Molecular Transmission Dynamics of Primary HIV Infections in Lazio Region, Years 2013–2020
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Population, HIV Serodiagnosis, and Therapy
- (a)
- HIV-1 RNA levels >10,000 copies/mL and negative fourth generation HIV assay (Ab/Ag Combo), or positive fourth generation HIV assay with negative or indeterminate WB test;
- (b)
- positive HIV Ab/Ag Combo test accompanied by positive WB without p31 reactivity, associated with an antibody avidity index ≤0.80;
- (c)
- documented negative HIV-1 test performed within 6 months before the current HIV-1 diagnosis.
2.2. HIV-1 Drug Resistance Testing, Subtype Assignment, TC Identification, and Infection Dating by pol Population Sequencing
2.3. UDS of HIV Env Region
2.4. Statistical Analysis
3. Results
3.1. PHI Description
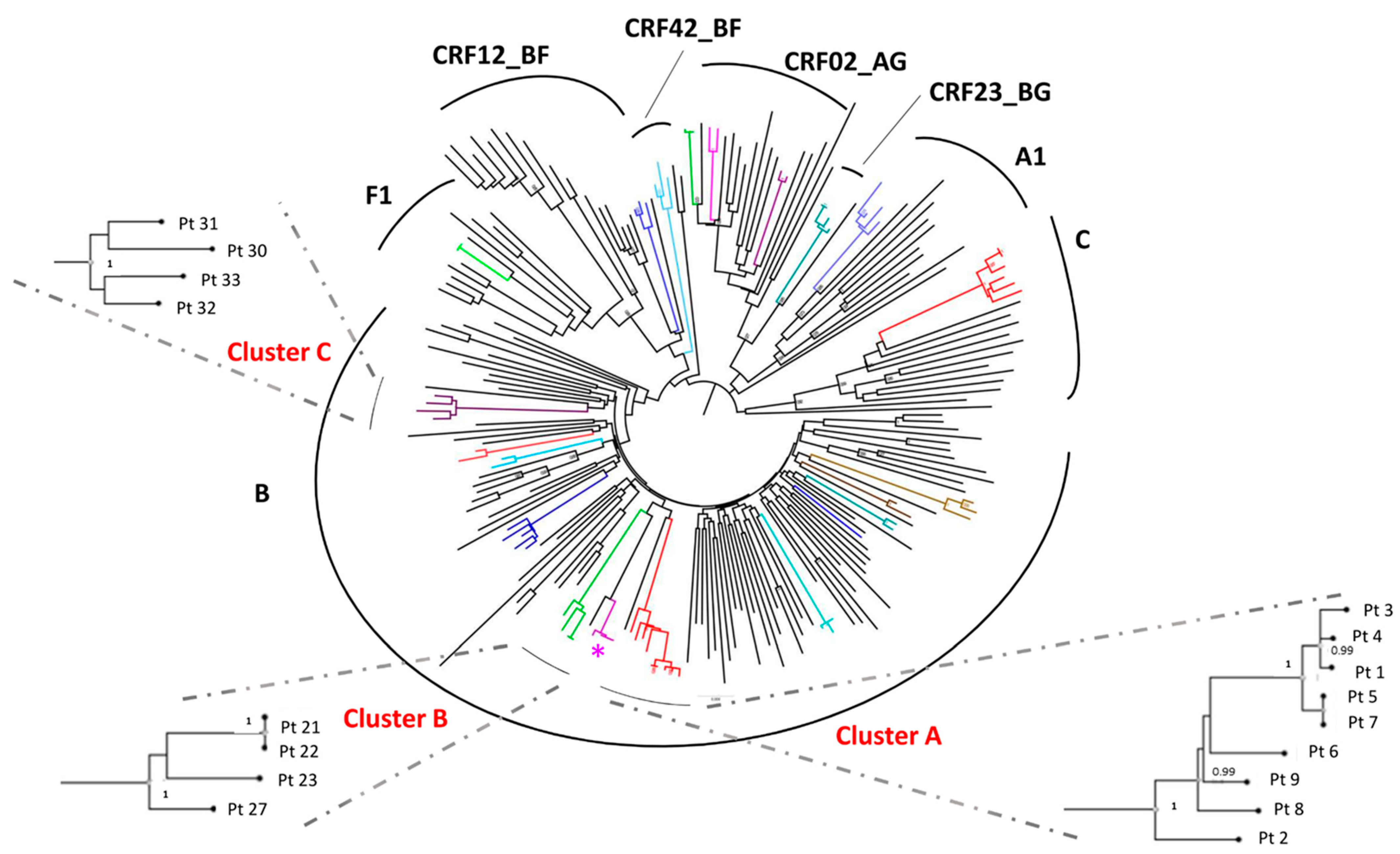
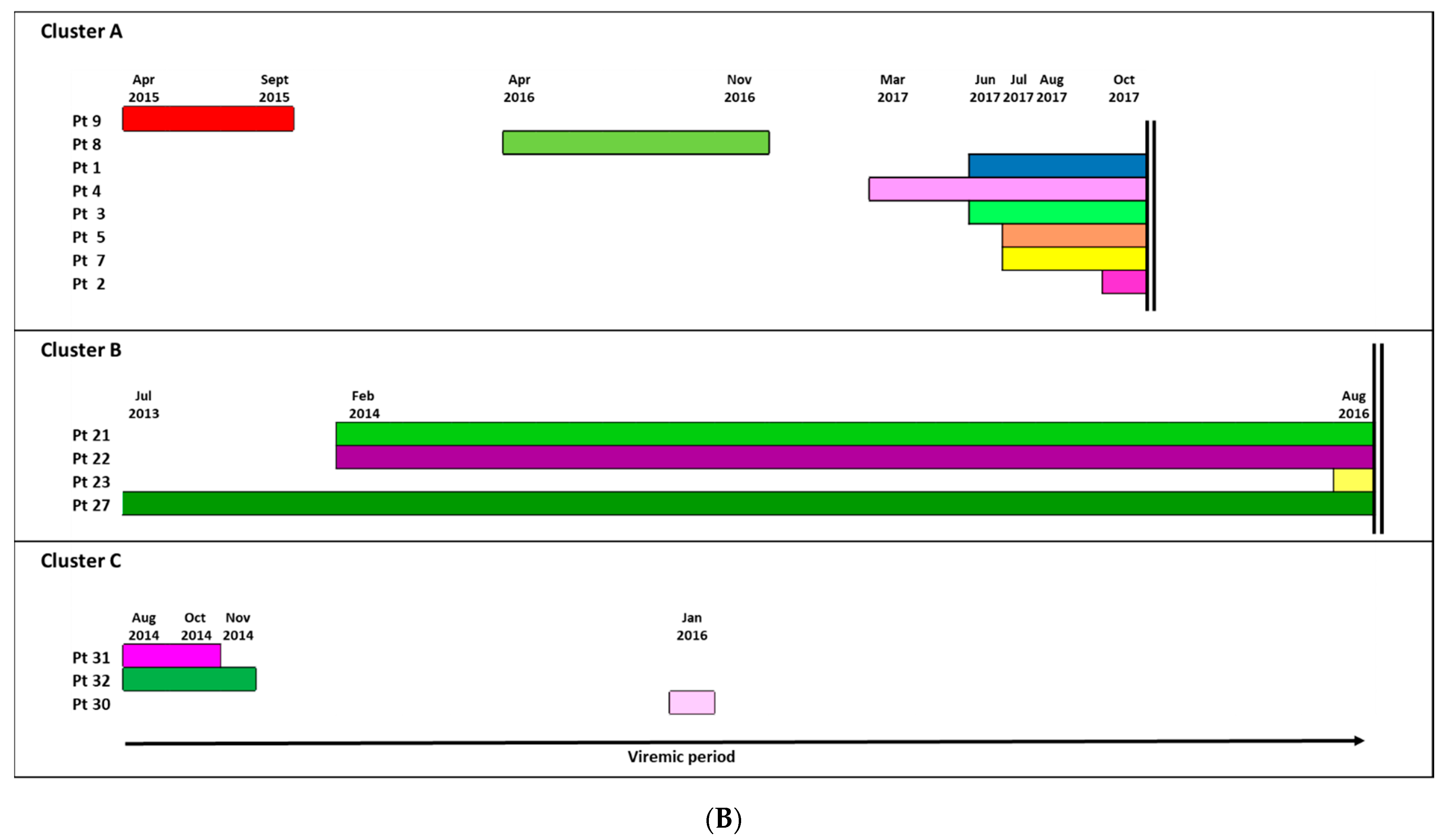
3.2. Identification of TC and Study of the Factors Associated with the Inclusion in a TC
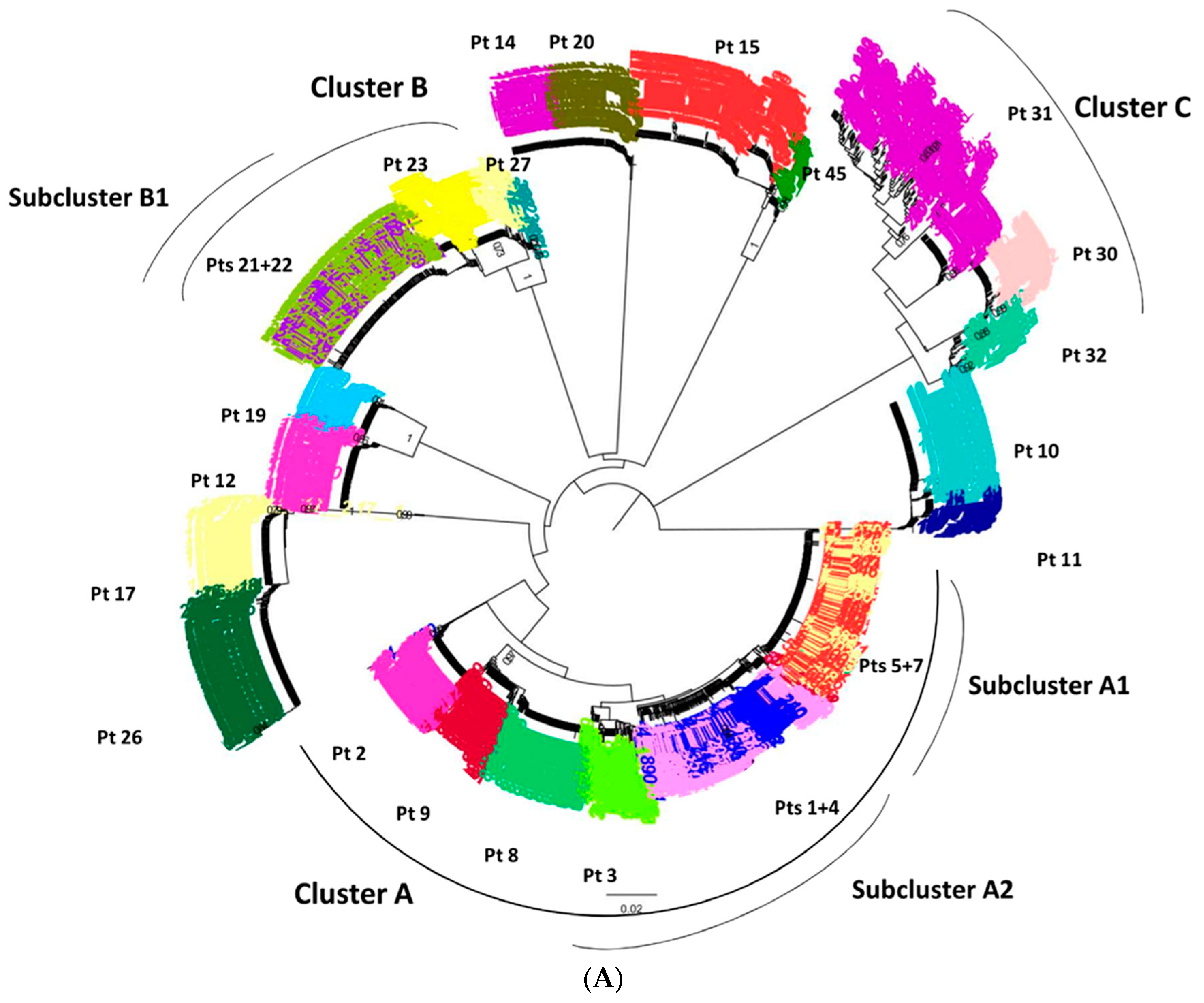
3.3. Ultra-Deep Analysis of TC
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Grant, R.M.; Lama, J.R.; Anderson, P.L.; Mcmahan, V.; Liu, A.Y.; Vargas, L.; Goicochea, P.; Casapía, M.; Guanira-Carranza, J.V.; Ramirez-Cardich, M.E.; et al. Preexposure Chemoprophylaxis for HIV Prevention in Men Who Have Sex with Men. N. Engl. J. Med. 2010, 363, 2587–2599. [Google Scholar] [CrossRef] [PubMed]
- Fonner, V.A.; Dalglish, S.L.; Kennedy, C.E.; Baggaley, R.; O’Reilly, K.R.; Koechlin, F.M.; Rodolph, M.; Hodges-Mameletzis, I.; Grant, R.M. Effectiveness and safety of oral HIV preexposure prophylaxis for all populations. AIDS 2016, 30, 1973–1983. [Google Scholar] [CrossRef] [PubMed]
- Hue, S.; Clewley, J.P.; Cane, P.A.; Pillay, D. Investigation of HIV-1 transmission events by phylogenetic methods: Requirement for scientific rigour. AIDS 2005, 19, 4. [Google Scholar] [CrossRef] [PubMed]
- Brenner, B.; Wainberg, M.A.; Roger, M. Phylogenetic inferences on HIV-1 transmission: Implications for the design of prevention and treatment interventions. AIDS 2013, 27, 1045–1057. [Google Scholar] [CrossRef] [PubMed]
- Gibson, K.M.; Jair, K.; Castel, A.D.; Bendall, M.L.; Wilbourn, B.; Jordan, J.A.; Crandall, K.A.; Pérez-Losada, M.; Subramanian, T.; Binkley, J.; et al. A cross-sectional study to characterize local HIV-1 dynamics in Washington, DC using next-generation sequencing. Sci. Rep. 2020, 10, 1989. [Google Scholar] [CrossRef]
- Leigh Brown, A.J.; Lycett, S.J.; Weinert, L.; Hughes, G.J.; Fearnhill, E.; Dunn, D.T. Transmission network parameters estimated from HIV sequences for a nationwide epidemic. J. Infect. Dis. 2011, 204, 1463–1469. [Google Scholar] [CrossRef]
- Beyrer, C.; Sullivan, P.; Sanchez, J.; Baral, S.D.; Collins, C.; Wirtz, A.L.; Altman, D.; Trapence, G.; Mayer, K. The increase in global HIV epidemics in MSM. AIDS 2013, 27, 2665–2678. [Google Scholar] [CrossRef]
- Fabeni, L.; Alteri, C.; Di Carlo, D.; Orchi, N.; Carioti, L.; Bertoli, A.; Gori, C.; Forbici, F.; Continenza, F.; Maffongelli, G.; et al. Dynamics and phylogenetic relationships of HIV-1 transmitted drug resistance according to subtype in Italy over the years 2000-14. J. Antimicrob. Chemother. 2017, 72, 2837–2845. [Google Scholar] [CrossRef]
- Fabeni, L.; Alteri, C.; Berno, G.; Scutari, R.; Orchi, N.; De Carli, G.; Bertoli, A.; Carioti, L.; Gori, C.; Forbici, F.; et al. Characterisation of HIV-1 molecular transmission clusters among newly diagnosed individuals infected with non-B subtypes in Italy. Sex. Transm. Infect. 2019, 95, 619–625. [Google Scholar] [CrossRef]
- Brenner, B.G.; Roger, M.; Routy, J.P.; Moisi, D.; Ntemgwa, M.; Matte, C.; Baril, J.G.; Thomas, R.; Rouleau, D.; Bruneau, J.; et al. High rates of forward transmission events after acute/early HIV-1 infection. J. Infect. Dis. 2007, 195, 951–959. [Google Scholar] [CrossRef]
- Pao, D.; Fisher, M.; Hué, S.; Dean, G.; Murphy, G.; Cane, P.A.; Sabin, C.A.; Pillay, D. Transmission of HIV-1 during primary infection: Relationship to sexual risk and sexually transmitted infections. AIDS 2005, 19, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Cohen, M.S.; Shaw, G.M.; McMichael, A.J.; Haynes, B.F. Acute HIV-1 infection. N. Engl. J. Med. 2011, 364, 1943–1954. [Google Scholar] [CrossRef] [PubMed]
- Hollingsworth, T.D.; Anderson, R.M.; Fraser, C. HIV-1 transmission, by stage of infection. J. Infect. Dis. 2008, 198, 687–693. [Google Scholar] [CrossRef] [PubMed]
- Miller, W.C.; Rosenberg, N.E.; Rutstein, S.E.; Powers, K.A. Role of acute and early HIV infection in the sexual transmission of HIV. Curr. Opin. HIV AIDS 2010, 5, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Pilcher, C.D.; Shugars, D.C.; Fiscus, S.A.; Miller, W.C.; Menezes, P.; Giner, J.; Dean, B.; Robertson, K.; Hart, C.E.; Lennox, J.L.; et al. HIV in body fluids during primary HIV infection: Implications for pathogenesis, treatment and public health. AIDS 2001, 15, 837–845. [Google Scholar] [CrossRef]
- Abbate, I.; Vlassi, C.; Rozera, G.; Bruselles, A.; Bartolini, B.; Giombini, E.; Corpolongo, A.; D’Offizi, G.; Narciso, P.; Desideri, A.; et al. Detection of quasispecies variants predicted to use CXCR4 by ultra-deep pyrosequencing during early HIV infection. AIDS 2011, 25, 611–617. [Google Scholar] [CrossRef]
- Rozera, G.; Abbate, I.; Giombini, E.; Castagna, A.; de Luca, A.; Ceccherini-Silberstein, F.; Lepri, A.C.; Cassola, G.; Torti, C.; d’Arminio Monforte, A.; et al. Evolution of HIV-1 tropism at quasispecies level after 5 years of combination antiretroviral therapy in patients always suppressed or experiencing episodes of virological failure. J. Antimicrob. Chemother. 2014, 69, 3085–3094. [Google Scholar] [CrossRef][Green Version]
- Alteri, C.; Santoro, M.M.; Abbate, I.; Rozera, G.; Bruselles, A.; Bartolini, B.; Gori, C.; Forbici, F.; Orchi, N.; Tozzi, V.; et al. “Sentinel” mutations in standard population sequencing can predict the presence of HIV-1 reverse transcriptase major mutations detectable only by ultra-deep pyrosequencing. J. Antimicrob. Chemother. 2011, 66, 2615–2623. [Google Scholar] [CrossRef]
- Simen, B.B.; Braverman, M.S.; Abbate, I.; Aerssens, J.; Bidet, Y.; Bouchez, O.; Gabriel, C.; Izopet, J.; Kessler, H.H.; Stelzl, E.; et al. An international multicenter study on HIV-1 drug resistance testing by 454 ultra-deep pyrosequencing. J. Virol. Methods 2014, 204, 31–37. [Google Scholar] [CrossRef]
- Montoya, V.; Olmstead, A.; Tang, P.; Cook, D.; Janjua, N.; Grebely, J.; Jacka, B.; Poon, A.F.Y.; Krajden, M. Deep sequencing increases hepatitis C virus phylogenetic cluster detection compared to Sanger sequencing. Infect. Genet. Evol. 2016, 43, 329–337. [Google Scholar] [CrossRef]
- Yu, F.; Wen, Y.; Wang, J.; Gong, Y.; Feng, K.; Ye, R.; Jiang, Y.; Zhao, Q.; Pan, P.; Wu, H.; et al. The Transmission and Evolution of HIV-1 Quasispecies within One Couple: A Follow-up Study based on Next-Generation Sequencing. Sci. Rep. 2018, 8, 1404. [Google Scholar] [CrossRef] [PubMed]
- Rosea, R.; Crossa, S.; Lamersa, S.L.; Astemborskib, J.; Kirkb, G.D.; Mehtab, S.H.; Sieversc, M.; Martense, G.; Brunoe, D.; Reddc, A.D.; et al. Persistence of HIV transmission clusters among people who inject drugs. AIDS 2020, 34, 2037–2044. [Google Scholar] [CrossRef] [PubMed]
- Antinori, A.; Di Biagio, A.; Marcotullio, S.; Andreoni, M.; Chirianni, A.; d’Arminio Monforte, A.; Galli, M.; Mazzotta, F.; Mussini, C.; Puoti, M.; et al. Italian HIV Guidelines Working Group. Italian guidelines for the use of antiretroviral agents and the diagnostic-clinical management of HIV-1 infected persons. Update 2016. New Microbiol. 2017, 40, 86–98. [Google Scholar] [PubMed]
- Suligoi, B.; Massi, M.; Galli, C.; Sciandra, M.; Di Sora, F.; Pezzotti, P.; Recchia, O.; Montella, F.; Sinicco, A.; Rezza, G. Identifying recent HIV infections using the avidity index and an automated enzyme immunoassay. J. Acquir. Immune Defic. Syndr. 2003, 32, 424–428. [Google Scholar] [CrossRef]
- Fiebig, E.W.; Wright, D.J.; Rawal, B.D.; Garrett, P.E.; Schumacher, R.T.; Peddada, L.; Heldebrant, C.; Smith, R.; Conrad, A.; Kleinman, S.H.; et al. Dynamics of HIV viremia and antibody seroconversion in plasma donors: Implications for diagnosis and staging of primary HIV infection. AIDS 2003, 17, 1871–1879. [Google Scholar] [CrossRef]
- Santoro, M.M.; Fabeni, L.; Armenia, D.; Alteri, C.; Di Pinto, D.; Forbici, F.; Bertoli, A.; Di Carlo, D.; Gori, C.; Carta, S.; et al. Reliability and clinical relevance of the HIV-1 drug resistance test in patients with low viremia levels. Clin. Infect. Dis. 2014, 58, 1156–1164. [Google Scholar] [CrossRef]
- Bennett, D.E.; Camacho, R.J.; Otelea, D.; Kuritzkes, D.R.; Fleury, H.; Kiuchi, M.; Heneine, W.; Kantor, R.; Jordan, M.R.; Schapiro, J.M.; et al. Drug resistance mutations for surveillance of transmitted HIV-1 drug-resistance: 2009 update. PLoS ONE 2009, 4, e4724. [Google Scholar] [CrossRef]
- Wensing, A.M.; Calvez, V.; Ceccherini-Silberstein, F.; Charpentier, C.; Günthard, H.F.; Paredes, R.; Shafer, R.W.; Richman, D.D. 2019 Update of the Drug Resistance Mutations in HIV-1. Top. Antivir. Med. 2019, 27, 111–112. [Google Scholar]
- Fabeni, L.; Berno, G.; Fokam, J.; Bertoli, A.; Alteri, C.; Gori, C.; Forbici, F.; Takou, D.; Vergori, A.; Zaccarelli, M.; et al. Comparative evaluation of subtyping tools for surveillance of newly emerging HIV-1 strains. J. Clin. Microbiol. 2017, 55, 2827–2837. [Google Scholar] [CrossRef]
- Tavaré, S. Some probabilistic and statistical problems in the analysis of DNA sequences. Am. Math. Soc. Lect. Math. Life Sci. 1986, 17, 57–86. [Google Scholar]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef] [PubMed]
- Drummond, A.J.; Rambaut, A. BEAST: Bayesian evolutionary analysis by sampling trees. BMC Evol. Biol. 2007, 7, 214. [Google Scholar] [CrossRef] [PubMed]
- Drummond, A.J.; Rambaut, A.; Shapiro, B.; Pybus, O.G. Bayesian coalescent inference of past population dynamics from molecular sequences. Mol. Biol. Evol. 2005, 22, 1185–1192. [Google Scholar] [CrossRef] [PubMed]
- Suchard, M.A.; Weiss, R.E.; Sinsheimer, J.S. Bayesian selection of continuous-time Markov chain evolutionary models. Mol. Biol. Evol. 2001, 18, 1001–1013. [Google Scholar] [CrossRef]
- Tajima, F.; Nei, M. Estimation of evolutionary distance between nucleotide sequences. Mol. Biol. Evol. 1984, 1, 269–285. [Google Scholar]
- Lengauer, T.; Sander, O.; Sierra, S.; Thielen, A.; Kaiser, R. Bioinformatics prediction of HIV coreceptor usage. Nat. Biotechnol. 2007, 25, 1407–1410. [Google Scholar] [CrossRef]
- Servizio Regionale Epidemiologia Sorveglianza e controllo delle Malattie Infettive (SERESMI). Nuove diagnosi Infezione da HIV. Report 2018. Available online: https://www.inmi.it/wp-content/uploads/2019/11/Report-HIV_2018_nuova-sorveglianza.pdf (accessed on 19 January 2021).
- Kroon, E.; Pham, P.T.; Sirivichayakul, S.; Trichavaroj, R.; Colby, D.J.; Pinyakorn, S.; Phanuphak, N.; Sanders-Buell, E.; van Griensven, F.; Kijak, G.H.; et al. Transmission dynamics among participants initiating antiretroviral therapy upon diagnosis of early acute HIV-1 infection in Thailand. AIDS 2018, 32, 2373–2381. [Google Scholar]
- Dennis, A.M.; Volz, E.; Frost, S.D.W.; Mukarram Hossain, A.S.M.; Poon, A.F.Y.; Rebeiro, P.F.; Vermund, S.H.; Sterling, T.R.; Kalish, M.L. HIV-1 Transmission Clustering and Phylodynamics Highlight the Important Role of Young Men Who Have Sex with Men. AIDS Res. Hum. Retrov 2018, 34, 10. [Google Scholar] [CrossRef]
- Chaillon, A.; Essat, A.; Frange, P.; Smith, D.M.; Delaugerre, C.; Barin, F.; Ghosn, J.; Pialoux, G.; Robineau, O.; Rouzioux, C.; et al. Spatiotemporal dynamics of HIV-1 transmission in France (1999–2014) and impact of targeted prevention strategies. Retrovirology 2017, 14, 15. [Google Scholar] [CrossRef]
- Patiño-Galindo, J.A.; Thomson, M.M.; Pérez-Álvarez, L.; Delgado, E.; Cuevas, M.T.; Fernández-García, A.; Nájera, R.; Iribarren, J.A.; Cilla, G.; López-Soria, L.; et al. Transmission dynamics of HIV-1 subtype B in the Basque Country, Spain. Infect. Genet. Evol. 2016, 40, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Ragonnet-Cronin, M.L.; Shilaih, M.; Günthard, H.F.; Hodcroft, E.B.; Böni, J.; Fearnhill, E.; Dunn, D.; Yerly, S.; Klimkait, T.; Aubert, V.; et al. A Direct Comparison of Two Densely Sampled HIV Epidemics: The UK and Switzerland. Sci. Rep. 2016, 6, 32251. [Google Scholar] [CrossRef] [PubMed]
- Aldous, J.L.; Pond, S.K.; Poon, A.; Jain, S.; Qin, H.; Kahn, J.S.; Kitahata, M.; Rodriguez, B.; Dennis, A.M.; Boswell, S.L.; et al. Characterizing HIV transmission networks across the United States. Clin. Infect. Dis. 2012, 55, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Yousef, K.P.; Meixenberger, K.; Smith, M.R.; Somogyi, S.; Gromöller, S.; Schmidt, D.; Gunsenheimer-Bartmeyer, B.; Hamouda, O.; Kücherer, C.; Kleist, M.v. Inferring HIV-1 Transmission Dynamics in Germany From Recently Transmitted Viruses. J. Acquir. Immune Defic. Syndr. 2016, 73, 356–363. [Google Scholar] [CrossRef] [PubMed]
- Pineda-Peña, A.C.; Schrooten, Y.; Vinken, L.; Ferreira, F.; Li, G.; Trovão, N.S.; Khouri, R.; Derdelinckx, I.; De Munter, P.; Kücherer, C.; et al. Trends and predictors of transmitted drug resistance (TDR) and clusters with TDR in a local Belgian HIV-1 epidemic. PLoS ONE 2014, 9, e101738. [Google Scholar] [CrossRef]
- Vega, Y.; Delgado, E.; Fernández-García, A.; Cuevas, M.T.; Thomson, M.M.; Montero, V.; Sánchez, M.; Sánchez, A.M.; Pérez-Álvarez, L. Epidemiological surveillance of HIV-1 transmitted drug resistance in Spain in 2004-2012: Relevance of transmission clusters in the propagation of resistance mutations. PLoS ONE 2015, 10, e0125699. [Google Scholar] [CrossRef]
- Frange, P.; Assoumou, L.; Descamps, D.; Chéret, A.; Goujard, C.; Tran, L.; Gousset, M.; Avettand-Fenoël, V.; Bocket, L.; Fafi-Kremer, S.; et al. HIV-1 subtype B-infected MSM may have driven the spread of transmitted resistant strains in France in 2007–12: Impact on susceptibility to first-line strategies. J. Antimicrob. Chemother. 2015, 70, 2084–2089. [Google Scholar] [CrossRef][Green Version]
- Lunar, M.M.; Židovec Lepej, S.; Abecasis, A.B.; Tomažič, J.; Vidmar, L.; Karner, P.; Vovko, T.D.; Pečavar, B.; Maver, P.J.; Seme, K.; et al. Short communication: Prevalence of HIV type 1 transmitted drug resistance in Slovenia: 2005-2010. AIDS Res. Hum. Retroviruses 2013, 29, 343–349. [Google Scholar] [CrossRef]
- Lundgren, J.D.; Babiker, A.G.; Gordin, F.; Emery, S.; Grund, B.; Sharma, S.; Avihingsanon, A.; Cooper, D.A.; Fätkenheuer, G.; Llibre, J.M.; et al. Initiation of antiretroviral therapy in early asymptomatic HIV infection. N. Engl. J. Med. 2015, 373, 795–807. [Google Scholar]
- Danel, C.; Moh, R.; Gabillard, D.; Badje, A.; Le Carrou, J.; Ouassa, T.; Ouattara, E.; Anzian, A.; Ntakpé, J.B.; Minga, A.; et al. A trial of early antiretrovirals and isoniazid preventive therapy in Africa. N. Engl. J. Med. 2015, 373, 808–822. [Google Scholar]
- Todesco, E.; Wirden, M.; Calin, R.; Simon, A.; Sayon, S.; Barin, F.; Katlama, C.; Calvez, V.; Marcelin, A.G.; Hué, S. Caution is needed in interpreting HIV transmission chains by ultradeep sequencing. AIDS 2019, 33, 691–699. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Overall | In Cluster | Out of Cluster | Univariate Analysis | p Value a |
|---|---|---|---|---|---|
| n = 229 | n = 68 (29.7%) | n = 161 (70.3%) | OR (95% CI) | ||
| Gender (male vs. female †), n (%) | 217 (94.8) | 66 (97.1) | 151 (93.8) | 0.46 (0.09–2.15) | 0.32 |
| Age (years), median (IQR) | 39 (32–48) | 37 (32–44) | 39 (32–49) | 0.98 (0.96–1.00) | 0.18 |
| Year of diagnosis, n (%) | |||||
| 2013–2014 | 77 (33.6) | 31 (45.6) | 46 (28.6) | 2.21 (1.10–4.44) | 0.03 |
| 2015–2016 † | 77 (33.6) | 18 (26.5) | 59 (36.6) | 1 | |
| 2017–2018 | 61 (26.6) | 16 (23.5) | 45 (28.0) | 1.17 (0.54–2.54) | 0.70 |
| 2019–2020 | 14 (6.1) | 3 (4.4) | 11 (6.8) | 0.89 (0.23–3.56) | 0.87 |
| Nationality, n (%) | |||||
| Italians † | 198 (86.5) | 63 (92.6) | 135 (83.9) | 1 | |
| Foreigners | 31 (13.5) | 5 (7.4) | 26 (16.1) | 2.43 (0.89–6.61) | 0.08 |
| Risk factor, n (%) | |||||
| MSM | 191 (83.4) | 59 (86.8) | 132 (82.0) | 1.44 (0.64–3.23) | 0.38 |
| Heterosexual † | 35 (15.3) | 8 (11.8) | 27 (16.8) | 1 | |
| IV drug addict | 3 (1.3) | 1 (1.5) | 2 (1.2) | 1.19 (0.11–13.3) | 0.89 |
| Fiebig stage, n (%) | |||||
| II-IV † | 117 (51.1) | 35 (51.5) | 82 (50.9) | 1 | |
| V-VI | 112 (48.9) | 33 (48.5) | 79 (49.1) | 1.02 (0.58–1.80) | 0.94 |
| CD4 cell count (cell/mm3), median (IQR) | 557 (389–712) | 548 (370–743) | 557 (391–705) | 1.00 (0.99–1.00) | 0.98 |
| Viral load (log10 copies/mL), median (IQR) | 5.5 (4.6–6.4) | 5.6 (4.6–6.5) | 5.4 (4.6–6.3) | 1.04 (0.83–1.30) | 0.74 |
| Subtype b, n (%) | |||||
| B † | 130 (56.8) | 40 (58.8) | 90 (55.9) | 1 | |
| Non B | 99 (43.2) | 28 (41.2) | 71 (44.1) | 1.13 (0.63–2.00) | 0.68 |
| Transmitted drug resistance, n (%) | |||||
| Any drug class | 23 (10.0) | 4 (5.9) | 19 (11.8) | 0.47 (0.15–1.43) | 0.18 |
| Time from diagnosis to virological suppression, n (%) of patients undergoing early ART c | |||||
| Overall | 190 (83.0) | 57 (83.8) | 133 (82.6) | ||
| ≤20 weeks † | 100 (52.6) | 29 (50.9) | 71 (53.4) | 1 | |
| >20 weeks | 90 (47.4) | 28 (49.1) | 62 (46.6) | 0.90 (0.49–1.68) | 0.75 |
| Patients who did not start ART within 24 weeks from diagnosis, n (%) | 17 (8.2) | 7 (10.4) | 10 (7.0) | 1.63 (0.59–4.50) | 0.34 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fabeni, L.; Rozera, G.; Berno, G.; Giombini, E.; Gori, C.; Orchi, N.; De Carli, G.; Pittalis, S.; Puro, V.; Pinnetti, C.; et al. Molecular Transmission Dynamics of Primary HIV Infections in Lazio Region, Years 2013–2020. Viruses 2021, 13, 176. https://doi.org/10.3390/v13020176
Fabeni L, Rozera G, Berno G, Giombini E, Gori C, Orchi N, De Carli G, Pittalis S, Puro V, Pinnetti C, et al. Molecular Transmission Dynamics of Primary HIV Infections in Lazio Region, Years 2013–2020. Viruses. 2021; 13(2):176. https://doi.org/10.3390/v13020176
Chicago/Turabian StyleFabeni, Lavinia, Gabriella Rozera, Giulia Berno, Emanuela Giombini, Caterina Gori, Nicoletta Orchi, Gabriella De Carli, Silvia Pittalis, Vincenzo Puro, Carmela Pinnetti, and et al. 2021. "Molecular Transmission Dynamics of Primary HIV Infections in Lazio Region, Years 2013–2020" Viruses 13, no. 2: 176. https://doi.org/10.3390/v13020176
APA StyleFabeni, L., Rozera, G., Berno, G., Giombini, E., Gori, C., Orchi, N., De Carli, G., Pittalis, S., Puro, V., Pinnetti, C., Mondi, A., Camici, M., Plazzi, M. M., Antinori, A., Capobianchi, M. R., & Abbate, I. (2021). Molecular Transmission Dynamics of Primary HIV Infections in Lazio Region, Years 2013–2020. Viruses, 13(2), 176. https://doi.org/10.3390/v13020176








