Epigallocatechin Gallate (EGCG), a Green Tea Polyphenol, Reduces Coronavirus Replication in a Mouse Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and Infection to Cells
2.2. Mouse Experiment
2.3. Quantitative RT-PCR and Western Blots
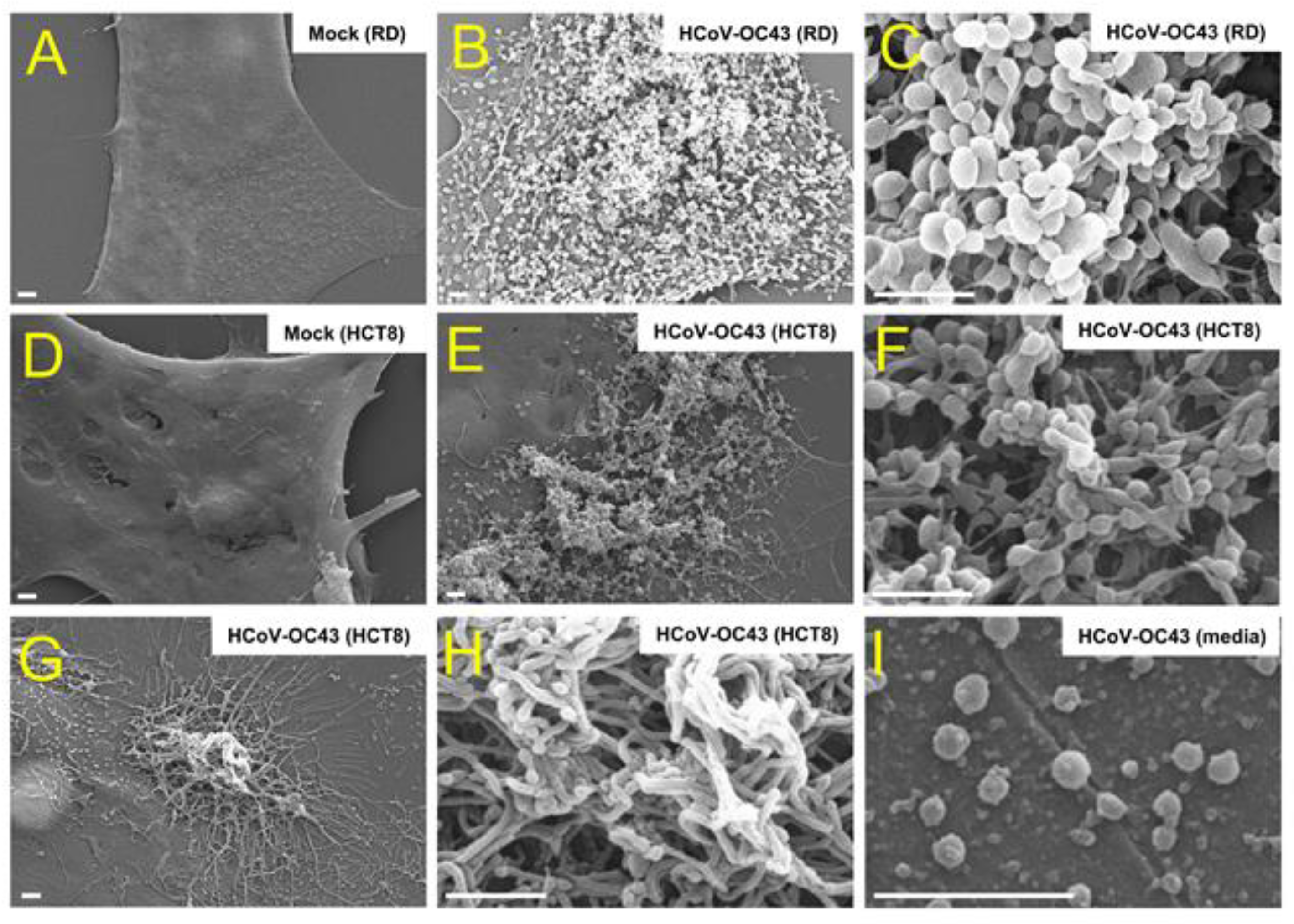
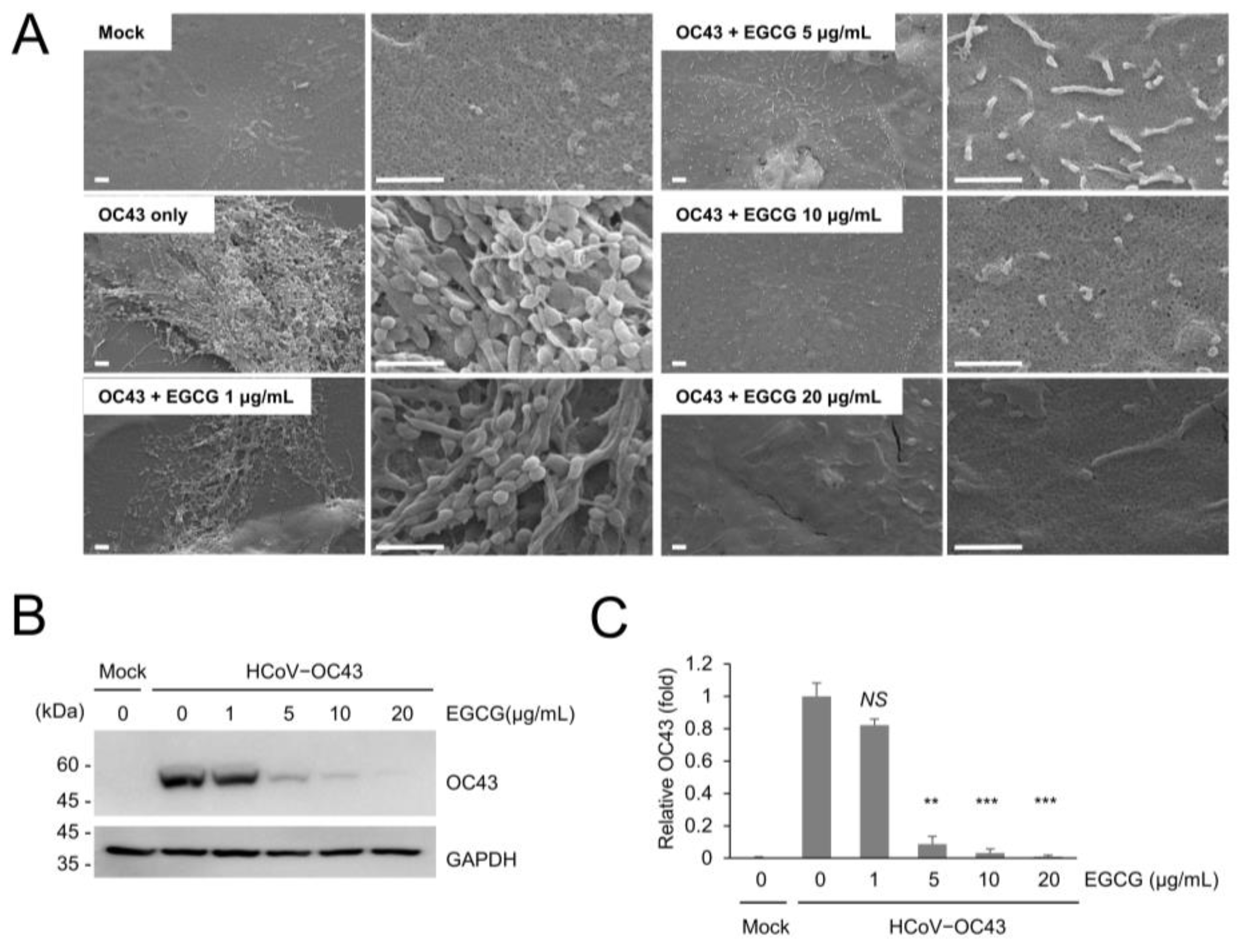
2.4. Scanning Electron Microscopy
2.5. Statistical Analysis
3. Results
3.1. Coronavirus Infection Results in Cell Surface Alteration
3.2. EGCG and Green Tea Polyphenols Inhibits Coronavirus Replication
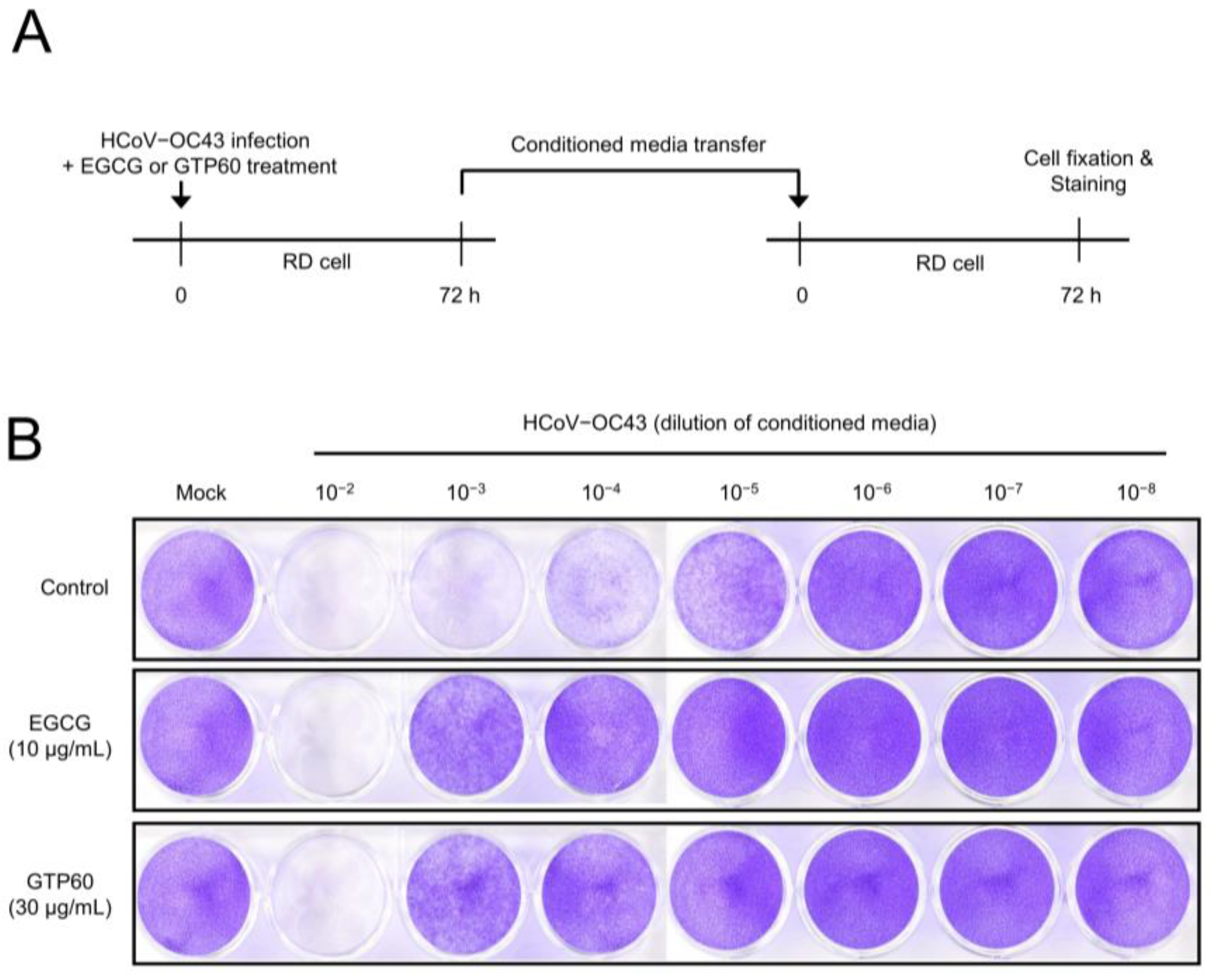
3.3. EGCG and Green Tea Polyphenols Reduce Coronavirus Replication in Mouse
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baud, D.; Qi, X.; Nielsen-Saines, K.; Musso, D.; Pomar, L.; Favre, G. Real estimates of mortality following COVID-19 infection. Lancet Infect. Dis. 2020, 20, 773. [Google Scholar] [CrossRef] [Green Version]
- Harvey, W.T.; Carabelli, A.M.; Jackson, B.; Gupta, R.K.; Thomson, E.C.; Harrison, E.M.; Ludden, C.; Reeve, R.; Rambaut, A.; Consortium, C.-G.U.; et al. SARS-CoV-2 variants, spike mutations and immune escape. Nat. Rev. Microbiol. 2021, 19, 409–424. [Google Scholar] [CrossRef] [PubMed]
- Reygaert, W.C. Green Tea Catechins: Their Use in Treating and Preventing Infectious Diseases. BioMed Res. Int. 2018, 2018, 9105261. [Google Scholar] [CrossRef]
- Yang, C.S.; Wang, X. Green tea and cancer prevention. Nutr. Cancer 2010, 62, 931–937. [Google Scholar] [CrossRef]
- Park, J.; Park, R.; Jang, M.; Park, Y.I. Therapeutic Potential of EGCG, a Green Tea Polyphenol, for Treatment of Coronavirus Diseases. Life 2021, 11, 197. [Google Scholar] [CrossRef]
- Jang, M.; Park, R.; Park, Y.I.; Cha, Y.E.; Yamamoto, A.; Lee, J.I.; Park, J. EGCG, a green tea polyphenol, inhibits human coronavirus replication in vitro. Biochem. Biophys. Res. Commun. 2021, 547, 23–28. [Google Scholar] [CrossRef]
- Yost, S.A.; Marcotrigiano, J. Viral precursor polyproteins: Keys of regulation from replication to maturation. Curr. Opin. Virol. 2013, 3, 137–142. [Google Scholar] [CrossRef] [Green Version]
- Drag, M.; Salvesen, G.S. Emerging principles in protease-based drug discovery. Nat. Rev. Drug Discov. 2010, 9, 690–701. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsu, M.F.; Kuo, C.J.; Chang, K.T.; Chang, H.C.; Chou, C.C.; Ko, T.P.; Shr, H.L.; Chang, G.G.; Wang, A.H.; Liang, P.H. Mechanism of the maturation process of SARS-CoV 3CL protease. J. Biol. Chem. 2005, 280, 31257–31266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, M.; Park, Y.I.; Cha, Y.E.; Park, R.; Namkoong, S.; Lee, J.I.; Park, J. Tea polyphenols EGCG and theaflavin inhibit the activity of SARS-CoV-2 3CL-protease in vitro. Evid.-Based Complement. Altern. Med. 2020, 2020, 5630838. [Google Scholar] [CrossRef] [PubMed]
- Chiou, W.C.; Chen, J.C.; Chen, Y.T.; Yang, J.M.; Hwang, L.H.; Lyu, Y.S.; Yang, H.Y.; Huang, C. The inhibitory effects of PGG and EGCG against the SARS-CoV-2 3C-like protease. Biochem. Biophys. Res. Commun. 2021, in press. [Google Scholar] [CrossRef]
- Zhu, Y.; Xie, D.Y. Docking Characterization and in vitro Inhibitory Activity of Flavan-3-ols and Dimeric Proanthocyanidins Against the Main Protease Activity of SARS-Cov-2. Front. Plant Sci. 2020, 11, 601316. [Google Scholar] [CrossRef] [PubMed]
- Du, A.; Zheng, R.; Disoma, C.; Li, S.; Chen, Z.; Li, S.; Liu, P.; Zhou, Y.; Shen, Y.; Liu, S.; et al. Epigallocatechin-3-gallate, an active ingredient of Traditional Chinese Medicines, inhibits the 3CLpro activity of SARS-CoV-2. Int. J. Biol. Macromol. 2021, 176, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Q.; Li, Q.S.; Zheng, X.Q.; Lu, J.L.; Liang, Y.R. Antiviral Effects of Green Tea EGCG and Its Potential Application against COVID-19. Molecules 2021, 26, 3962. [Google Scholar] [CrossRef] [PubMed]
- LeBlanc, E.V.; Colpitts, C.C. The green tea catechin EGCG provides proof-of-concept for a pan-coronavirus entry inhibitor. bioRxiv 2021. [Google Scholar] [CrossRef]
- Hong, S.; Seo, S.H.; Woo, S.J.; Kwon, Y.; Song, M.; Ha, N.C. Epigallocatechin Gallate Inhibits the Uridylate-Specific Endoribonuclease Nsp15 and Efficiently Neutralizes the SARS-CoV-2 Strain. J. Agric. Food Chem. 2021, 69, 5948–5954. [Google Scholar] [CrossRef]
- Storozhuk, M. COVID-19: Could green tea catechins reduce the risks? medRxiv 2020. [Google Scholar] [CrossRef]
- Bettuzzi, S.; Gabba, L.; Cataldo, S. Efficacy of a Polyphenolic, Standardized Green Tea Extract for the Treatment of COVID-19 Syndrome: A Proof-of-Principle Study. COVID 2021, 1, 2–12. [Google Scholar] [CrossRef]
- Velavan, T.P.; Meyer, C.G. The COVID-19 epidemic. Trop. Med. Int. Health 2020, 25, 278–280. [Google Scholar] [CrossRef] [Green Version]
- Andersen, K.G.; Rambaut, A.; Lipkin, W.I.; Holmes, E.C.; Garry, R.F. The proximal origin of SARS-CoV-2. Nat. Med. 2020, 26, 450–452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rojo, M.A.; Garrosa, M.; Jimenez, P.; Girbes, T.; Garcia-Recio, V.; Cordoba-Diaz, M.; Cordoba-Diaz, D. Unexpected Toxicity of Green Tea Polyphenols in Combination with the Sambucus RIL Ebulin. Toxins 2020, 12, 542. [Google Scholar] [CrossRef] [PubMed]
- Butler, N.; Pewe, L.; Trandem, K.; Perlman, S. Murine encephalitis caused by HCoV-OC43, a human coronavirus with broad species specificity, is partly immune-mediated. Virology 2006, 347, 410–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patterson, E.; Ryan, P.M.; Wiley, N.; Carafa, I.; Sherwin, E.; Moloney, G.; Franciosi, E.; Mandal, R.; Wishart, D.S.; Tuohy, K.; et al. Gamma-aminobutyric acid-producing lactobacilli positively affect metabolism and depressive-like behaviour in a mouse model of metabolic syndrome. Sci. Rep. 2019, 9, 16323. [Google Scholar] [CrossRef] [PubMed]
- Meyer, U.; Spoerri, E.; Yee, B.K.; Schwarz, M.J.; Feldon, J. Evaluating Early Preventive Antipsychotic and Antidepressant Drug Treatment in an Infection-Based Neurodevelopmental Mouse Model of Schizophrenia. Schizophr. Bull. 2008, 36, 607–623. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, R.; Jang, M.; Park, Y.-I.; Park, Y.; Jung, W.; Park, J.; Park, J. Epigallocatechin Gallate (EGCG), a Green Tea Polyphenol, Reduces Coronavirus Replication in a Mouse Model. Viruses 2021, 13, 2533. https://doi.org/10.3390/v13122533
Park R, Jang M, Park Y-I, Park Y, Jung W, Park J, Park J. Epigallocatechin Gallate (EGCG), a Green Tea Polyphenol, Reduces Coronavirus Replication in a Mouse Model. Viruses. 2021; 13(12):2533. https://doi.org/10.3390/v13122533
Chicago/Turabian StylePark, Rackhyun, Minsu Jang, Yea-In Park, Yeonjeong Park, Woochul Jung, Jayhyun Park, and Junsoo Park. 2021. "Epigallocatechin Gallate (EGCG), a Green Tea Polyphenol, Reduces Coronavirus Replication in a Mouse Model" Viruses 13, no. 12: 2533. https://doi.org/10.3390/v13122533
APA StylePark, R., Jang, M., Park, Y.-I., Park, Y., Jung, W., Park, J., & Park, J. (2021). Epigallocatechin Gallate (EGCG), a Green Tea Polyphenol, Reduces Coronavirus Replication in a Mouse Model. Viruses, 13(12), 2533. https://doi.org/10.3390/v13122533






