Zika RNA and Flavivirus-Like Antigens in the Sperm Cells of Symptomatic and Asymptomatic Subjects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Examination of Conventional Semen Parameters
2.3. Semen Preparation for Immunofluorescence (IF)
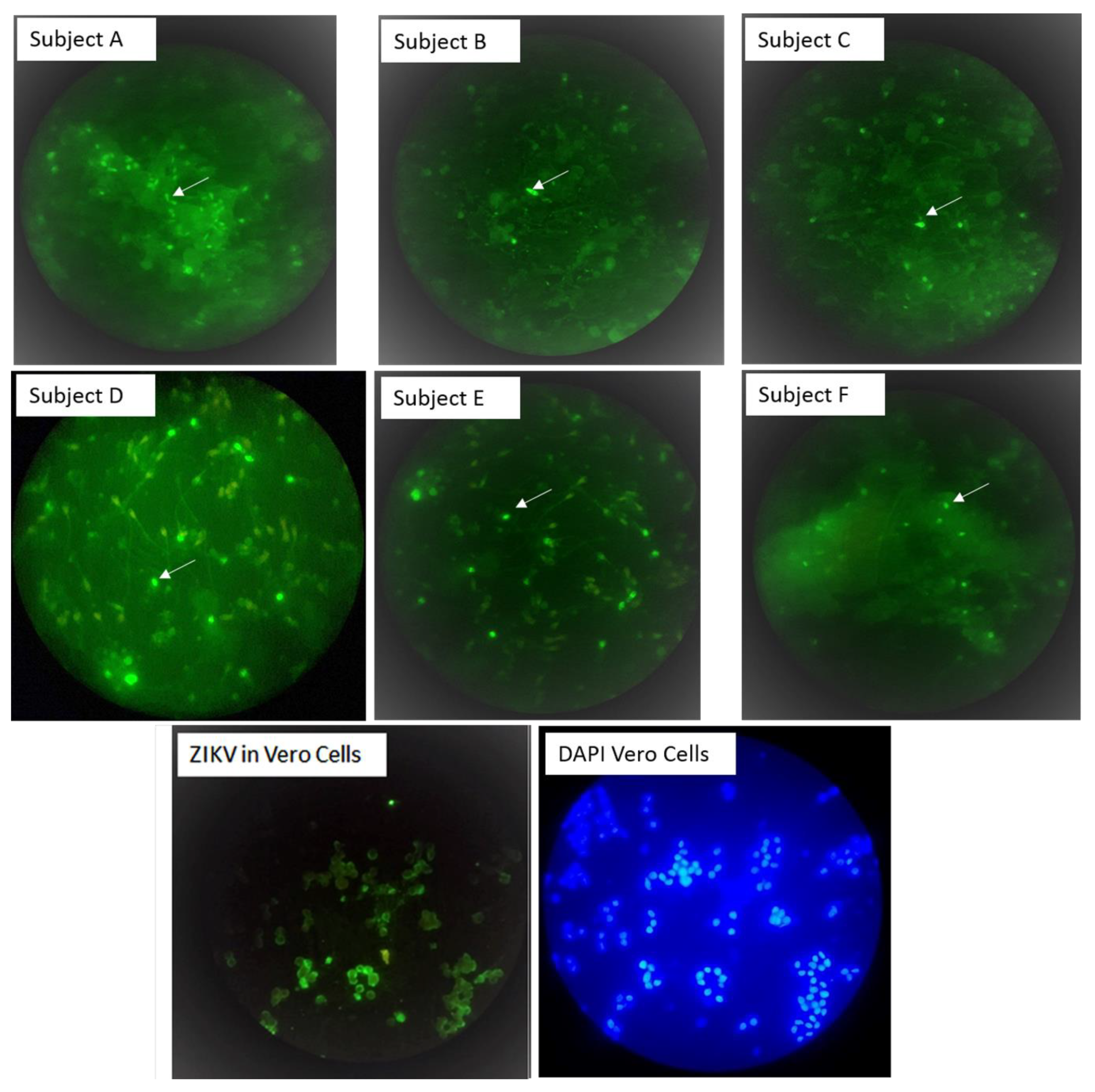
2.4. Immunofluorescence (IF) for Detection of Flavivirus Antigens
2.5. RNA Purification
2.6. Reverse Transcriptase and Quantitative Polymerase Chain Reaction (RT-qPCR)
2.7. PCR for E-Gene Amplification and Sequencing
2.8. Flavivirus IgG and IgM Detection
2.9. Virus Isolation
2.10. Statistical Analysis
3. Results
3.1. Flavivirus Antigens Were Detected in Sperm Cells from Symptomatic Subjects
3.2. Flavivirus Antigens Were Found in Asymptomatic Subjects
3.3. Correlation between Antigen and RNA Detection
3.4. Immunofluorescent Patterns
3.5. Virus Isolation
3.6. Partial Sequence Analysis of the E Gene
3.7. Correlation between Fresh Semen Analyses of Subjects ZIKV-Positive and ZIKV-Negative
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Duffy, M.R.; Chen, T.H.; Hancock, W.T.; Powers, A.M.; Kool, J.L.; Lanciotti, R.S.; Pretrick, M.; Marfel, M.; Holzbauer, S.; Dubray, C.; et al. Zika virus outbreak on Yap Island, Federated States of Micronesia. N. Engl. J. Med. 2009, 360, 2536–2543. [Google Scholar] [CrossRef] [PubMed]
- Becker-Dreps, S.; Stringer, E.M.; Bucardo, F.; Bowman, N.M.; Boivin, M.J. Is there a silver lining to the Zika virus epidemic in the Americas? Lancet Infect. Dis. 2020, 20, 14–15. [Google Scholar] [CrossRef] [Green Version]
- Govero, J.; Esakky, P.; Scheaffer, S.M.; Fernandez, E.; Drury, A.; Platt, D.J.; Gorman, M.J.; Richner, J.M.; Caine, E.A.; Salazar, V.; et al. Zika virus infection damages the testes in mice. Nature 2016, 540, 438–442. [Google Scholar] [CrossRef] [Green Version]
- Ma, W.; Li, S.; Ma, S.; Jia, L.; Zhang, F.; Zhang, Y.; Zhang, J.; Wong, G.; Zhang, S.; Lu, X.; et al. Zika Virus Causes Testis Damage and Leads to Male Infertility in Mice. Cell 2017, 168, 542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Robinson, C.L.; Chong, A.C.N.; Ashbrook, A.W.; Jeng, G.; Jin, J.; Chen, H.; Tang, E.I.; Martin, L.A.; Kim, R.S.; Kenyon, R.M.; et al. Male germ cells support long-term propagation of Zika virus. Nat. Commun. 2018, 9, 2090. [Google Scholar] [CrossRef]
- Matusali, G.; Houzet, L.; Satie, A.P.; Mahe, D.; Aubry, F.; Couderc, T.; Frouard, J.; Bourgeau, S.; Bensalah, K.; Lavoue, S.; et al. Zika virus infects human testicular tissue and germ cells. J. Clin. Investig. 2018, 128, 4697–4710. [Google Scholar] [CrossRef] [PubMed]
- Mlera, L.; Bloom, M.E. Differential Zika Virus Infection of Testicular Cell Lines. Viruses 2019, 11, 42. [Google Scholar] [CrossRef] [Green Version]
- Counotte, M.J.; Kim, C.R.; Wang, J.; Bernstein, K.; Deal, C.D.; Broutet, N.J.N.; Low, N. Sexual transmission of Zika virus and other flaviviruses: A living systematic review. PLoS Med. 2018, 15, e1002611. [Google Scholar] [CrossRef] [Green Version]
- Joguet, G.; Mansuy, J.M.; Matusali, G.; Hamdi, S.; Walschaerts, M.; Pavili, L.; Guyomard, S.; Prisant, N.; Lamarre, P.; Dejucq-Rainsford, N.; et al. Effect of acute Zika virus infection on sperm and virus clearance in body fluids: A prospective observational study. Lancet Infect. Dis. 2017, 17, 1200–1208. [Google Scholar] [CrossRef] [Green Version]
- Paz-Bailey, G.; Rosenberg, E.S.; Doyle, K.; Munoz-Jordan, J.; Santiago, G.A.; Klein, L.; Perez-Padilla, J.; Medina, F.A.; Waterman, S.H.; Gubern, C.G.; et al. Persistence of Zika Virus in Body Fluids—Preliminary Report. N. Engl. J. Med. 2017, 379, 1234–1243. [Google Scholar] [CrossRef]
- Moreira, J.; Peixoto, T.M.; Siqueira, A.M.; Lamas, C.C. Sexually acquired Zika virus: A systematic review. Clin. Microbiol. Infect. 2017, 23, 296–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mead, P.S.; Duggal, N.K.; Hook, S.A.; Delorey, M.; Fischer, M.; Olzenak McGuire, D.; Becksted, H.; Max, R.J.; Anishchenko, M.; Schwartz, A.M.; et al. Zika Virus Shedding in Semen of Symptomatic Infected Men. N. Engl. J. Med. 2018, 378, 1377–1385. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, B.; Thorburn, F.; Petridou, C.; Bailey, D.; Hewson, R.; Simpson, A.J.; Brooks, T.J.; Aarons, E.J. Presence and Persistence of Zika Virus RNA in Semen, United Kingdom, 2016. Emerg. Infect. Dis. 2017, 23, 611–615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huits, R.; De Smet, B.; Grard, G.; Eggermont, K.; Minto-Bain, C.; Jess, N.; Leparc-Goffart, I.; Malvy, D.; Cnops, L. Detection of Zika virus replication in human semen by reverse transcription polymerase chain reaction targeting of antisense RNA. J. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- De Laval, F.; Matheus, S.; Labrousse, T.; Enfissi, A.; Rousset, D.; Briolant, S. Kinetics of Zika Viral Load in Semen. N. Engl. J. Med. 2017, 377, 697–699. [Google Scholar] [CrossRef]
- Medina, F.A.; Torres, G.; Acevedo, J.; Fonseca, S.; Casiano, L.; De Leon-Rodriguez, C.M.; Santiago, G.A.; Doyle, K.; Sharp, T.M.; Alvarado, L.I.; et al. Duration of the Presence of Infectious Zika Virus in Semen and Serum. J. Infect. Dis. 2019, 219, 31–40. [Google Scholar] [CrossRef] [Green Version]
- Musso, D.; Richard, V.; Teissier, A.; Stone, M.; Lanteri, M.C.; Latoni, G.; Alsina, J.; Reik, R.; Busch, M.P. Detection of Zika virus RNA in semen of asymptomatic blood donors. Clin. Microbiol. Infect. 2017, 23, 1001e1–1001e3. [Google Scholar] [CrossRef] [Green Version]
- Mansuy, J.M.; Pasquier, C.; Daudin, M.; Chapuy-Regaud, S.; Moinard, N.; Chevreau, C.; Izopet, J.; Mengelle, C.; Bujan, L. Zika virus in semen of a patient returning from a non-epidemic area. Lancet Infect. Dis. 2016, 16, 894–895. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.; Jovel, J.; Lopez-Orozco, J.; Limonta, D.; Airo, A.M.; Hou, S.; Stryapunina, I.; Fibke, C.; Moore, R.B.; Hobman, T.C. Human Sertoli cells support high levels of Zika virus replication and persistence. Sci. Rep. 2018, 8, 5477. [Google Scholar] [CrossRef]
- Almeida, R.D.N.; Braz-de-Melo, H.A.; Santos, I.O.; Correa, R.; Kobinger, G.P.; Magalhaes, K.G. The Cellular Impact of the ZIKA Virus on Male Reproductive Tract Immunology and Physiology. Cells 2020, 9, 1006. [Google Scholar] [CrossRef] [Green Version]
- Atkinson, B.; Hearn, P.; Afrough, B.; Lumley, S.; Carter, D.; Aarons, E.J.; Simpson, A.J.; Brooks, T.J.; Hewson, R. Detection of Zika Virus in Semen. Emerg. Infect. Dis. 2016, 22, 940. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foy, B.D.; Kobylinski, K.C.; Chilson Foy, J.L.; Blitvich, B.J.; Travassos da Rosa, A.; Haddow, A.D.; Lanciotti, R.S.; Tesh, R.B. Probable non-vector-borne transmission of Zika virus, Colorado, USA. Emerg. Infect. Dis. 2011, 17, 880–882. [Google Scholar] [CrossRef] [PubMed]
- Musso, D.; Roche, C.; Robin, E.; Nhan, T.; Teissier, A.; Cao-Lormeau, V.M. Potential sexual transmission of Zika virus. Emerg. Infect. Dis. 2015, 21, 359–361. [Google Scholar] [CrossRef] [PubMed]
- Oliveira Souto, I.; Alejo-Cancho, I.; Gascon Brustenga, J.; Peiro Mestres, A.; Munoz Gutierrez, J.; Martinez Yoldi, M.J. Persistence of Zika virus in semen 93 days after the onset of symptoms. Enferm. Infecc. Microbiol. Clin. 2018, 36, 21–23. [Google Scholar] [CrossRef] [Green Version]
- Zambrana, J.V.; Bustos Carrillo, F.; Burger-Calderon, R.; Collado, D.; Sanchez, N.; Ojeda, S.; Carey Monterrey, J.; Plazaola, M.; Lopez, B.; Arguello, S.; et al. Seroprevalence, risk factor, and spatial analyses of Zika virus infection after the 2016 epidemic in Managua, Nicaragua. Proc. Natl. Acad. Sci. USA 2018, 115, 9294–9299. [Google Scholar] [CrossRef] [Green Version]
- Haby, M.M.; Pinart, M.; Elias, V.; Reveiz, L. Prevalence of asymptomatic Zika virus infection: A systematic review. Bull. World Health Organ. 2018, 96, 402D–413D. [Google Scholar] [CrossRef]
- World Health Organization. WHO Laboratory Manual for the Examination of Human Semen and Sperm-Cervical Mucus Interaction, 5th ed.; WHO Press: Geneva, Switzerland, 2010; p. 286. [Google Scholar]
- Gentry, M.K.; Henchal, E.A.; McCown, J.M.; Brandt, W.E.; Dalrymple, J.M. Identification of distinct antigenic determinants on dengue-2 virus using monoclonal antibodies. Am. J. Trop. Med. Hyg. 1982, 31, 548–555. [Google Scholar] [CrossRef]
- Lanciotti, R.S.; Kosoy, O.L.; Laven, J.J.; Velez, J.O.; Lambert, A.J.; Johnson, A.J.; Stanfield, S.M.; Duffy, M.R. Genetic and serologic properties of Zika virus associated with an epidemic, Yap State, Micronesia, 2007. Emerg. Infect. Dis. 2008, 14, 1232–1239. [Google Scholar] [CrossRef]
- Faye, O.; Faye, O.; Dupressoir, A.; Weidmann, M.; Ndiaye, M.; Alpha Sall, A. One-step RT-PCR for detection of Zika virus. J. Clin. Virol. Off. Publ. Pan Am. Soc. Clin. Virol. 2008, 43, 96–101. [Google Scholar] [CrossRef]
- Fonseca, V.; Libin, P.J.K.; Theys, K.; Faria, N.R.; Nunes, M.R.T.; Restovic, M.I.; Freire, M.; Giovanetti, M.; Cuypers, L.; Nowe, A.; et al. A computational method for the identification of Dengue, Zika and Chikungunya virus species and genotypes. PLoS Negl. Trop. Dis. 2019, 13, e0007231. [Google Scholar] [CrossRef] [Green Version]
- Collins, M.H.; McGowan, E.; Jadi, R.; Young, E.; Lopez, C.A.; Baric, R.S.; Lazear, H.M.; de Silva, A.M. Lack of Durable Cross-Neutralizing Antibodies Against Zika Virus from Dengue Virus Infection. Emerg. Infect. Dis. 2017, 23, 773–781. [Google Scholar] [CrossRef] [PubMed]
- Bujan, L.; Mansuy, J.M.; Hamdi, S.; Pasquier, C.; Joguet, G. 1 year after acute Zika virus infection in men. Lancet Infect. Dis. 2020, 20, 25–26. [Google Scholar] [CrossRef] [Green Version]
- Muller, J.A.; Harms, M.; Kruger, F.; Gross, R.; Joas, S.; Hayn, M.; Dietz, A.N.; Lippold, S.; von Einem, J.; Schubert, A.; et al. Semen inhibits Zika virus infection of cells and tissues from the anogenital region. Nat. Commun. 2018, 9, 2207. [Google Scholar] [CrossRef] [PubMed]
- Huits, R.; De Smet, B.; Arien, K.K.; Van Esbroeck, M.; Bottieau, E.; Cnops, L. Zika virus in semen: A prospective cohort study of symptomatic travellers returning to Belgium. Bull. World Health Organ. 2017, 95, 802–809. [Google Scholar] [CrossRef] [PubMed]

| Subjects a | Clinical Status | Age (Years) | Date of Enrollment | Flavivirus Serology b | DPSO c | ZIKV Antigen in Sperm Cells by IF | % of IF Positive Sperm Cells | ZIKV RNA in Sperm Cells (Ct1/Ct2) e |
|---|---|---|---|---|---|---|---|---|
| A | Symptomatic | 21 | Nov/2016 | IgM-positiveIgG-negative | 7 | Yes | 3 (11/337) | No |
| 14 | Yes | 2.4 (18/737) | No | |||||
| 21 | Yes | 25 (321/1266) | No | |||||
| 28 | No | 0 (0/663) | Yes (21/40) | |||||
| 180 | No | 0/1603 | Yes 40/31 | |||||
| B | Symptomatic | 22 | Nov/2016 | IgM-positiveIgG-positive | 7 | Yes | 4.3 (4/916) | Yes (22/21) |
| 14 | No | 0 (0/226) | Yes (32/36) | |||||
| 21 | No | 0 (0/723) | Yes (32/40) | |||||
| 28 | No | 0 (0/370) | No (40/40) | |||||
| 60 | No | 0 (0/507) | No (40/40) | |||||
| C | Symptomatic | 43 | Aug/2017 | IgM-inconclusive | 21 | No | 0 (0/486) | No (40/40) |
| IgG-positive | 28 | Yes | 1.9 (18/935) | Yes (22/24) | ||||
| D | Asymptomatic | 21 | Jan/2017 | IgM-negative | NA d | Yes | 0.8 (4/487) | Yes (33/35) |
| IgG-positive | ||||||||
| E | Asymptomatic | 22 | Jan/2017 | IgM-negative | NA | Yes | 1.4 (10/724) | Yes (40/37) |
| IgG-positive | ||||||||
| F | Asymptomatic | 19 | Jan/2017 | IgM-positive | NA | Yes | 3.8 (18/472) | Yes (40/36) |
| IgG-positive |
| Semen Parameters | Zika Immunofluorescence | t-Test, p | |
|---|---|---|---|
| ZIKV-Negative (n = 16) | ZIKV-Positive (n = 14) | ||
| Median age in years (min and max) | 21.5 (17 and 42) | 21 (18 and 24) | 0.999, (0.330) |
| Median of semen volume (ml) | 2.3 (IQR 1.1–3) | 2.5 (IQR 2–3.5) | −1.031, (0.359) |
| Median of the sperm concentration (million/mL) a | 63.5 (IQR 36–104) | 45 (IQR 27–54) | 2.4 (0.041) |
| Mean motility (%, range) | |||
| Total motility (%, range) b | 75 (50–100) | 54 (27–90) | 2.883 (0.009) |
| Progressive motility (%, range) | 60 (30–80) | 46 (20–81) | 2.020, (0.063) |
| Nonprogressive motility (%, range) | 15 (0–30) | 8 (1–20) | 1.99, (0.050) |
| Nonmotile b (%, range) | 25 (0–65) | 46 (10–73) | −2.870, (0.009) |
| Mean viability (%, range) | 86 (60–98) | 72 (10–98) | 1.492, (0.159) |
| Normal morphology (%, range) | 3 (0–15) | 2 (0–9) | 0.456, (0.656) |
| Abnormal distributions (%, range) | |||
| Head defects (%, range) | 16 (0–100) | 42 (0–86) | −2.043, (0.051) |
| Neck mid-piece defects | 5 (0–16) | 6 (0–51) | 0.112, (0.909) |
| Tail defects | 8 (0–34) | 7 (0–25) | −0.431, (0.674) |
| Excess residual cytoplasm | 11 (0–66) | 5 (0–35) | −0.936, (0.369) |
| Median of the round cells concentration (million/mL) | 4.8 (IQR 2.7–10.6) | 5.1 (IQR 0.6–7.7) | 1.1 (0.286) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vanegas, H.; González, F.; Reyes, Y.; Centeno, E.; Palacios, J.; Zepeda, O.; Hagbom, M.; Collins, M.H.; Coward, R.M.; Becker-Dreps, S.; et al. Zika RNA and Flavivirus-Like Antigens in the Sperm Cells of Symptomatic and Asymptomatic Subjects. Viruses 2021, 13, 152. https://doi.org/10.3390/v13020152
Vanegas H, González F, Reyes Y, Centeno E, Palacios J, Zepeda O, Hagbom M, Collins MH, Coward RM, Becker-Dreps S, et al. Zika RNA and Flavivirus-Like Antigens in the Sperm Cells of Symptomatic and Asymptomatic Subjects. Viruses. 2021; 13(2):152. https://doi.org/10.3390/v13020152
Chicago/Turabian StyleVanegas, Hernan, Fredman González, Yaoska Reyes, Edwing Centeno, Jayrintzina Palacios, Omar Zepeda, Marie Hagbom, Matthew H. Collins, R. Matthew Coward, Sylvia Becker-Dreps, and et al. 2021. "Zika RNA and Flavivirus-Like Antigens in the Sperm Cells of Symptomatic and Asymptomatic Subjects" Viruses 13, no. 2: 152. https://doi.org/10.3390/v13020152
APA StyleVanegas, H., González, F., Reyes, Y., Centeno, E., Palacios, J., Zepeda, O., Hagbom, M., Collins, M. H., Coward, R. M., Becker-Dreps, S., Bowman, N., & Bucardo, F. (2021). Zika RNA and Flavivirus-Like Antigens in the Sperm Cells of Symptomatic and Asymptomatic Subjects. Viruses, 13(2), 152. https://doi.org/10.3390/v13020152








