the C-Terminal Domains of Sendai Virus N and P Proteins: Comparison of Polymerase-Nucleocapsid Interactions within the Paramyxovirus Family. J. Virol. 2007, 81, 6807–6816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tawar, R.G.; Duquerroy, S.; Vonrhein, C.; Varela, P.F.; Damier-Piolle, L.; Castagné, N.; MacLellan, K.; Bedouelle, H.; Bricogne, G.; Bhella, D.; et al. Crystal Structure of a Nucleocapsid-Like Nucleoprotein-RNA Complex of Respiratory Syncytial Virus. Science 2009, 326, 1279–1283. [Google Scholar] [CrossRef]
- Bakker, S.E.; Duquerroy, S.; Galloux, M.; Loney, C.; Conner, E.; Eléouët, J.-F.; Rey, F.; Bhella, D. The respiratory syncytial virus nucleoprotein-RNA complex forms a left-handed helical nucleocapsid. J. Gen. Virol. 2013, 94, 1734–1738. [Google Scholar] [CrossRef]
- Renner, M.; Bertinelli, M.; Leyrat, C.; Paesen, G.C.; De Oliveira, L.F.S.; Huiskonen, J.T.; Grimes, J.M. Nucleocapsid assembly in pneumoviruses is regulated by conformational switching of the N protein. eLife 2016, 5, e12627. [Google Scholar] [CrossRef]
- Dong, S.; Yang, P.; Li, G.; Liu, B.; Wang, W.; Liu, X.; Xia, B.; Yang, C.; Lou, Z.; Guo, Y.; et al. Insight into the Ebola virus nucleocapsid assembly mechanism: Crystal structure of Ebola virus nucleoprotein core domain at 1.8 Å resolution. Protein Cell 2015, 6, 351–362. [Google Scholar] [CrossRef] [Green Version]
- Wan, W.; Kolesnikova, L.; Clarke, M.; Koehler, A.; Noda, T.; Becker, S.; Briggs, J.A.G. Structure and assembly of the Ebola virus nucleocapsid. Nature 2017, 551, 394–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugita, Y.; Matsunami, H.; Kawaoka, Y.; Noda, T.; Wolf, M. Cryo-EM structure of the Ebola virus nucleoprotein-RNA complex at 3.6 A resolution. Nature 2018, 563, 137–140. [Google Scholar] [CrossRef] [Green Version]
- Su, Z.; Wu, C.; Shi, L.; Luthra, P.; Pintilie, G.D.; Johnson, B.; Porter, J.R.; Ge, P.; Chen, M.; Liu, G.; et al. Electron Cryo-microscopy Structure of Ebola Virus Nucleoprotein Reveals a Mechanism for Nucleocapsid-like Assembly. Cell 2018, 172, 966–978.e12. [Google Scholar] [CrossRef] [Green Version]
- Kirchdoerfer, R.N.; Saphire, E.O.; Ward, A.B. Cryo-EM structure of the Ebola virus nucleoprotein-RNA complex. Acta Crystallogr. Sect. F Struct. Biol. Commun. 2019, 75, 340–347. [Google Scholar] [CrossRef]
- Liu, B.; Dong, S.; Li, G.; Wang, W.; Liu, X.; Wang, Y.; Yang, C.; Rao, Z.; Guo, Y. Structural Insight into Nucleoprotein Conformation Change Chaperoned by VP35 Peptide in Marburg Virus. J. Virol. 2017, 91, e00825-17. [Google Scholar] [CrossRef] [Green Version]
- Rudolph, M.G.; Kraus, I.; Dickmanns, A.; Eickmann, M.; Garten, W.; Ficner, R. Crystal Structure of the Borna Disease Virus Nucleoprotein. Structure 2003, 11, 1219–1226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, T.J.; Zhang, X.; Wertz, G.W.; Luo, M. Structure of the Vesicular Stomatitis Virus Nucleoprotein-RNA Complex. Science 2006, 313, 357–360. [Google Scholar] [CrossRef]
- Albertini, A.A.V.; Wernimont, A.K.; Muziol, T.M.; Ravelli, R.B.G.; Clapier, C.R.; Schoehn, G.; Weissenhorn, W.; Ruigrok, R.W.H. Crystal Structure of the Rabies Virus Nucleoprotein-RNA Complex. Science 2006, 313, 360–363. [Google Scholar] [CrossRef]
- Luo, M.; Terrell, J.R.; McManus, S.A. Nucleocapsid Structure of Negative Strand RNA Virus. Viruses 2020, 12, 835. [Google Scholar] [CrossRef]
- Ge, P.; Tsao, J.; Schein, S.; Green, T.J.; Luo, M.; Zhou, Z.H. Cryo-EM Model of the Bullet-Shaped Vesicular Stomatitis Virus. Science 2010, 327, 689–693. [Google Scholar] [CrossRef] [Green Version]
- Riedel, C.; Vasishtan, D.; Pražák, V.; Ghanem, A.; Conzelmann, K.-K.; Rümenapf, T. Cryo EM structure of the rabies virus ribonucleoprotein complex. Sci. Rep. 2019, 9, 9639. [Google Scholar] [CrossRef] [Green Version]
- Iseni, F.; Baudin, F.; Garcin, D.; Marq, J.-B.; Ruigrok, R.W.; Kolakofsky, D. Chemical modification of nucleotide bases and mRNA editing depend on hexamer or nucleoprotein phase in Sendai virus nucleocapsids. RNA 2002, 8, 1056–1067. [Google Scholar] [CrossRef] [Green Version]
- Pelet, T.; Delenda, C.; Gubbay, O.; Garcin, D.; Kolakofsky, D. Partial Characterization of a Sendai Virus Replication Promoter and the Rule of Six. Virology 1996, 224, 405–414. [Google Scholar] [CrossRef] [Green Version]
- Tapparel, C.; Maurice, D.; Roux, L. The activity of Sendai virus genomic and antigenomic promoters requires a second element past the leader template regions: A motif (GNNNNN)3 is essential for replication. J. Virol. 1998, 72, 3117–3128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, S.K.; Parks, G.D. RNA replication for the paramyxovirus simian virus 5 requires an internal repeated (CGNNNN) sequence motif. J. Virol. 1999, 73, 805–809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marcos, F.; Ferreira, L.; Cros, J.; Park, M.-S.; Nakaya, T.; García-Sastre, A.; Villar, E. Mapping of the RNA promoter of Newcastle disease virus. Virology 2005, 331, 396–406. [Google Scholar] [CrossRef] [Green Version]
- Matsumoto, Y.; Ohta, K.; Kolakofsky, D.; Nishio, M. A Point Mutation in the RNA-Binding Domain of Human Parainfluenza Virus Type 2 Nucleoprotein Elicits Abnormally Enhanced Polymerase Activity. J. Virol. 2017, 91, e02203-16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsumoto, Y.; Ohta, K.; Kolakofsky, D.; Nishio, M. The control of paramyxovirus genome hexamer length and mRNA editing. RNA 2018, 24, 461–467. [Google Scholar] [CrossRef] [Green Version]
- Vidal, S.; Curran, J.; Kolakofsky, D. A stuttering model for paramyxovirus P mRNA editing. EMBO J. 1990, 9, 2017–2022. [Google Scholar] [CrossRef] [PubMed]
- Jensen, M.R.; Communie, G.; Ribeiro, E.A.; Martinez, N.; Desfosses, A.; Salmon, L.; Mollica, L.; Gabel, F.; Jamin, M.; Longhi, S.; et al. Intrinsic disorder in measles virus nucleocapsids. Proc. Natl. Acad. Sci. USA 2011, 108, 9839–9844. [Google Scholar] [CrossRef] [Green Version]
- Ryan, K.W.; Portner, A.; Murti, K. Antibodies to Paramyxovirus Nucleoproteins Define Regions Important for Immunogenicity and Nucleocapsid Assembly. Virology 1993, 193, 376–384. [Google Scholar] [CrossRef]
- Zhang, X.; Glendening, C.; Linke, H.; Parks, C.L.; Brooks, C.; Udem, S.A.; Oglesbee, M. Identification and Characterization of a Regulatory Domain on the Carboxyl Terminus of the Measles Virus Nucleocapsid Protein. J. Virol. 2002, 76, 8737–8746. [Google Scholar] [CrossRef] [Green Version]
- Chan, Y.P.; Koh, C.L.; Lam, S.K.; Wang, L.-F. Mapping of domains responsible for nucleocapsid protein-phosphoprotein interaction of henipaviruses. J. Gen. Virol. 2004, 85, 1675–1684. [Google Scholar] [CrossRef] [PubMed]
- Bourhis, J.-M.; Johansson, K.; Receveur-Bréchot, V.; Oldfield, C.J.; Dunker, K.A.; Canard, B.; Longhi, S. The C-terminal domain of measles virus nucleoprotein belongs to the class of intrinsically disordered proteins that fold upon binding to their physiological partner. Virus Res. 2004, 99, 157–167. [Google Scholar] [CrossRef] [PubMed]
- Dosnon, M.; Bonetti, D.; Morrone, A.; Erales, J.; Di Silvio, E.; Longhi, S.; Gianni, S. Demonstration of a Folding after Binding Mechanism in the Recognition between the Measles Virus NTAIL and X Domains. ACS Chem. Biol. 2014, 10, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Coronel, E.C.; Takimoto, T.; Murti, K.G.; Varich, N.; Portner, A. Nucleocapsid Incorporation into Parainfluenza Virus Is Regulated by Specific Interaction with Matrix Protein. J. Virol. 2001, 75, 1117–1123. [Google Scholar] [CrossRef] [Green Version]
- Iwasaki, M.; Takeda, M.; Shirogane, Y.; Nakatsu, Y.; Nakamura, T.; Yanagi, Y. The matrix protein of measles virus regulates viral RNA synthesis and assembly by interacting with the nucleocapsid protein. J. Virol. 2009, 83, 10374–10383. [Google Scholar] [CrossRef] [Green Version]
- Schmitt, P.T.; Ray, G.; Schmitt, A. The C-Terminal End of Parainfluenza Virus 5 NP Protein Is Important for Virus-Like Particle Production and M-NP Protein Interaction. J. Virol. 2010, 84, 12810–12823. [Google Scholar] [CrossRef] [Green Version]
- Liljeroos, L.; Huiskonen, J.; Ora, A.; Susi, P.; Butcher, S.J. Electron cryotomography of measles virus reveals how matrix protein coats the ribonucleocapsid within intact virions. Proc. Natl. Acad. Sci. USA 2011, 108, 18085–18090. [Google Scholar] [CrossRef] [Green Version]
- Ray, G.; Schmitt, P.T.; Schmitt, A.P. C-Terminal DxD-Containing Sequences within Paramyxovirus Nucleocapsid Proteins Determine Matrix Protein Compatibility and Can Direct Foreign Proteins into Budding Particles. J. Virol. 2016, 90, 3650–3660. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.; Bourhis, J.-M.; Longhi, S.; Carsillo, T.; Buccellato, M.; Morin, B.; Canard, B.; Oglesbee, M. Hsp72 recognizes a P binding motif in the measles virus N protein C-terminus. Virology 2005, 337, 162–174. [Google Scholar] [CrossRef] [Green Version]
- Couturier, M.; Buccellato, M.; Costanzo, S.; Bourhis, J.-M.; Shu, Y.; Nicaise, M.; Desmadril, M.; Flaudrops, C.; Longhi, S.; Oglesbee, M. High affinity binding between Hsp70 and the C-terminal domain of the measles virus nucleoprotein requires an Hsp40 co-chaperone. J. Mol. Recognit. 2009, 23, 301–315. [Google Scholar] [CrossRef]
- Watanabe, A.; Yoneda, M.; Ikeda, F.; Sugai, A.; Sato, H.; Kai, C. Peroxiredoxin 1 Is Required for Efficient Transcription and Replication of Measles Virus. J. Virol. 2010, 85, 2247–2253. [Google Scholar] [CrossRef] [Green Version]
- Mavrakis, M.; Méhouas, S.; Réal, E.; Iseni, F.; Blondel, D.; Tordo, N.; Ruigrok, R.W. Rabies virus chaperone: Identification of the phosphoprotein peptide that keeps nucleoprotein soluble and free from non-specific RNA. Virology 2006, 349, 422–429. [Google Scholar] [CrossRef] [Green Version]
- Karlin, D.; Belshaw, R. Detecting Remote Sequence Homology in Disordered Proteins: Discovery of Conserved Motifs in the N-Termini of Mononegavirales phosphoproteins. PLoS ONE 2012, 7, e31719. [Google Scholar] [CrossRef] [Green Version]
- Leyrat, C.; Yabukarski, F.; Tarbouriech, N.; Ribeiro, E.A., Jr.; Jensen, M.R.; Blackledge, M.; Ruigrok, R.W.H.; Jamin, M. Structure of the Vesicular Stomatitis Virus N0-P Complex. PLoS Pathog. 2011, 7, e1002248. [Google Scholar] [CrossRef]
- Kirchdoerfer, R.N.; Abelson, D.M.; Li, S.; Wood, M.R.; Saphire, E.O. Assembly of the Ebola Virus Nucleoprotein from a Chaperoned VP35 Complex. Cell Rep. 2015, 12, 140–149. [Google Scholar] [CrossRef] [Green Version]
- Leung, D.W.; Borek, D.; Luthra, P.; Binning, J.M.; Anantpadma, M.; Liu, G.; Harvey, I.B.; Su, Z.; Endlich-Frazier, A.; Pan, J.; et al. An Intrinsically Disordered Peptide from Ebola Virus VP35 Controls Viral RNA Synthesis by Modulating Nucleoprotein-RNA Interactions. Cell Rep. 2015, 11, 376–389. [Google Scholar] [CrossRef] [Green Version]
- Zhu, T.; Song, H.; Peng, R.; Shi, Y.; Qi, J.; Gao, G.F. Crystal Structure of the Marburg Virus Nucleoprotein Core Domain Chaperoned by a VP35 Peptide Reveals a Conserved Drug Target for Filovirus. J. Virol. 2017, 91, e00996-17. [Google Scholar] [CrossRef] [Green Version]
- Landeras-Bueno, S.; Oda, S.-I.; Norris, M.J.; Salie, Z.L.; Guenaga, J.; Wyatt, R.T.; Saphire, E.O. Sudan Ebolavirus VP35-NP Crystal Structure Reveals a Potential Target for Pan-Filovirus Treatment. mBio 2019, 10, e00734-19. [Google Scholar] [CrossRef] [Green Version]
- Milles, S.; Jensen, M.R.; Lazert, C.; Guseva, S.; Ivashchenko, S.; Communie, G.; Maurin, D.; Gerlier, D.; Ruigrok, R.W.H.; Blackledge, M. An ultraweak interaction in the intrinsically disordered replication machinery is essential for measles virus function. Sci. Adv. 2018, 4, eaat7778. [Google Scholar] [CrossRef] [Green Version]
- Schiavina, M.; Salladini, E.; Murrali, M.G.; Tria, G.; Felli, I.C.; Pierattelli, R.; Longhi, S. Ensemble description of the intrinsically disordered N-terminal domain of the Nipah virus P/V protein from combined NMR and SAXS. Sci. Rep. 2020, 10, 19574. [Google Scholar] [CrossRef]
- Cevik, B.; Kaesberg, J.; Smallwood, S.; Feller, J.A.; Moyer, S.A. Mapping the phosphoprotein binding site on Sendai virus NP protein assembled into nucleocapsids. Virology 2004, 325, 216–224. [Google Scholar] [CrossRef] [Green Version]
- Milles, S.; Jensen, M.R.; Communie, G.; Maurin, D.; Schoehn, G.; Ruigrok, R.W.; Blackledge, M. Self-Assembly of Measles Virus Nucleocapsid-like Particles: Kinetics and RNA Sequence Dependence. Angew. Chem. Int. Ed. 2016, 55, 9356–9360. [Google Scholar] [CrossRef] [Green Version]
- Blumberg, B.M.; Giorgi, C.; Kolakofsky, D. N protein of vesicular stomatitis virus selectively encapsidates leader RNA in vitro. Cell 1983, 32, 559–567. [Google Scholar] [CrossRef]
- Moyer, S.A.; Smallwood-Kentro, S.; Haddad, A.; Prevec, L. Assembly and transcription of synthetic vesicular stomatitis virus nucleocapsids. J. Virol. 1991, 65, 2170–2178. [Google Scholar] [CrossRef] [Green Version]
- Green, T.J.; Rowse, M.; Tsao, J.; Kang, J.; Ge, P.; Zhou, Z.H.; Luo, M. Access to RNA Encapsidated in the Nucleocapsid of Vesicular Stomatitis Virus. J. Virol. 2010, 85, 2714–2722. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Cao, D.; Ahn, H.M.; Swain, A.; Hill, S.; Ogilvie, C.; Kurien, M.; Rahmatullah, T.; Liang, B. In vitro trackable assembly of RNA-specific nucleocapsids of the respiratory syncytial virus. J. Biol. Chem. 2020, 295, 883–895. [Google Scholar] [CrossRef]
- Guseva, S.; Milles, S.; Jensen, M.R.; Salvi, N.; Kleman, J.-P.; Maurin, D.; Ruigrok, R.W.H.; Blackledge, M. Measles virus nucleo- and phosphoproteins form liquid-like phase-separated compartments that promote nucleocapsid assembly. Sci. Adv. 2020, 6, eaaz7095. [Google Scholar] [CrossRef] [Green Version]
- Gérard, F.C.A.; Jamin, M.; Blackledge, M.; Blondel, D.; Bourhis, J.-M. Vesicular Stomatitis Virus Phosphoprotein Dimerization Domain Is Dispensable for Virus Growth. J. Virol. 2020, 94, e01789-19. [Google Scholar] [CrossRef] [PubMed]
- Bloyet, L.-M.; Morin, B.; Brusic, V.; Gardner, E.; Ross, R.A.; Vadakkan, T.; Kirchhausen, T.; Whelan, S.P.J. Oligomerization of the Vesicular Stomatitis Virus Phosphoprotein Is Dispensable for mRNA Synthesis but Facilitates RNA Replication. J. Virol. 2020, 94, e00115-20. [Google Scholar] [CrossRef] [PubMed]
- Curran, J. A role for the Sendai virus P protein trimer in RNA synthesis. J. Virol. 1998, 72, 4274–4280. [Google Scholar] [CrossRef] [Green Version]
- Kolakofsky, D.; Le Mercier, P.; Iseni, F.; Garcin, D. Viral DNA polymerase scanning and the gymnastics of Sendai virus RNA synthesis. Virology 2004, 318, 463–473. [Google Scholar] [CrossRef] [Green Version]
- Kingston, R.L.; Gay, L.S.; Baase, W.S.; Matthews, B.W. Structure of the Nucleocapsid-Binding Domain from the Mumps Virus Polymerase; an Example of Protein Folding Induced by Crystallization. J. Mol. Biol. 2008, 379, 719–731. [Google Scholar] [CrossRef] [Green Version]
- Gely, S.; Lowry, D.F.; Bernard, C.; Jensen, M.R.; Blackledge, M.; Costanzo, S.; Bourhis, J.-M.; Darbon, H.; Daughdrill, G.; Longhi, S. Solution structure of the C-terminal X domain of the measles virus phosphoprotein and interaction with the intrinsically disordered C-terminal domain of the nucleoprotein. J. Mol. Recognit. 2010, 23, 435–447. [Google Scholar] [CrossRef]
- Bloyet, L.-M.; Schramm, A.; Lazert, C.; Raynal, B.; Hologne, M.; Walker, O.; Longhi, S.; Gerlier, D. Regulation of measles virus gene expression by P protein coiled-coil properties. Sci. Adv. 2019, 5, eaaw3702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du Pont, V.; Jiang, Y.; Plemper, R.K. Bipartite interface of the measles virus phosphoprotein X domain with the large polymerase protein regulates viral polymerase dynamics. PLoS Pathog. 2019, 15, e1007995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kingston, R.L.; Baase, W.A.; Gay, L.S. Characterization of nucleocapsid binding by the measles virus and mumps virus phosphoproteins. J. Virol. 2004, 78, 8630–8640. [Google Scholar] [CrossRef] [Green Version]
- Sourimant, J.; Thakkar, V.D.; Cox, R.M.; Plemper, R.K. Viral evolution identifies a regulatory interface between paramyxovirus polymerase complex and nucleocapsid that controls replication dynamics. Sci. Adv. 2020, 6, eaaz1590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Omi-Furutani, M.; Yoneda, M.; Fujita, K.; Ikeda, F.; Kai, C. Novel Phosphoprotein-Interacting Region in Nipah Virus Nucleocapsid Protein and Its Involvement in Viral Replication. J. Virol. 2010, 84, 9793–9799. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yegambaram, K.; Kingston, R.L. The feet of the measles virus polymerase bind the viral nucleocapsid protein at a single site. Protein Sci. 2010, 19, 893–899. [Google Scholar] [CrossRef]
- Bourhis, J.-M.; Receveur-Brechot, V.; Oglesbee, M.; Zhang, X.; Buccellato, M.; Darbon, H.; Canard, B.; Finet, S.; Longhi, S. The intrinsically disordered C-terminal domain of the measles virus nucleoprotein interacts with the C-terminal domain of the phosphoprotein via two distinct sites and remains predominantly unfolded. Protein Sci. 2005, 14, 1975–1992. [Google Scholar] [CrossRef]
- Belle, V.; Rouger, S.; Costanzo, S.; Liquière, E.; Strancar, J.; Guigliarelli, B.; Fournel, A.; Longhi, S. Mapping α-helical induced folding within the intrinsically disordered C-terminal domain of the measles virus nucleoprotein by site-directed spin-labeling EPR spectroscopy. Proteins Struct. Funct. Bioinform. 2008, 73, 973–988. [Google Scholar] [CrossRef]
- Kavalenka, A.; Urbancic, I.; Belle, V.; Rouger, S.; Costanzo, S.; Kure, S.; Fournel, A.; Longhi, S.; Guigliarelli, B.; Strancar, J. Conformational analysis of the partially disordered measles virus N(TAIL)-XD complex by SDSL EPR spectroscopy. Biophys. J. 2010, 98, 1055–1064. [Google Scholar] [CrossRef] [Green Version]
- Martinho, M.; Habchi, J.; El Habre, Z.; Nesme, L.; Guigliarelli, B.; Belle, V.; Longhi, S. Assessing induced folding within the intrinsically disordered C-terminal domain of the Henipavirus nucleoproteins by site-directed spin labeling EPR spectroscopy. J. Biomol. Struct. Dyn. 2013, 31, 453–471. [Google Scholar] [CrossRef]
- Tompa, P.; Fuxreiter, M. Fuzzy complexes: Polymorphism and structural disorder in protein-protein interactions. Trends Biochem. Sci. 2008, 33, 2–8. [Google Scholar] [CrossRef]
- Gruet, A.; Dosnon, M.; Blocquel, D.; Brunel, J.; Gerlier, D.; Das, R.; Bonetti, D.; Gianni, S.; Fuxreiter, M.; Longhi, S.; et al. Fuzzy regions in an intrinsically disordered protein impair protein-protein interactions. FEBS J. 2016, 283, 576–594. [Google Scholar] [CrossRef] [Green Version]
- Troilo, F.; Bonetti, D.; Bignon, C.; Longhi, S.; Gianni, S. Understanding Intramolecular Crosstalk in an Intrinsically Disordered Protein. ACS Chem. Biol. 2019, 14, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Gruet, A.; Dosnon, M.; Vassena, A.; Lombard, V.; Gerlier, D.; Bignon, C.; Longhi, S. Dissecting Partner Recognition by an Intrinsically Disordered Protein Using Descriptive Random Mutagenesis. J. Mol. Biol. 2013, 425, 3495–3509. [Google Scholar] [CrossRef]
- Shu, Y.; Habchi, J.; Costanzo, S.; Padilla, A.; Brunel, J.; Gerlier, D.; Oglesbee, M.; Longhi, S. Plasticity in Structural and Functional Interactions between the Phosphoprotein and Nucleoprotein of Measles Virus. J. Biol. Chem. 2012, 287, 11951–11967. [Google Scholar] [CrossRef] [Green Version]
- Brunel, J.; Chopy, D.; Dosnon, M.; Bloyet, L.-M.; Devaux, P.; Urzua, E.; Cattaneo, R.; Longhi, S.; Gerlier, D. Sequence of Events in Measles Virus Replication: Role of Phosphoprotein-Nucleocapsid Interactions. J. Virol. 2014, 88, 10851–10863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bloyet, L.-M.; Brunel, J.; Dosnon, M.; Hamon, V.; Erales, J.; Gruet, A.; Lazert, C.; Bignon, C.; Roche, P.; Longhi, S.; et al. Modulation of Re-initiation of Measles Virus Transcription at Intergenic Regions by PXD to NTAIL Binding Strength. PLoS Pathog. 2016, 12, e1006058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krumm, S.A.; Takeda, M.; Plemper, R.K. The Measles Virus Nucleocapsid Protein Tail Domain Is Dispensable for Viral Polymerase Recruitment and Activity. J. Biol. Chem. 2013, 288, 29943–29953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thakkar, V.; Cox, R.M.; Sawatsky, B.; Budaszewski, R.D.F.; Sourimant, J.; Wabbel, K.; Makhsous, N.; Greninger, A.; von Messling, V.; Plemper, R.K. The Unstructured Paramyxovirus Nucleocapsid Protein Tail Domain Modulates Viral Pathogenesis through Regulation of Transcriptase Activity. J. Virol. 2018, 92, e02064-17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cox, R.M.; Krumm, S.A.; Thakkar, V.D.; Sohn, M.; Plemper, R.K. The structurally disordered paramyxovirus nucleocapsid protein tail domain is a regulator of the mRNA transcription gradient. Sci. Adv. 2017, 3, e1602350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galloux, M.; Tarus, B.; Blazevic, I.; Fix, J.; Duquerroy, S.; Eléouët, J.-F. Characterization of a Viral Phosphoprotein Binding Site on the Surface of the Respiratory Syncytial Nucleoprotein. J. Virol. 2012, 86, 8375–8387. [Google Scholar] [CrossRef] [Green Version]
- Ouizougun-Oubari, M.; Pereira, N.; Tarus, B.; Galloux, M.; Lassoued, S.; Fix, J.; Tortorici, M.A.; Hoos, S.; Baron, B.; England, P.; et al. A Druggable Pocket at the Nucleocapsid/Phosphoprotein Interaction Site of Human Respiratory Syncytial Virus. J. Virol. 2015, 89, 11129–11143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, T.J.; Luo, M. Structure of the vesicular stomatitis virus nucleocapsid in complex with the nucleocapsid-binding domain of the small polymerase cofactor, P. Proc. Natl. Acad. Sci. USA 2009, 106, 11713–11718. [Google Scholar] [CrossRef] [Green Version]
- Oglesbee, M.J.; Liu, Z.; Kenney, H.; Brooks, C.L. The Highly Inducible Member of the 70 kDa Family of Heat Shock Proteins Increases Canine Distemper Virus Polymerase Activity. J. Gen. Virol. 1996, 77, 2125–2135. [Google Scholar] [CrossRef]
- Oglesbee, M.; Tatalick, L.; Rice, J.; Krakowka, S. Isolation and Characterization of Canine Distemper Virus Nucleocapsid Variants. J. Gen. Virol. 1989, 70, 2409–2419. [Google Scholar] [CrossRef]
- Oglesbee, M.; Ringler, S.; Krakowka, S. Interaction of canine distemper virus nucleocapsid variants with 70K heat-shock proteins. J. Gen. Virol. 1990, 71, 1585–1590. [Google Scholar] [CrossRef] [PubMed]
- Jamin, M.; Yabukarski, F. Nonsegmented Negative-Sense RNA Viruses—Structural Data Bring New Insights into Nucleocapsid Assembly. Adv. Virus Res. 2017, 97, 143–185. [Google Scholar] [CrossRef]
- Severin, C.; Terrell, J.R.; Zengel, J.R.; Cox, R.; Plemper, R.K.; He, B.; Luo, M. Releasing the Genomic RNA Sequestered in the Mumps Virus Nucleocapsid. J. Virol. 2016, 90, 10113–10119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curran, J. Reexamination of the Sendai Virus P Protein Domains Required for RNA Synthesis: A Possible Supplemental Role for the P Protein. Virology 1996, 221, 130–140. [Google Scholar] [CrossRef] [Green Version]
- De, B.P.; Hoffman, M.A.; Choudhary, S.; Huntley, C.C.; Banerjee, A.K. Role of NH2- and COOH-Terminal Domains of the P Protein of Human Parainfluenza Virus Type 3 in Transcription and Replication. J. Virol. 2000, 74, 5886–5895. [Google Scholar] [CrossRef] [Green Version]
- Ogino, T.; Green, T.J. RNA Synthesis and Capping by Non-segmented Negative Strand RNA Viral Polymerases: Lessons from a Prototypic Virus. Front. Microbiol. 2019, 10, 1490. [Google Scholar] [CrossRef] [PubMed]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bloyet, L.-M. The Nucleocapsid of Paramyxoviruses: Structure and Function of an Encapsidated Template. Viruses 2021, 13, 2465. https://doi.org/10.3390/v13122465
Bloyet L-M. The Nucleocapsid of Paramyxoviruses: Structure and Function of an Encapsidated Template. Viruses. 2021; 13(12):2465. https://doi.org/10.3390/v13122465
Chicago/Turabian StyleBloyet, Louis-Marie. 2021. "The Nucleocapsid of Paramyxoviruses: Structure and Function of an Encapsidated Template" Viruses 13, no. 12: 2465. https://doi.org/10.3390/v13122465
APA StyleBloyet, L.-M. (2021). The Nucleocapsid of Paramyxoviruses: Structure and Function of an Encapsidated Template. Viruses, 13(12), 2465. https://doi.org/10.3390/v13122465






