Abstract
The SAM and HD domain-containing protein 1 (SAMHD1) is a dNTP triphosphohydrolase that plays a crucial role for a variety of different cellular functions. Besides balancing intracellular dNTP concentrations, facilitating DNA damage repair, and dampening excessive immune responses, SAMHD1 has been shown to act as a major restriction factor against various virus species. In addition to its well-described activity against retroviruses such as HIV-1, SAMHD1 has been identified to reduce the infectivity of different DNA viruses such as the herpesviruses CMV and EBV, the poxvirus VACV, or the hepadnavirus HBV. While some viruses are efficiently restricted by SAMHD1, others have developed evasion mechanisms that antagonize the antiviral activity of SAMHD1. Within this review, we summarize the different cellular functions of SAMHD1 and highlight the countermeasures viruses have evolved to neutralize the restriction factor SAMHD1.
Keywords:
SAMHD1; restriction factor; viral antagonism; HIV; herpesviruses; viral kinases; Vpx; dNTP hydrolase; viral interference 1. The dNTPase SAMHD1
The SAM and HD domain-containing protein 1 (SAMHD1) is a ubiquitously expressed deoxynucleotide triphosphohydrolase (dNTPase) of 626 amino acids (Figure 1). In general, sterile alpha motif (SAM) domains have been shown to mediate protein–protein interaction or nucleic acid binding; however, its function in SAMHD1 is still unclear. The enzymatically active HD domain, defined by two pairs of histidine and aspartate residues in its active center, on the other hand is essential for retroviral restriction and tetramerization of the protein [1,2,3]. Although nuclear localization of SAMHD1 is mediated through an N-terminal nuclear localization signal (NLS), neither the antiviral activity nor the dNTPase function of SAMHD1 seem to be influenced by its localization [4,5,6]. Oligomerization of SAMHD1 is required for its catalytic dNTPase activity and is induced upon cofactor binding at two allosteric sites within the protein [7,8,9,10,11,12]. At allosteric site 1, dGTP or GTP binding leads to SAMHD1 dimerization, while the subsequent binding of any dNTP to allosteric site 2 induces tetramer formation [1,13,14].
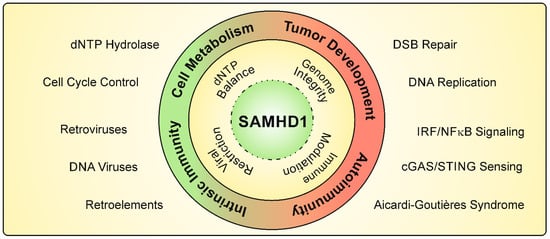
Figure 1.
Cellular functions of SAMHD1. The dNTP triphosphohydrolase SAMHD1 is an important regulator of dNTP levels. A correct balance of the cellular dNTP pool has been shown to be important for cell-cycle control and genome integrity. SAMHD1 is a mediator of double-strand break (DSB) repair and ensures the progression of DNA replication. Mutations in the SAMHD1 gene have been identified in various types of cancer. Furthermore, SAMHD1 potently restricts viral infectivity of retroviruses and DNA viruses, and blocks the activity of endogenous retroelements. Mutations in SAMHD1 are correlated with autoimmune diseases such as Aicardi–Goutières syndrome, resulting in elevated type I IFN levels. SAMHD1 also impairs the innate immune sensing of viral infections by interfering with cGAS/STING nucleic acid recognition and NFκB/IRF signaling pathways.
SAMHD1 converts dNTPs into deoxynucleosides (dN) and inorganic triphosphates (PPPi) and thereby counteracts the de novo dNTP synthesis, primarily conducted by ribonucleotide reductase (RNR) and cellular deoxynucleoside kinases, which are mainly active during the S phase of the cell cycle [15,16,17,18]. RNR catalyzes the formation of dNTPs from ribonucleotides, while deoxynucleoside kinases, such as thymidine kinases (TK), add phosphates to nucleosides to form deoxynucleoside triphosphates [19,20]. This ensures a highly balanced dNTP pool during the progression of the cell cycle, with a sufficient supply of dNTPs for efficient genome replication in dividing cells and limited levels of dNTPs in nondividing and resting cells [1,14,21]. The cyclin-dependent kinases (CDK) 1 and 2, together with cyclin A, phosphorylate SAMHD1 at the threonine residue 592 (T592) during the S and G2 phase of the cell cycle [22,23]. SAMHD1 is dephosphorylated during the M/G1 phase transition by the PP2A-B55α phosphatase and is dephosphorylated in the G0 and G1 phases [24]. Thus, SAMHD1 is phosphorylated during the progression of the cell cycle, which correlates with the demand of cellular dNTPs. However, at this point it is unclear whether the phosphorylation at T592 indeed regulates the dNTPase activity of SAMHD1 [25,26,27,28].
2. DNA Replication and DNA Damage Repair
By degrading cellular dNTPs, SAMHD1 is a major regulator of nucleotide homeostasis. A highly balanced dNTP pool is essential for genomic integrity, including proper DNA replication and efficient repair of DNA breaks. An imbalance can lead to a deregulated cell-cycle progression and therefore induce replication stress, which might eventually result in the accumulation of genomic mutations [29,30]. During host genome replication, SAMHD1 was found to be recruited to replication foci to regulate the progression of the replication fork. Here, SAMHD1 activates the 3′-5′-exonuclease MRE11, thereby promoting the MRE11-mediated degradation of nascent DNA at stalled replication forks, which has been suggested to prevent the induction of type I interferons (IFN) through the aberrant accumulation of single-stranded DNA (ssDNA). In addition, stimulation of MRE11 by SAMHD1 leads to activation of the ATR-CHK1 checkpoint, resulting in restart and progression of stalled replication forks [31].
SAMHD1 has also been described to be important for DNA damage repair by promoting homologous recombination (HR) [32]. SAMHD1 was identified to localize to DNA double-strand breaks (DSBs) and to recruit members of the DSB repair (DSBR) machinery [32,33]. It directly binds to the C-terminal binding protein interacting protein (CtIP), leading to its recruitment to DSBs and consequently to the activation of MRE11 as part of the MRE11-Rad50-NBS1 (MRN) DSBR complex, which is responsible for the resection of DNA ends via the 3′-5′-exonuclease function of MRE11 [32,33]. Due to its role in DSBR, it is conceivable that mutations in the SAMHD1 gene, as well as silencing hypermethylation of the SAMHD1 promoter, might play a role in tumorigenesis of various forms of cancer such as colon cancer, chronic lymphocytic leukemia, and lung adenocarcinoma [30,34,35,36].
3. Role of SAMHD1 in Intrinsic Immunity
Mutations in SAMHD1 are associated with Aicardi–Goutières syndrome (AGS)—an autoimmune disorder characterized by a progressive inflammatory encephalopathy, spontaneous type I IFN production in the cerebrospinal fluid, and upregulated IFN-stimulated gene (ISG) expression [6]. AGS highly resembles the phenotype of congenital viral infections, with signs of neurological dysfunction, physical and mental retardation, and basal ganglia calcification [37,38]. The development of AGS is linked to mutations in several genes involved in nucleic acid metabolism or sensing, including ADAR1, TREX1, RNaseH2, MDA5, and SAMHD1 [6,37,39]. Defects or alterations in these genes can lead to enhanced accumulation or sensing of endogenous nucleic acids, which in turn drive inflammatory immune responses in AGS patients [6,38,40]. In line with its role in AGS, depletion of SAMHD1 results in a highly elevated type I IFN production in human macrophages, as well as in an upregulation of ISGs in the mouse model [41,42,43].
Besides its protective role in autoimmunity, SAMHD1 was also shown to suppress the antiviral immune response upon infection [44]. HIV-1 reverse-transcription products have been shown to be recognized by the pattern recognition receptor cyclic GMP-AMP synthase (cGAS) in myeloid cell lines such as dendritic cells (DC) [45,46,47]. Stimulation of cGAS, and subsequently of the adaptor protein STING, leads to the activation and nuclear translocation of the transcription factors nuclear factor κB (NFκB) and IFN regulatory factor 3 (IRF3), culminating in the production of proinflammatory cytokines [44,48]. Interestingly and somewhat counterintuitive, SAMHD1 restricts reverse transcription of the HIV-1 genome in nondividing cells, and therefore dampens the sensing of HIV DNA products by cGAS in myeloid cells such as DCs [45,46,47]. In addition, SAMHD1 inhibits the activation of NFκB and type I IFN upon viral infection through direct binding to NFκB as well as IRF7 [49]. Furthermore, SAMHD1 reduces the phosphorylation of NFκB inhibitor IκBα, and interacts with the inhibitor κB kinase ε (IKKε), which prevents IKKε-dependent phosphorylation and activation of IRF7 [49]. Thus, SAMHD1 interferes with several mediators of immune signaling pathways and prevents an excessive antiviral and proinflammatory response with a secretion of cytokines such as tumor necrosis factor α (TNFα), interleukin 1β and -6 (IL-1β/-6), IFN α and β induction, resulting in a downregulated recruitment of immune cells [44,49,50,51,52].
5. Viral Antagonisms to SAMHD1
While SAMHD1 experienced positive selection during host–pathogen coevolution, several viruses have also developed evasion strategies to antagonize the restriction by SAMHD1 [78,79,80]. Thus, the following chapter will focus on the different countermeasures that viruses have evolved to circumvent the restrictive activity of SAMHD1 (Figure 2; Table 1).
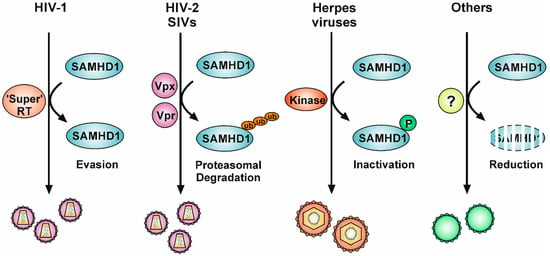
Figure 2.
Viral countermeasures to circumvent the intrinsic restriction factor SAMHD1. Retroviruses without a Vpx-like function, such as HIV-1, encode a highly efficient reverse transcriptase (“Super” RT) that enables the virus to reverse-transcribe its genome despite the presence of active SAMHD1 and the resulting low dNTP level. HIV-2 and related SIVs encode the small accessory protein Vpx (or Vpr with a Vpx-like function), which ties SAMHD1 to a Cullin E3 ubiquitin ligase complex, resulting in ubiquitination and subsequent proteasomal degradation of SAMHD1. Herpesviruses, especially β- and γ-herpesviruses, rely on their conserved serine/threonine protein kinases to phosphorylate and thereby inactivate SAMHD1. In addition, HCMV infection also induces the proteasomal degradation of SAMHD1. Infection with other viruses, such as HSV-2, HBV, and HPV16, has been reported to cause a reduction in SAMHD1 RNA or protein levels, suggesting that additional unknown mechanisms exist to circumvent the SAMHD1-mediated restriction. Ub: ubiquitination, P: phosphorylation.
5.1. The Viral Accessory Proteins Vpx and Vpr
A hallmark of lentiviral genomes is the presence of small accessory open reading frames. While both HIV-1 and HIV-2 encode the accessory protein Vpr, only HIV-2 and related SIVs contain an additional open reading frame for Vpx [81,82]. Vpx arose from Vpr by an ancient gene duplication event in a lentiviral precursor virus, suggesting two similar but not identical functions in both proteins [83]. SIV from sooty mangabeys (SIVsmm) is the zoonotic precursor of HIV-2 and gave rise to SIV found in macaques in captivity (SIVmac). Thus, Vpx and Vpr proteins of these viruses share a high sequence homology. SIVsmm infection of pigtailed macaques revealed that Vpx and Vpr are important for dissemination and pathogenesis of the virus in vivo [84]. Both Vpx and Vpr are actively packaged into virions by binding to the p6 domain of the Gag polyprotein and were therefore thought to play an important role during the early steps of infection, prior to integration into the host genome [85]. Vpx, but not Vpr, is critical for the ability of HIV-2/SIVsmm to efficiently infect monocytes, macrophages, and DCs [86,87,88]. Thus, it has been suggested that Vpx inactivates an early post-entry block in these cells. Indeed, in 2011, Vpx of HIV-2/SIVsmm was shown to counteract the HIV-1 restriction factor SAMHD1 in myeloid cells and resting CD4+ T cells [54,55,56,89]. Interestingly, Vpx is not only enhancing SIV infectivity, but also boosts the infection of different retroviruses, including HIV-1, in trans, when delivered into cells by virus-like particles [2,62,90]. In target cells, Vpx induces the proteasomal degradation of SAMHD1 by tying it to the ubiquitin–proteasome system [54,55]. Cullin E3 ubiquitin ligase complexes consist of a central Cullin scaffold protein, a catalytic RING subunit, and varying adapter proteins mediating interaction with specific target proteins [91]. In case of Vpx and Vpr, coisolation approaches revealed that both proteins interact with a Cullin4 E3 ubiquitin ligase complex via the adapter proteins damage-specific DNA binding protein 1 (DDB1) and DDB1 Cullin-associated factor 1 (DCAF1) [92,93,94,95,96,97,98]. By binding to SAMHD1, Vpx and some SIV Vpr proteins render SAMHD1 a substrate for polyubiquitination and thereby induce its proteasomal degradation.
The genetic conflict between innate immunity and viruses results in the rapid selection of mutations in proteins involved in direct host–pathogen interaction. The groups of Monsef Benkirane and Michael Emerman described this “arms race” for SAMHD1 and Vpx/Vpr [83,99]. While Vpr is expressed by all primate lentiviruses, a Vpx gene is only present in two out of eight SIV lineages, the HIV-2/SIVsmm/SIVmac lineage and the SIV rcm/SIVmnd2 (SIV of red-capped mangabeys, SIV of mandrill) lineage. Lim and colleagues analyzed SAMHD1 degradation upon cotransfection with Vpr and Vpx proteins from different SIVs [83]. While Vpx from SIVmac did degrade human and all simian SAMHD1 proteins tested, other Vpx proteins only counteracted SAMHD1 from certain species. In a similar approach, Laguette and colleagues demonstrated that the interaction of Vpx and SAMHD1 is species-specific [99]. Both studies also identified specific SIV Vpr proteins that were able to degrade SAMHD1. These Vpr proteins are found in viruses lacking Vpx, and belong to the two SIV lineages that include SIV infecting African green monkeys (SIVagm) or Sykes monkeys (SIVsyk) and greater spot-nosed monkeys (SIVgsn). Comparative analysis showed that the neofunctionalization of Vpr to degrade SAMHD1 has occurred once in an ancient predecessor and, importantly, prior to the gene duplication or recombination event that gave rise to Vpx. This suggests that viruses with an additional Vpx reading frame had an evolutionary advantage, perhaps driven by positive selection events in SAMHD1. Indeed, both studies showed that the antagonism by Vpx and Vpr proteins drove positive selection of SAMHD1 in Old World monkeys. Sites of strong positive selection can be found in the N-terminal and C-terminal regions of SAMHD1. Fregoso et al. determined that different Vpx and Vpr proteins have evolved to recognize distinct N- or C-terminal interfaces of SAMHD1, which is in line with the idea of a rapidly evolving SAMHD1 [100]. Schwefel and colleagues were able to corroborate these findings and solved the crystal structures of SIV Vpx of the mandrill lineage together with the N-terminus of SAMHD1 and DCAF1, as well as SIVsmm together with the C-terminus of SAMHD1 and DCAF1 [101,102].
Interestingly, another lentivirus, EIAV, has evolved a different strategy to circumvent SAMHD1 restriction in equine macrophages [103]. While equine SAMHD1 restricts replication at the reverse-transcription step, EIAV seems to employ its transcriptional regulator Rev to counteract SAMHD1 by downregulating the equine SAMHD1 level via a lysosomal pathway to ensure efficient EIAV replication [103].
5.2. “Super-RT”: Highly Efficient Lentiviral Reverse Transcriptases
Nondividing myeloid cells, such as macrophages or microglia, are targeted by many lentiviruses during the course of infection. However, not all lentiviruses encode Vpx, or a protein with a Vpx-like function, to inactivate SAMHD1. Instead, to overcome the SAMHD1-driven shortage of dNTPs in nondividing cells, viruses lacking Vpx harbor very efficient reverse transcriptases that polymerize viral cDNA at low dNTP concentrations [104,105,106,107]. For instance, Lenzi and colleagues biochemically characterized RTs from HIV-1, HIV-2, and SIV isolates and determined the Km values of HIV-1 RTs to be very low in general and in the range of the low dNTP concentrations found in nondividing macrophages. In contrast, enzymes encoded by viruses that trigger SAMHD1 degradation, such as HIV-2, displayed significantly higher Km values and required higher dNTP concentrations to efficiently generate cDNA [104]. Interestingly, the Kim laboratory also cloned and analyzed the sequences of RT genes derived from Rhesus macaques infected with either SIVmac WT virus encoding Vpx or a genetically engineered variant lacking the accessory protein [106]. The authors compared RT sequences isolated from these monkeys at 6 to 7 months postinfection (mpi) and at 36 mpi, and found an enhanced accumulation of mutations in RT genes from samples infected with virus lacking Vpx. Biochemical analysis revealed that at 36 mpi, RT variants from (−)Vpx viruses displayed higher catalytic and nucleotide incorporation efficacies compared to RTs from (+)Vpx virus-infected animals or compared to samples from early time points [106]. These findings corroborate the idea that lentiviruses without a Vpx-like function may have evolved more efficient RT molecules to enable cDNA synthesis in nondividing cells.
Interestingly, however, pretreatment of nondividing macrophages with Vpx-containing virus-like particles further boosts HIV-1 infectivity in macrophages in vitro, despite optimized RT enzymes [87,108]. At this stage, it is still unclear why HIV-1 and other lentiviruses do not encode a Vpx-like function to degrade SAMHD1 in order to increase infectivity in macrophages. One intriguing idea is that the advantage to infect macrophages more efficiently by degrading SAMHD1 may be opposed in vivo by a simultaneously enhanced infection of DCs, resulting in a stronger antiviral immune response [46,47].
5.3. Conserved Herpesviral Protein Kinases
Human herpesviruses (HHV) encode conserved herpesvirus protein kinases (CHPK). This family of serine/threonine kinases includes UL13 of HSV-1 and -2, ORF47 of varicella-zoster virus (VZV), UL97 of HCMV, U69 of HHV-6 and -7, BGLF4 of EBV, and ORF36 of Kaposi’s sarcoma-associated herpesvirus (KSHV) [109,110,111]. These kinases are also referred to as viral CDKs (v-CDKs), since they structurally resemble cellular CDKs and target many of their substrates to modulate cell-cycle-dependent processes to favor viral replication [110,112,113].
In 2019, Kim et al. and Businger et al. analyzed the role of SAMHD1 in HCMV infection of myeloid cell lines [73,76]. They found that SAMHD1 inhibits HCMV gene expression as well as viral genome replication, respectively. In addition, Businger and colleagues observed that the SAMHD1-mediated restriction of HCMV is potently antagonized by the viral kinase pUL97, as well as by activation of cellular CDKs following HCMV infection. SAMHD1 was shown to be strongly phosphorylated at the regulatory T592 site upon HCMV infection, as well as upon transient pUL97 coexpression [73]. Of note, Kim et al. and Chen and colleagues found that the inhibitory effect of SAMHD1 on NFκB-mediated MIE promoter activation and innate immune signaling is independent of T592 phosphorylation, even though phosphorylation is induced upon HCMV infection [49,76]. In an affinity purification and mass spectrometry approach, Bogdanow et al. confirmed SAMHD1 as a target of HCMV pUL97 and of the homologous kinase U69 of HHV7 [114]. In addition, De Meo et al. substantiated previous findings that SAMHD1 is also phosphorylated through induction of CDK2 upon HCMV infection [115].
The murine homolog of SAMHD1 occurs in two isoforms. Isoform 2, which is expressed at low levels, is differentially spliced, and therefore lacks the regulatory C-terminal phosphorylation site. Isoform 1, however, highly resembles the human protein with an equally abundant expression pattern [42,116,117,118,119]. The antiretroviral activity of isoform 1 is negatively regulated by phosphorylation at threonine residue 603 (T603), which corresponds to T592 in human SAMHD1 [42,118,119]. Recently, we found that also the MCMV orthologue of pUL97—M97—strongly phosphorylates murine SAMHD1 at T603 upon infection of murine macrophages, causing the loss of its antiviral activity [74]. In addition, infection with MCMV WT but not with an M97 kinase-deficient (KD) virus significantly increased intracellular dNTP concentrations. Importantly, our study revealed that SAMHD1 restricts MCMV genome replication in vivo and that WT virus replication, and more pronounced M97 KD virus replication, is enhanced in SAMHD1 KO mice [74].
In line with these findings, a recent study by Zhang and colleagues found that the EBV kinase BGLF4 phosphorylates human SAMHD1 at the T592 site. The phosphorylation abrogated EBV inhibition by SAMHD1 as well as its dNTPase activity [75]. In their manuscript, Zhang et al. also analyzed SAMHD1 phosphorylation upon coexpression of other herpesviral CHPKs. The authors found that all kinases of β- and γ-herpesviruses phosphorylate SAMHD1 at T592, while the α-herpesviral kinases UL13 of HSV-1 and -2 and ORF47 of VZV do not affect SAMHD1 phosphorylation [75]. Of note, α-herpesviruses encode an additional serine/threonine kinase, which might inactivate SAMHD1 in these viruses instead of the CHPKs [112,120]. However, it remains unclear whether SAMHD1 phosphorylation plays a role in α-herpesviral infection at all, since Kim and colleagues found that the phosphorylation of SAMHD1 upon infection does not affect the SAMHD1-mediated restriction of HSV-1 [72]. Another interesting feature of several herpesviruses is that they encode viral proteins supporting de novo dNTP synthesis. All members of the α- and γ-herpesviruses express their own RNR and TK proteins, which drive dNTP anabolism and therefore antagonize SAMHD1 activity [121,122,123]. This suggests that these viruses are not as dependent on a kinase-mediated inactivation of SAMHD1 as β-herpesviruses. Of note, also other viruses have developed mechanisms to antagonize the dNTP-catabolizing activity of SAMHD1. VACV encodes its own TK, and Hollenbaugh et al. found that the deletion of TK enhances the inhibitory activity of SAMHD1 in VACV infection [71]. Another example is HBV, which was shown to induce the expression of the cellular RNR subunit R2 to ensure a sufficient dNTP supply upon infection [124,125].
5.4. Downregulation of Expression Levels and Protein Relocalization
Aside from the phosphorylation-dependent inactivation of SAMHD1, the downregulation of SAMHD1 expression levels has been observed as a viral countermeasure mediated by several DNA viruses. In case of HCMV, Businger et al. found that the infection of primary MDMs results in a decreased steady state expression of SAMHD1 [73]. This is in line with results from Cheung et al., who reported the expression of several antiviral restriction factors, among them SAMHD1, to be reduced upon infection of CD34+ hematopoietic progenitor cells [126]. Hyeon and colleagues also analyzed SAMHD1 protein level and found that HCMV infection induces the proteasomal degradation of SAMHD1 via a Cullin2 E3 ubiquitin ligase complex [127]. In addition, De Meo et al. found that the HCMV-induced phosphorylation of SAMHD1 leads to its cytosolic relocalization and suggested that this relocalization might represent a novel evasion mechanism by HCMV [115]. Of note, the innate DNA sensor and HCMV inhibitory factor IFI16 was also shown to be phosphorylated by pUL97, leading to its inactivation and relocalization in a similar manner [128,129]. Also, infection with HSV-2 has been shown to reduce the expression levels of antiviral restriction factors, including SAMHD1, in DCs [130]. In case of HBV, Chen et al. described a reduced expression of SAMHD1 upon infection of hepatocytes, which could not be confirmed by other groups [67,69]. Also in the context of HPV-16 infection, SAMHD1 RNA as well as protein levels were found to be decreased in infected keratinocytes, suggesting that, similar to other DNA viruses, HPV found a way to circumvent the SAMHD1-mediated restriction in order to efficiently replicate its DNA genome [70].

Table 1.
Viral SAMHD1 evasion strategies.
Table 1.
Viral SAMHD1 evasion strategies.
| Virus | Mechanism | Antagonism | Reference | |
|---|---|---|---|---|
| Retroviruses | HIV-1 (Lenti) | dNTPase | Efficient RT in presence of SAMHD1 | [1,14,53,104,105,106,107] |
| HIV-2 (Lenti) | dNTPase | Vpx-induced proteasomal degradation | [54,56,90] | |
| SIV (Lenti) | dNTPase | Vpx/Vpr-induced proteasomal degradation | [54,55,56,57,87,89,90] | |
| FIV (Lenti) | dNTPase | [2,62] | ||
| BIV (Lenti) | dNTPase | [2] | ||
| EIAV (Lenti) | dNTPase | Rev-induced lysosomal degradation | [2,62,103] | |
| RSV (α) | [62] | |||
| MPMV (β) | [62] | |||
| HTLV (δ) | STING-mediated apoptosis | [62,63] | ||
| N-/B-MLV, FV (γ) | dNTPase | Replication in dividing cells | [2,42,62] | |
| Herpesviruses | HSV-1/2 (α) | dNTPase | SAMHD1 downregulation + viral RNR/TK expression | [71,72,130] |
| HCMV (β) | dNTPase, NFκB/IRF inhibition | Phosphorylation by pUL97 + Induction of cellular CDKs + Proteasomal degradation + Cytosolic relocalization | [73,76,115,126,127] | |
| MCMV (β) | dNTPase | Phosphorylation by M97 | [74] | |
| EBV (γ) | dNTPase | Phosphorylation by BGLF4 + viral RNR/TK expression | [75] | |
| Others | Hepadnavirus HBV | dNTPase | SAMHD1 downregulation + RNR induction | [67,68,69,124,125] |
| Poxvirus VACV | dNTPase | TK expression | [71] | |
| Papillomavirus HPV16 | dNTPase | SAMHD1 downregulation | [70] | |
| Retro Elements | LINE-1 | ORF2p binding, dNTPase, Sequestering RNPs | [64,65,66] | |
| Alu/SVA | [64,66] | |||
| IAP/MusD | [66] |
6. Conclusions
SAMHD1 is a potent restriction factor of various viruses with different replication strategies. The growing spectrum of viruses found to be inhibited by SAMHD1 accentuates its importance for the intrinsic immune defense. During mammalian evolution, SAMHD1 has been under positive selective pressure, and an antiviral activity has been described not only for human or primate SAMHD1, but also for proteins from other species, such as equine, feline, bovine, or murine SAMHD1 [42,74,80,103,119,131]. During virus–host coevolution, however, viruses have evolved various strategies to circumvent the restriction factor SAMHD1. In 2011, it became clear that lentiviruses, such as HIV-2 and several SIV strains, counteract SAMHD1 with the help of their accessory proteins Vpx and Vpr, resulting in the proteasomal degradation of SAMHD1 [54,55,56,89,96]. Another strategy to evade the dNTPase activity of SAMHD1, found in HIV-1 and other SIVs, is the use of highly efficient RTs that function at low dNTP concentrations in the presence of SAMHD1 [104,105,106]. In contrast, herpesviruses employ their conserved serine/threonine kinases to phosphorylate and inactivate SAMHD1 [73,74,75]. In addition, downregulation of SAMHD1 or its relocalization might be alternative ways to circumvent antiviral restriction [67,70,73,115,126,127,130]. The variety of the hitherto uncovered SAMHD1 countermeasures emphasizes the importance of the restriction factor SAMHD1 in antiviral immunity. Of note, some RNA viruses, such as Chickungunya or Zika virus, seem to rather benefit from SAMHD1 activity by a so far unknown mechanism, suggesting that the SAMHD1 restriction might be specifically aimed at viruses whose replication depends on dNTPs [132].
Viral antagonists are promising drug targets for the development of novel antiviral therapies. Many current antiviral therapies are based on nucleoside analogues (NA) [133]. NAs highly resemble their cellular dNTP counterparts, but lead to a premature termination of DNA synthesis, when incorporated into the viral genome as substrates for RTs and DNA polymerases. In case of HIV, the activity of SAMHD1 has been shown to increase the efficacy of nucleoside RT inhibitors (NTRI) such as zidovudine (AZT) or zalcitabine (ddC) [134,135,136]. Since NAs are not targeted by SAMHD1 or are only hydrolyzed at a significantly slower rate than cellular dNTPs, they have an increased efficacy in the presence of SAMHD1. Depletion of SAMHD1 in macrophages and CD4+ T cells leads to a dramatic decrease in HIV sensitivity against RT inhibitors, especially those that resemble thymidine. By degrading cellular dNTPs, SAMHD1 leads to an enhanced concentration of exogenous NAs and therefore to a more efficient viral inhibition [134,135]. Existing herpesviral NA therapies often exploit the ability of herpesviral kinases to phosphorylate their targets. Prodrugs such as ganciclovir or acyclovir, two guanosine analogues, are only activated through initial monophosphorylation by herpesviral kinases such as HCMV pUL97 or HSV TK, and are thereby highly specific to virus-infected cells [137,138,139,140]. However, since herpesviruses are known to accumulate drug-resistant mutations against nucleoside analogues, other therapeutics are needed in these cases to substitute for NA treatment. CHPK inhibitors are therefore promising candidates for alternative therapies to release the block in SAMHD1-mediated antiviral activity. The most advanced candidate, maribavir, was shown to potently reduce HCMV replication through inhibition of the viral kinase pUL97, and is currently under clinical investigation in a phase III study [141,142]. Thus, development of efficient strategies to block the various SAMHD1 evasion mechanisms such as the inhibition of viral kinases as well as SAMHD1-resistant NAs, will result in a higher efficacy and capability of antiviral therapies in the future.
Author Contributions
J.D. and T.G. wrote, reviewed, and edited the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
J.D. is funded by the German Federal Ministry for Education and Research (SENSECoV2). T.G. is funded University Hospital Erlangen Interdisciplinary Center for Clinical Research (IZKF) Grant A83 and by the Deutsche Forschungsgemeinschaft (GR 3355/6-1).
Acknowledgments
We thank Justine Lagisquet for helpful discussions and thoroughly proofreading the manuscript. Figure 2 is based on artwork provided by servier.com.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Goldstone, D.C.; Ennis-Adeniran, V.; Hedden, J.J.; Groom, H.C.T.; Rice, G.I.; Christodoulou, E.; Walker, P.A.; Kelly, G.; Haire, L.F.; Yap, M.W.; et al. HIV-1 restriction factor SAMHD1 is a deoxynucleoside triphosphate triphosphohydrolase. Nature 2011, 480, 379–382. [Google Scholar] [CrossRef]
- White, T.E.; Brandariz-Nuñez, A.; Valle-Casuso, J.C.; Amie, S.; Nguyen, L.; Kim, B.; Brojatsch, J.; Diaz-Griffero, F. Contribution of SAM and HD domains to retroviral restriction mediated by human SAMHD1. Virology 2013, 436, 81–90. [Google Scholar] [CrossRef]
- Beloglazova, N.; Flick, R.; Tchigvintsev, A.; Brown, G.; Popovic, A.; Nocek, B.; Yakunin, A.F. Nuclease Activity of the Human SAMHD1 Protein Implicated in the Aicardi-Goutières Syndrome and HIV-1 Restriction. J. Biol. Chem. 2013, 288, 8101–8110. [Google Scholar] [CrossRef]
- Brandariz-Nuñez, A.; Valle-Casuso, J.C.; White, T.E.; Laguette, N.; Benkirane, M.; Brojatsch, J.; Diaz-Griffero, F. Role of SAMHD1 nuclear localization in restriction of HIV-1 and SIVmac. Retrovirology 2012, 9, 1–12. [Google Scholar] [CrossRef]
- Hofmann, H.; Logue, E.C.; Bloch, N.; Daddacha, W.; Polsky, S.B.; Schultz, M.L.; Kim, B.; Landau, N.R. The Vpx Lentiviral Accessory Protein Targets SAMHD1 for Degradation in the Nucleus. J. Virol. 2012, 86, 12552–12560. [Google Scholar] [CrossRef] [PubMed]
- Rice, G.I.; Bond, J.; Asipu, A.; Brunette, R.L.; Manfield, I.W.; Carr, I.M.; Fuller, J.C.; Jackson, R.M.; Lamb, T.; Briggs, T.A.; et al. Mutations involved in Aicardi-Goutières syndrome implicate SAMHD1 as regulator of the innate immune response. Nat. Genet. 2009, 41, 829–832. [Google Scholar] [CrossRef]
- Koharudin, L.M.I.; Wu, Y.; DeLucia, M.; Mehrens, J.; Gronenborn, A.M.; Ahn, J. Structural Basis of Allosteric Activation of Sterile α Motif and Histidine-Aspartate Domain-containing Protein 1 (SAMHD1) by Nucleoside Triphosphates. J. Biol. Chem. 2014, 289, 32617–32627. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.-F.; Wei, W.; Peng, X.; Dong, Y.-H.; Gong, Y.; Yu, X.-F. The mechanism of substrate-controlled allosteric regulation of SAMHD1 activated by GTP. Acta Crystallogr. Sect. D Biol. Crystallogr. 2015, 71, 516–524. [Google Scholar] [CrossRef]
- Brandariz-Nuñez, A.; Valle-Casuso, J.C.; White, T.E.; Nguyen, L.; Bhattacharya, A.; Wang, Z.; Demeler, B.; Amie, S.; Knowlton, C.; Kim, B.; et al. Contribution of oligomerization to the anti-HIV-1 properties of SAMHD1. Retrovirology 2013, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Wu, Y.; Yan, J.; Mehrens, J.; Yang, H.; DeLucia, M.; Hao, C.; Gronenborn, A.M.; Skowronski, J.; Ahn, J.; et al. Mechanism of allosteric activation of SAMHD1 by dGTP. Nat. Struct. Mol. Biol. 2013, 20, 1304–1309. [Google Scholar] [CrossRef] [PubMed]
- Ji, X.; Tang, C.; Zhao, Q.; Wang, W.; Xiong, Y. Structural basis of cellular dNTP regulation by SAMHD1. Proc. Natl. Acad. Sci. USA 2014, 111, E4305–E4314. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Wang, Z.; White, T.E.; Buffone, C.; Nguyen, L.A.; Shepard, C.N.; Kim, B.; Demeler, B.; Diaz-Griffero, F. Effects of T592 phosphomimetic mutations on tetramer stability and dNTPase activity of SAMHD1 can not explain the retroviral restriction defect. Sci. Rep. 2016, 6, 1–12. [Google Scholar] [CrossRef]
- Patra, K.K.; Bhattacharya, A.; Bhattacharya, S. Uncovering allostery and regulation in SAMHD1 through molecular dynamics simulations. Proteins Struct. Funct. Bioinform. 2017, 85, 1266–1275. [Google Scholar] [CrossRef]
- Powell, R.D.; Holland, P.J.; Hollis, T.; Perrino, F.W. Aicardi-Goutières Syndrome Gene and HIV-1 Restriction Factor SAMHD1 Is a dGTP- regulated Deoxynucleotide Triphosphohydrolase. J. Biol. Chem. 2011, 286, 43596–43600. [Google Scholar] [CrossRef]
- Eriksson, S.; Gräslund, A.; Skog, S.; Thelander, L.; Tribukait, B. Cell cycle-dependent regulation of mammalian ribonucleotide reductase. The S phase-correlated increase in subunit M2 is regulated by de novo protein synthesis. J. Biol. Chem. 1984, 259, 11695–11700. [Google Scholar] [CrossRef]
- Johnson, L.F.; Rao, L.G.; Muench, A.J. Regulation of thymidine kinase enzyme level in serum-stimulated mouse 3T6 fibroblasts. Exp. Cell Res. 1982, 138, 79–85. [Google Scholar] [CrossRef]
- Kolberg, M.; Strand, K.R.; Graff, P.; Andersson, K.K. Structure, function, and mechanism of ribonucleotide reductases. Biochim. Biophys. Acta Proteins Proteom. 2004, 1699, 1–34. [Google Scholar] [CrossRef]
- Stewart, C.J.; Ito, M.; Conrad, S.E. Evidence for Transcriptional and Post-Transcriptional Control of the Cellular Thymidine Kinase Gene. Mol. Cell. Biol. 1987, 7, 1156–1163. [Google Scholar] [CrossRef]
- Nordlund, P.; Reichard, P. Ribonucleotide Reductases. Annu. Rev. Biochem. 2006, 75, 681–706. [Google Scholar] [CrossRef]
- Arnér, E.S.J.; Eriksson, S. Mammalian deoxyribonucleoside kinases. Pharmacol. Ther. 1995, 67, 155–186. [Google Scholar] [CrossRef]
- Franzolin, E.; Pontarin, G.; Rampazzo, C.; Miazzi, C.; Ferraro, P.; Palumbo, E.; Reichard, P.; Bianchi, V. The deoxynucleotide triphosphohydrolase SAMHD1 is a major regulator of DNA precursor pools in mammalian cells. Proc. Natl. Acad. Sci. USA 2013, 110, 14272–14277. [Google Scholar] [CrossRef]
- Cribier, A.; Descours, B.; Valadão, A.L.C.; Laguette, N.; Benkirane, M. Phosphorylation of SAMHD1 by Cyclin A2/CDK1 Regulates Its Restriction Activity toward HIV-1. Cell Rep. 2013, 3, 1036–1043. [Google Scholar] [CrossRef]
- St. Gelais, C.; de Silva, S.; Hach, J.C.; White, T.E.; Diaz-Griffero, F.; Yount, J.S.; Wu, L. Identification of Cellular Proteins Interacting with the Retroviral Restriction Factor SAMHD1. J. Virol. 2014, 88, 5834–5844. [Google Scholar] [CrossRef]
- Schott, K.; Fuchs, N.V.; Derua, R.; Mahboubi, B.; Schnellbächer, E.; Seifried, J.; Tondera, C.; Schmitz, H.; Shepard, C.; Brandariz-Nuñez, A.; et al. Dephosphorylation of the HIV-1 restriction factor SAMHD1 is mediated by PP2A-B55α holoenzymes during mitotic exit. Nat. Commun. 2018, 9. [Google Scholar] [CrossRef]
- White, T.E.; Brandariz-Nuñez, A.; Valle-Casuso, J.C.; Amie, S.; Nguyen, L.A.; Kim, B.; Tuzova, M.; Diaz-Griffero, F. The Retroviral Restriction Ability of SAMHD1, but Not Its Deoxynucleotide Triphosphohydrolase Activity, Is Regulated by Phosphorylation. Cell Host Microbe 2013, 13, 441–451. [Google Scholar] [CrossRef]
- Welbourn, S.; Dutta, S.M.; Semmes, O.J.; Strebel, K. Restriction of Virus Infection but Not Catalytic dNTPase Activity Is Regulated by Phosphorylation of SAMHD1. J. Virol. 2013, 87, 11516–11524. [Google Scholar] [CrossRef]
- Tang, C.; Ji, X.; Wu, L.; Xiong, Y. Impaired dNTPase activity of SAMHD1 by phosphomimetic mutation of Thr-592. J. Biol. Chem. 2015, 290, 26352–26359. [Google Scholar] [CrossRef]
- Tramentozzi, E.; Ferraro, P.; Hossain, M.; Stillman, B.; Bianchi, V.; Pontarin, G. The dNTP triphosphohydrolase activity of SAMHD1 persists during S-phase when the enzyme is phosphorylated at T592. Cell Cycle 2018, 17, 1102–1114. [Google Scholar] [CrossRef]
- Mathews, C.K. Deoxyribonucleotide metabolism, mutagenesis and cancer. Nat. Rev. Cancer 2015, 15, 528–539. [Google Scholar] [CrossRef]
- Rentoft, M.; Lindell, K.; Tran, P.; Chabes, A.L.; Buckland, R.J.; Watt, D.L.; Marjavaara, L.; Nilsson, A.K.; Melin, B.; Trygg, J.; et al. Heterozygous colon cancer-associated mutations of SAMHD1 have functional significance. Proc. Natl. Acad. Sci. USA 2016, 113, 4723–4728. [Google Scholar] [CrossRef]
- Coquel, F.; Silva, M.-J.; Técher, H.; Zadorozhny, K.; Sharma, S.; Nieminuszczy, J.; Mettling, C.; Dardillac, E.; Barthe, A.; Schmitz, A.L.; et al. SAMHD1 acts at stalled replication forks to prevent interferon induction. Nature 2018, 557, 57–61. [Google Scholar] [CrossRef]
- Daddacha, W.; Koyen, A.E.; Bastien, A.J.; Head, P.S.E.; Dhere, V.R.; Nabeta, G.N.; Connolly, E.C.; Werner, E.; Madden, M.Z.; Daly, M.B.; et al. SAMHD1 Promotes DNA End Resection to Facilitate DNA Repair by Homologous Recombination. Cell Rep. 2017, 20, 1921–1935. [Google Scholar] [CrossRef]
- Cabello-Lobato, M.J.; Wang, S.; Schmidt, C.K. SAMHD1 Sheds Moonlight on DNA Double-Strand Break Repair. Trends Genet. 2017, 33, 895–897. [Google Scholar] [CrossRef]
- Clifford, R.; Louis, T.; Robbe, P.; Ackroyd, S.; Burns, A.; Timbs, A.T.; Colopy, G.W.; Dreau, H.; Sigaux, F.; Judde, J.G.; et al. SAMHD1 is mutated recurrently in chronic lymphocytic leukemia and is involved in response to DNA damage. Blood 2014, 123, 1021–1031. [Google Scholar] [CrossRef]
- Amin, N.A.; Seymour, E.; Saiya-Cork, K.; Parkin, B.; Shedden, K.; Malek, S.N. A Quantitative Analysis of Subclonal and Clonal Gene Mutations before and after Therapy in Chronic Lymphocytic Leukemia. Clin. Cancer Res. 2016, 22, 4525–4535. [Google Scholar] [CrossRef]
- Wang, J.L.; Lu, F.Z.; Shen, X.Y.; Wu, Y.; Zhao, L.T. SAMHD1 is down regulated in lung cancer by methylation and inhibits tumor cell proliferation. Biochem. Biophys. Res. Commun. 2014, 455, 229–233. [Google Scholar] [CrossRef]
- Rice, G.; Patrick, T.; Parmar, R.; Taylor, C.F.; Aeby, A.; Aicardi, J.; Artuch, R.; Montalto, S.A.; Bacino, C.A.; Barroso, B.; et al. Clinical and molecular phenotype of Aicardi-Goutières syndrome. Am. J. Hum. Genet. 2007, 81, 713–725. [Google Scholar] [CrossRef]
- Crow, Y.J.; Rehwinkel, J. Aicardi-Goutières syndrome and related phenotypes: Linking nucleic acid metabolism with autoimmunity. Hum. Mol. Genet. 2009, 18, R130–R136. [Google Scholar] [CrossRef]
- Rice, G.I.; Kasher, P.R.; Forte, G.M.A.; Mannion, N.M.; Greenwood, S.M.; Szynkiewicz, M.; Dickerson, J.E.; Bhaskar, S.S.; Zampini, M.; Briggs, T.A.; et al. Mutations in ADAR1 cause Aicardi-Goutières syndrome associated with a type I interferon signature. Nat. Genet. 2012, 44, 1243–1248. [Google Scholar] [CrossRef]
- Lim, Y.W.; Sanz, L.A.; Xu, X.; Hartono, S.R.; Chédin, F. Genome-wide DNA hypomethylation and RNA:DNA hybrid accumulation in Aicardi-Goutières syndrome. Elife 2015, 4, e08007. [Google Scholar] [CrossRef]
- Rehwinkel, J.; Maelfait, J.; Bridgeman, A.; Rigby, R.; Hayward, B.; Liberatore, R.A.; Bieniasz, P.D.; Towers, G.J.; Moita, L.F.; Crow, Y.J.; et al. SAMHD1-dependent retroviral control and escape in mice. EMBO J. 2013, 32, 2454–2462. [Google Scholar] [CrossRef] [PubMed]
- Behrendt, R.; Schumann, T.; Gerbaulet, A.; Nguyen, L.A.; Schubert, N.; Alexopoulou, D.; Berka, U.; Lienenklaus, S.; Peschke, K.; Gibbert, K.; et al. Mouse SAMHD1 Has Antiretroviral Activity and Suppresses a Spontaneous Cell-Intrinsic Antiviral Response. Cell Rep. 2013, 4, 689–696. [Google Scholar] [CrossRef]
- Martinez-Lopez, A.; Martin-Fernandez, M.; Buta, S.; Kim, B.; Bogunovic, D.; Diaz-Griffero, F. SAMHD1 deficient human monocytes autonomously trigger type I interferon. Mol. Immunol. 2018, 101, 450–460. [Google Scholar] [CrossRef]
- Qin, Z.; Bonifati, S.; St. Gelais, C.; Li, T.-W.; Kim, S.-H.; Antonucci, J.M.; Mahboubi, B.; Yount, J.S.; Xiong, Y.; Kim, B.; et al. The dNTPase activity of SAMHD1 is important for its suppression of innate immune responses in differentiated monocytic cells. J. Biol. Chem. 2020, 295, 1575–1586. [Google Scholar] [CrossRef]
- Gao, D.; Wu, J.; Wu, Y.T.; Du, F.; Aroh, C.; Yan, N.; Sun, L.; Chen, Z.J. Cyclic GMP-AMP synthase is an innate immune sensor of HIV and other retroviruses. Science 2013, 341, 903–906. [Google Scholar] [CrossRef]
- Manel, N.; Hogstad, B.; Wang, Y.; Levy, D.E.; Unutmaz, D.; Littman, D.R. A cryptic sensor for HIV-1 activates antiviral innate immunity in dendritic cells. Nature 2010, 467, 214–217. [Google Scholar] [CrossRef]
- Maelfait, J.; Bridgeman, A.; Benlahrech, A.; Cursi, C.; Rehwinkel, J. Restriction by SAMHD1 Limits cGAS/STING-Dependent Innate and Adaptive Immune Responses to HIV-1. Cell Rep. 1501, 16, 1492–1501. [Google Scholar] [CrossRef]
- Ablasser, A.; Goldeck, M.; Cavlar, T.; Deimling, T.; Witte, G.; Röhl, I.; Hopfner, K.-P.; Ludwig, J.; Hornung, V. cGAS produces a 2′-5′-linked cyclic dinucleotide second messenger that activates STING. Nature 2013, 498, 380–384. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Bonifati, S.; Qin, Z.; St. Gelais, C.; Kodigepalli, K.M.; Barrett, B.S.; Kim, S.H.; Antonucci, J.M.; Ladner, K.J.; Buzovetsky, O.; et al. SAMHD1 suppresses innate immune responses to viral infections and inflammatory stimuli by inhibiting the NF-κB and interferon pathways. Proc. Natl. Acad. Sci. USA 2018, 115, E3798–E3807. [Google Scholar] [CrossRef]
- Oeckinghaus, A.; Hayden, M.S.; Ghosh, S. Crosstalk in NF-κB signaling pathways. Nat. Immunol. 2011, 12, 695–708. [Google Scholar] [CrossRef]
- Oh, C.; Ryoo, J.; Park, K.; Kim, B.; Daly, M.B.; Cho, D.; Ahn, K. A central role for PI3K-AKT signaling pathway in linking SAMHD1-deficiency to the type i interferon signature. Sci. Rep. 2018, 8, 1–13. [Google Scholar] [CrossRef]
- Espada, C.E.; St. Gelais, C.; Bonifati, S.; Maksimova, V.V.; Cahill, M.P.; Kim, S.H.; Wu, L. TRAF6 and TAK1 Contribute to SAMHD1-Mediated Negative Regulation of NF-κB Signaling. J. Virol. 2020, 95. [Google Scholar] [CrossRef] [PubMed]
- Lahouassa, H.; Daddacha, W.; Hofmann, H.; Ayinde, D.; Logue, E.C.; Dragin, L.; Bloch, N.; Maudet, C.; Bertrand, M.; Gramberg, T.; et al. SAMHD1 restricts the replication of human immunodeficiency virus type 1 by depleting the intracellular pool of deoxynucleoside triphosphates. Nat. Immunol. 2012, 13, 223–228. [Google Scholar] [CrossRef]
- Hrecka, K.; Hao, C.; Gierszewska, M.; Swanson, S.K.; Kesik-Brodacka, M.; Srivastava, S.; Florens, L.; Washburn, M.P.; Skowronski, J. Vpx relieves inhibition of HIV-1 infection of macrophages mediated by the SAMHD1 protein. Nature 2011, 474, 658–661. [Google Scholar] [CrossRef]
- Laguette, N.; Sobhian, B.; Casartelli, N.; Ringeard, M.; Chable-Bessia, C.; Ségéral, E.; Yatim, A.; Emiliani, S.; Schwartz, O.; Benkirane, M. SAMHD1 is the dendritic- and myeloid-cell-specific HIV-1 restriction factor counteracted by Vpx. Nature 2011, 474, 654–657. [Google Scholar] [CrossRef] [PubMed]
- Baldauf, H.-M.; Pan, X.; Erikson, E.; Schmidt, S.; Daddacha, W.; Burggraf, M.; Schenkova, K.; Ambiel, I.; Wabnitz, G.; Gramberg, T.; et al. SAMHD1 restricts HIV-1 infection in resting CD4+ T cells. Nat. Med. 2012, 18, 1682–1688. [Google Scholar] [CrossRef] [PubMed]
- Descours, B.; Cribier, A.; Chable-Bessia, C.; Ayinde, D.; Rice, G.; Crow, Y.; Yatim, A.; Schwartz, O.; Laguette, N.; Benkirane, M. SAMHD1 restricts HIV-1 reverse transcription in quiescent CD4 + T-cells. Retrovirology 2012, 9, 1. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.; Ryoo, J.; Oh, C.; Hwang, S.; Ahn, K. SAMHD1 specifically restricts retroviruses through its RNase activity. Retrovirology 2015, 12, 1–12. [Google Scholar] [CrossRef]
- Ryoo, J.; Choi, J.; Oh, C.; Kim, S.; Seo, M.; Kim, S.-Y.; Seo, D.; Kim, J.; White, T.E.; Brandariz-Nuñez, A.; et al. The ribonuclease activity of SAMHD1 is required for HIV-1 restriction. Nat. Med. 2014, 20, 936–941. [Google Scholar] [CrossRef]
- Antonucci, J.M.; St. Gelais, C.; de Silva, S.; Yount, J.S.; Tang, C.; Ji, X.; Shepard, C.; Xiong, Y.; Kim, B.; Wu, L. SAMHD1-mediated HIV-1 restriction in cells does not involve ribonuclease activity. Nat. Med. 2016, 22, 1074–1075. [Google Scholar] [CrossRef] [PubMed]
- Seamon, K.J.; Sun, Z.; Shlyakhtenko, L.S.; Lyubchenko, Y.L.; Stivers, J.T. SAMHD1 is a single-stranded nucleic acid binding protein with no active site-associated nuclease activity. Nucleic Acids Res. 2015, 43, 6486–6499. [Google Scholar] [CrossRef] [PubMed]
- Gramberg, T.; Kahle, T.; Bloch, N.; Wittmann, S.; Müllers, E.; Daddacha, W.; Hofmann, H.; Kim, B.; Lindemann, D.; Landau, N.R. Restriction of diverse retroviruses by SAMHD1. Retrovirology 2013, 10, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sze, A.; Belgnaoui, S.M.; Olagnier, D.; Lin, R.; Hiscott, J.; Van Grevenynghe, J. Host restriction factor SAMHD1 limits human T cell leukemia virus type 1 infection of monocytes via STING-mediated apoptosis. Cell Host Microbe 2013, 14, 422–434. [Google Scholar] [CrossRef] [PubMed]
- Zhao, K.; Du, J.; Han, X.; Goodier, J.L.; Li, P.; Zhou, X.; Wei, W.; Evans, S.L.; Li, L.; Zhang, W.; et al. Modulation of LINE-1 and Alu/SVA Retrotransposition by Aicardi-Goutieres Syndrome-Related SAMHD1. Cell Rep. 2013, 4, 1108–1115. [Google Scholar] [CrossRef]
- Hu, S.; Li, J.; Xu, F.; Mei, S.; Le Duff, Y.; Yin, L.; Pang, X.; Cen, S.; Jin, Q.; Liang, C.; et al. SAMHD1 Inhibits LINE-1 Retrotransposition by Promoting Stress Granule Formation. PLoS Genet. 2015, 11, e1005367. [Google Scholar] [CrossRef]
- Herrmann, A.; Wittmann, S.; Thomas, D.; Shepard, C.N.; Kim, B.; Ferreirós, N.; Gramberg, T. The SAMHD1-mediated block of LINE-1 retroelements is regulated by phosphorylation. Mob. DNA 2018, 9, 11. [Google Scholar] [CrossRef]
- Chen, Z.; Zhu, M.; Pan, X.; Zhu, Y.; Yan, H.; Jiang, T.; Shen, Y.; Dong, X.; Zheng, N.; Lu, J.; et al. Inhibition of Hepatitis B virus replication by SAMHD1. Biochem. Biophys. Res. Commun. 2014, 450, 1462–1468. [Google Scholar] [CrossRef]
- Jeong, G.U.; Park, I.-H.; Ahn, K.; Ahn, B.-Y. Inhibition of hepatitis B virus replication by a dNTPase-dependent function of the host restriction factor SAMHD1. Virology 2016, 495, 71–78. [Google Scholar] [CrossRef]
- Sommer, A.F.R.; Rivière, L.; Qu, B.; Schott, K.; Riess, M.; Ni, Y.; Shepard, C.; Schnellbächer, E.; Finkernagel, M.; Himmelsbach, K.; et al. Restrictive influence of SAMHD1 on Hepatitis B Virus life cycle. Sci. Rep. 2016, 6, 1–14. [Google Scholar] [CrossRef]
- James, C.D.; Prabhakar, A.T.; Otoa, R.; Evans, M.R.; Wang, X.; Bristol, M.L.; Zhang, K.; Li, R.; Morgan, I.M. SAMHD1 Regulates Human Papillomavirus 16-Induced Cell Proliferation and Viral Replication during Differentiation of Keratinocytes. mSphere 2019, 4, 1–15. [Google Scholar] [CrossRef]
- Hollenbaugh, J.A.; Gee, P.; Baker, J.; Daly, M.B.; Amie, S.M.; Tate, J.; Kasai, N.; Kanemura, Y.; Kim, D.H.; Ward, B.M.; et al. Host Factor SAMHD1 Restricts DNA Viruses in Non-Dividing Myeloid Cells. PLoS Pathog. 2013, 9, e1003481. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.T.; White, T.E.; Brandariz-Nuñez, A.; Diaz-Griffero, F.; Weitzman, M.D. SAMHD1 Restricts Herpes Simplex Virus 1 in Macrophages by Limiting DNA Replication. J. Virol. 2013, 87, 12949–12956. [Google Scholar] [CrossRef]
- Businger, R.; Deutschmann, J.; Gruska, I.; Milbradt, J.; Wiebusch, L.; Gramberg, T.; Schindler, M. Human cytomegalovirus overcomes SAMHD1 restriction in macrophages via pUL97. Nat. Microbiol. 2019, 4, 2260–2272. [Google Scholar] [CrossRef] [PubMed]
- Deutschmann, J.; Schneider, A.; Gruska, I.; Vetter, B.; Thomas, D.; Kießling, M.; Wittmann, S.; Herrmann, A.; Schindler, M.; Milbradt, J.; et al. A viral kinase counteracts in vivo restriction of murine cytomegalovirus by SAMHD1. Nat. Microbiol. 2019, 4, 2273–2284. [Google Scholar] [CrossRef]
- Zhang, K.; Lv, D.-W.; Li, R. Conserved Herpesvirus Protein Kinases Target SAMHD1 to Facilitate Virus Replication. Cell Rep. 2019, 28, 449–459. [Google Scholar] [CrossRef]
- Kim, E.T.; Roche, K.L.; Kulej, K.; Spruce, L.A.; Seeholzer, S.H.; Coen, D.M.; Diaz-Griffero, F.; Murphy, E.A.; Weitzman, M.D. SAMHD1 Modulates Early Steps during Human Cytomegalovirus Infection by Limiting NF-κB Activation. Cell Rep. 2019, 28, 434–448.e6. [Google Scholar] [CrossRef]
- DeMeritt, I.B.; Podduturi, J.P.; Tilley, A.M.; Nogalski, M.T.; Yurochko, A.D. Prolonged activation of NF-κB by human cytomegalovirus promotes efficient viral replication and late gene expression. Virology 2006, 346, 15–31. [Google Scholar] [CrossRef] [PubMed]
- Daugherty, M.D.; Malik, H.S. Rules of Engagement: Molecular Insights from Host-Virus Arms Races. Annu. Rev. Genet. 2012, 46, 677–700. [Google Scholar] [CrossRef]
- Harris, R.S.; Hultquist, J.F.; Evans, D.T. The Restriction Factors of Human Immunodeficiency Virus. J. Biol. Chem. 2012, 287, 40875–40883. [Google Scholar] [CrossRef] [PubMed]
- Monit, C.; Morris, E.R.; Ruis, C.; Szafran, B.; Thiltgen, G.; Tsai, M.H.C.; Mitchison, N.A.; Bishop, K.N.; Stoye, J.P.; Taylor, I.A.; et al. Positive selection in dNTPase SAMHD1 throughout mammalian evolution. Proc. Natl. Acad. Sci. USA. 2019, 116, 18647–18654. [Google Scholar] [CrossRef]
- Tristem, M.; Marshall, C.; Karpas, A.; Hill, F. Evolution of the primate lentiviruses: Evidence from vpx and vpr. EMBO J. 1992, 11, 3405–3412. [Google Scholar] [CrossRef] [PubMed]
- Sharp, P.M.; Balles, E.; Stevenson, M.; Emerman, M.; Hahn, B.H. Gene acquisition in HIV and SIV. Nature 1996, 383, 586–587. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.S.; Fregoso, O.I.; McCoy, C.O.; Matsen, F.A.; Malik, H.S.; Emerman, M. The Ability of Primate Lentiviruses to Degrade the Monocyte Restriction Factor SAMHD1 Preceded the Birth of the Viral Accessory Protein Vpx. Cell Host Microbe 2012, 11, 194–204. [Google Scholar] [CrossRef] [PubMed]
- Hirsch, V.M.; Sharkey, M.E.; Brown, C.R.; Brichacek, B.; Goedstein, S.; Wakefield, J.; Byrum, R.; Elkins, W.R.; Hahn, B.H.; Lifson, J.D.; et al. Vpx is required for dissemination and pathogenesis of SIV(SM) PBj: Evidence of macrophage-dependent viral amplification. Nat. Med. 1998, 4, 1401–1408. [Google Scholar] [CrossRef]
- Accola, M.A.; Bukovsky, A.A.; Jones, M.S.; Göttlinger, H.G. A Conserved Dileucine-Containing Motif in p6gag Governs the Particle Association of Vpx and Vpr of Simian Immunodeficiency Viruses SIVmac and SIVagm. J. Virol. 1999, 73, 9992–9999. [Google Scholar] [CrossRef]
- Yu, X.F.; Yu, Q.C.; Essex, M.; Lee, T.H. The vpx Gene of Simian Immunodeficiency Virus Facilitates Efficient Viral Replication in Fresh Lymphocytes and Macrophages. J. Virol. 1991, 65, 5088–5091. [Google Scholar] [CrossRef]
- Goujon, C.; Jarrosson-Wuillème, L.; Bernaud, J.; Rigal, D.; Darlix, J.L.; Cimarelli, A. With a little help from a friend: Increasing HIV transduction of monocyte-derived dendritic cells with virion-like particles of SIVMAC. Gene Ther. 2006, 13, 991–994. [Google Scholar] [CrossRef]
- Goujon, C.; Arfi, V.; Pertel, T.; Luban, J.; Lienard, J.; Rigal, D.; Darlix, J.-L.; Cimarelli, A. Characterization of Simian Immunodeficiency Virus SIVSM/Human Immunodeficiency Virus Type 2 Vpx Function in Human Myeloid Cells. J. Virol. 2008, 82, 12335–12345. [Google Scholar] [CrossRef]
- Berger, A.; Sommer, A.F.R.; Zwarg, J.; Hamdorf, M.; Welzel, K.; Esly, N.; Panitz, S.; Reuter, A.; Ramos, I.; Jatiani, A.; et al. SAMHD1-deficient CD14+ cells from individuals with Aicardi-Goutières syndrome are highly susceptible to HIV-1 infection. PLoS Pathog. 2001, 7, e1002425. [Google Scholar] [CrossRef]
- Goujon, C.; Rivière, L.; Jarrosson-Wuilleme, L.; Bernaud, J.; Rigal, D.; Darlix, J.-L.; Cimarelli, A. SIVSM/HIV-2 Vpx proteins promote retroviral escape from a proteasome-dependent restriction pathway present in human dendritic cells. Retrovirology 2007, 4, 2. [Google Scholar] [CrossRef]
- Zimmerman, E.S.; Schulman, B.A.; Zheng, N. Structural assembly of cullin-RING ubiquitin ligase complexes. Curr. Opin. Struct. Biol. 2010, 20, 714–721. [Google Scholar] [CrossRef]
- Hrecka, K.; Gierszewska, M.; Srivastava, S.; Kozaczkiewicz, L.; Swanson, S.K.; Florens, L.; Washburn, M.P.; Skowronski, J. Lentiviral Vpr usurps Cul4–DDB1[VprBP] E3 ubiquitin ligase to modulate cell cycle. Proc. Natl. Acad. Sci. USA 2007, 104, 11778–11783. [Google Scholar] [CrossRef]
- Schröfelbauer, B.; Hakata, Y.; Landau, N.R. HIV-1 Vpr function is mediated by interaction with the damage-specific DNA-binding protein DDB1. Proc. Natl. Acad. Sci. USA 2007, 104, 4130–4135. [Google Scholar] [CrossRef] [PubMed]
- Belzile, J.P.; Duisit, G.; Rougeau, N.; Mercier, J.; Finzi, A.; Cohen, É.A. HIV-1 Vpr-mediated G2 arrest involves the DDB1-CUL4AVPRBP E3 ubiquitin ligase. PLoS Pathog. 2007, 3, 0882–0893. [Google Scholar] [CrossRef] [PubMed]
- Le Rouzic, E.; Belaïdouni, N.; Estrabaud, E.; Morel, M.; Rain, J.C.; Transy, C.; Margottin-Goguet, F. HIV1 Vpr Arrests the Cell Cycle by Recruiting DCAF1/VprBP, a Receptor of the Cul4-DDB1 Ubiquitin Ligase. Cell Cycle 2007, 6, 182–188. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, S.; Swanson, S.K.; Manel, N.; Florens, L.; Washburn, M.P.; Skowronski, J. Lentiviral Vpx accessory factor targets VprBP/DCAF1 substrate adaptor for cullin 4 E3 ubiquitin ligase to enable macrophage infection. PLoS Pathog. 2008, 4, e1000059. [Google Scholar] [CrossRef]
- Sharova, N.; Wu, Y.; Zhu, X.; Stranska, R.; Kaushik, R.; Sharkey, M.; Stevenson, M. Primate lentiviral Vpx commandeers DDB1 to counteract a macrophage restriction. PLoS Pathog. 2008, 4, e100057. [Google Scholar] [CrossRef]
- Wen, X.; Duus, K.M.; Friedrich, T.D.; de Noronha, C.M.C. The HIV1 protein Vpr acts to promote G2 cell cycle arrest by engaging a DDB1 and cullin4A-containing ubiquitin ligase complex using VprBP/DCAF1 as an adaptor. J. Biol. Chem. 2007, 282, 27046–27051. [Google Scholar] [CrossRef] [PubMed]
- Laguette, N.; Rahm, N.; Sobhian, B.; Chable-Bessia, C.; Münch, J.; Snoeck, J.; Sauter, D.; Switzer, W.M.; Heneine, W.; Kirchhoff, F.; et al. Evolutionary and Functional Analyses of the Interaction between the Myeloid Restriction Factor SAMHD1 and the Lentiviral Vpx Protein. Cell Host Microbe 2012, 11, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Fregoso, O.I.; Ahn, J.; Wang, C.; Mehrens, J.; Skowronski, J.; Emerman, M. Evolutionary Toggling of Vpx/Vpr Specificity Results in Divergent Recognition of the Restriction Factor SAMHD1. PLoS Pathog. 2013, 9, e1003496. [Google Scholar] [CrossRef]
- Schwefel, D.; Groom, H.C.T.; Boucherit, V.C.; Christodoulou, E.; Walker, P.A.; Stoye, J.P.; Bishop, K.N.; Taylor, I.A. Structural basis of lentiviral subversion of a cellular protein degradation pathway. Nature 2014, 505, 234–238. [Google Scholar] [CrossRef]
- Schwefel, D.; Boucherit, V.C.; Christodoulou, E.; Walker, P.A.; Stoye, J.P.; Bishop, K.N.; Taylor, I.A. Molecular determinants for recognition of divergent SAMHD1 proteins by the lentiviral accessory protein Vpx. Cell Host Microbe 2015, 17, 489–499. [Google Scholar] [CrossRef]
- Ren, H.; Yin, X.; Su, C.; Guo, M.; Wang, X.-F.; Na, L.; Lin, Y.; Wang, X. Equine lentivirus counteracts SAMHD1 restriction by Rev-mediated degradation of SAMHD1 via the BECN1-dependent lysosomal pathway. Autophagy 2020. [Google Scholar] [CrossRef]
- Lenzi, G.M.; Domaoal, R.A.; Kim, D.; Schinazi, R.F.; Kim, B. Kinetic variations between reverse transcriptases of viral protein X coding and noncoding lentiviruses. Retrovirology 2014, 11, 111. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Coggins, S.A.; Holler, J.M.; Kimata, J.T.; Kim, D.H.; Schinazi, R.F.; Kim, B. Efficient pre-catalytic conformational change of reverse transcriptases from SAMHD1 non-counteracting primate lentiviruses during dNTP incorporation. Virology 2019, 537, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Coggins, S.A.; Kim, D.H.; Schinazi, R.F.; Desrosier, R.C.; Kim, B. Enhanced enzyme kinetics of reverse transcriptase variants cloned from animals infected with SIVmac239 lacking viral protein X. J. Biol. Chem. 2020, 295, 16975–16986. [Google Scholar] [CrossRef] [PubMed]
- Lenzi, G.M.; Domaoal, R.A.; Kim, D.H.; Schinazi, R.F.; Kim, B. Mechanistic and kinetic differences between reverse transcriptases of Vpx coding and non-coding lentiviruses. J. Biol. Chem. 2015, 290, 30078–30086. [Google Scholar] [CrossRef] [PubMed]
- Gramberg, T.; Sunseri, N.; Landau, N.R. Evidence for an Activation Domain at the Amino Terminus of Simian Immunodeficiency Virus Vpx. J. Virol. 2010, 84, 1387–1396. [Google Scholar] [CrossRef] [PubMed]
- Chee, M.S.; Lawrence, G.L.; Barrell, B.G. Alpha-, Beta- and Gammaherpesviruses Encode a Putative Phosphotransferase. J. Gen. Virol. 1989, 70, 1151–1160. [Google Scholar] [CrossRef] [PubMed]
- Kuny, C.V.; Chinchilla, K.; Culbertson, M.R.; Kalejta, R.F. Cyclin-dependent kinase-like function is shared by the beta and gamma subset of the conserved herpesvirus protein kinases. PLoS Pathog. 2010, 6, e1001092. [Google Scholar] [CrossRef] [PubMed]
- Gershburg, E.; Pagano, J.S. Conserved herpesvirus protein kinases. Biochim. Biophys. Acta Proteins Proteom. 2008, 1784, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Jacob, T.; van den Broeke, C.; Favoreel, H. Viral Serine/Threonine Protein Kinases. J. Virol. 2011, 85, 1158–1173. [Google Scholar] [CrossRef] [PubMed]
- Kawaguchi, Y.; Kato, K. Protein kinases conserved in herpesviruses potentially share a function mimicking the cellular protein kinase cdc2. Rev. Med. Virol. 2003, 13, 331–340. [Google Scholar] [CrossRef]
- Bogdanow, B.; Schmidt, M.; Weisbach, H.; Gruska, I.; Vetter, B.; Imami, K.; Ostermann, E.; Brune, W.; Selbach, M.; Hagemeier, C.; et al. Cross-regulation of viral kinases with cyclin A secures shutoff of host DNA synthesis. Nat. Commun. 2020, 11, 4845. [Google Scholar] [CrossRef] [PubMed]
- De Meo, S.; Dell’Oste, V.; Molfetta, R.; Tassinari, V.; Lotti, L.V.; Vespa, S.; Pignoloni, B.; Covino, D.A.; Fantuzzi, L.; Bona, R.; et al. Samhd1 phosphorylation and cytoplasmic relocalization after human cytomegalovirus infection limits its antiviral activity. PLoS Pathog. 2020, 16, e1008855. [Google Scholar] [CrossRef] [PubMed]
- Bloch, N.; Gläsker, S.; Sitaram, P.; Hofmann, H.; Shepard, C.N.; Schultz, M.L.; Kim, B.; Landau, N.R. A Highly Active Isoform of Lentivirus Restriction Factor SAMHD1 in Mouse. J. Biol. Chem. 2017, 292, 1068–1080. [Google Scholar] [CrossRef]
- Buzovetsky, O.; Tang, C.; Knecht, K.M.; Antonucci, J.M.; Wu, L.; Ji, X.; Xiong, Y. The SAM domain of mouse SAMHD1 is critical for its activation and regulation. Nat. Commun. 2018, 9, 411. [Google Scholar] [CrossRef]
- Wang, F.; St. Gelais, C.; de Silva, S.; Zhang, H.; Geng, Y.; Shepard, C.; Kim, B.; Yount, J.S.; Wu, L. Phosphorylation of mouse SAMHD1 regulates its restriction of human immunodeficiency virus type 1 infection, but not murine leukemia virus infection. Virology 2016, 487, 273–284. [Google Scholar] [CrossRef][Green Version]
- Wittmann, S.; Behrendt, R.; Eissmann, K.; Volkmann, B.; Thomas, D.; Ebert, T.; Cribier, A.; Benkirane, M.; Hornung, V.; Bouzas, N.F.; et al. Phosphorylation of murine SAMHD1 regulates its antiretroviral activity. Retrovirology 2015, 12, 1–15. [Google Scholar] [CrossRef]
- McGeoch, D.J.; Davison, A.J. Alphaherpesviruses possess a gene homologous to the protein kinase gene family of eukaryotes and retroviruses. Nucleic Acids Res. 1986, 14, 1765–1777. [Google Scholar] [CrossRef][Green Version]
- Conner, J.; Marsden, H.; Clements, J.B. Ribonucleotide Reductase of Herpesviruses. Rev. Med. Virol. 1994, 4, 25–34. [Google Scholar] [CrossRef]
- Lembo, D.; Brune, W. Tinkering with a viral ribonucleotide reductase. Trends Biochem. Sci. 2009, 34, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Lembo, D.; Gribaudo, G.; Hofer, A.; Riera, L.; Cornaglia, M.; Mondo, A.; Angeretti, A.; Gariglio, M.; Thelander, L.; Landolfo, S. Expression of an Altered Ribonucleotide Reductase Activity Associated with the Replication of Murine Cytomegalovirus in Quiescent Fibroblasts. J. Virol. 2000, 74, 11557–11565. [Google Scholar] [CrossRef] [PubMed]
- Cohen, D.; Adamovich, Y.; Reuven, N.; Shaul, Y. Hepatitis B virus activates deoxynucleotide synthesis in nondividing hepatocytes by targeting the R2 gene. Hepatology 2010, 51, 1538–1546. [Google Scholar] [CrossRef] [PubMed]
- Ricardo-Lax, I.; Ramanan, V.; Michailidis, E.; Shamia, T.; Reuven, N.; Rice, C.M.; Shlomai, A.; Shaul, Y. Hepatitis B virus induces RNR-R2 expression via DNA damage response activation. J. Hepatol. 2015, 63, 789–796. [Google Scholar] [CrossRef] [PubMed]
- Cheung, A.K.L.; Huang, Y.; Kwok, H.Y.; Chen, M.; Chen, Z. Latent human cytomegalovirus enhances HIV-1 infection in CD34+ progenitor cells. Blood Adv. 2017, 1, 306–318. [Google Scholar] [CrossRef] [PubMed]
- Hyeon, S.; Lee, M.K.; Kim, Y.E.; Lee, G.M.; Ahn, J.H. Degradation of SAMHD1 Restriction Factor Through Cullin-Ring E3 Ligase Complexes During Human Cytomegalovirus Infection. Front. Cell. Infect. Microbiol. 2020, 10, 391. [Google Scholar] [CrossRef]
- Dell’Oste, V.; Gatti, D.; Gugliesi, F.; De Andrea, M.; Bawadekar, M.; Lo Cigno, I.; Biolatti, M.; Vallino, M.; Marschall, M.; Gariglio, M.; et al. Innate Nuclear Sensor IFI16 Translocates into the Cytoplasm during the Early Stage of In Vitro Human Cytomegalovirus Infection and Is Entrapped in the Egressing Virions during the Late Stage. J. Virol. 2014, 88, 6970–6982. [Google Scholar] [CrossRef]
- Gariano, G.R.; Dell’Oste, V.; Bronzini, M.; Gatti, D.; Luganini, A.; De Andrea, M.; Gribaudo, G.; Gariglio, M.; Landolfo, S. The Intracellular DNA Sensor IFI16 Gene Acts as Restriction Factor for Human Cytomegalovirus Replication. PLoS Pathog. 2012, 8, e1002498. [Google Scholar] [CrossRef] [PubMed]
- Crisci, E.; Svanberg, C.; Ellegård, R.; Khalid, M.; Hellblom, J.; Okuyama, K.; Bhattacharya, P.; Nyström, S.; Shankar, E.M.; Eriksson, K.; et al. HSV-2 Cellular Programming Enables Productive HIV Infection in Dendritic Cells. Front. Immunol. 2019, 10, 2889. [Google Scholar] [CrossRef]
- Mereby, S.A.; Maehigashi, T.; Holler, J.M.; Kim, D.H.; Schinazi, R.F.; Kim, B. Interplay of ancestral non-primate lentiviruses with the virusrestricting SAMHD1 proteins of their hosts. J. Biol. Chem. 2018, 293, 16402–16412. [Google Scholar] [CrossRef]
- Wichit, S.; Hamel, R.; Zanzoni, A.; Diop, F.; Cribier, A.; Talignani, L.; Diack, A.; Ferraris, P.; Liegeois, F.; Urbach, S.; et al. SAMHD1 Enhances Chikungunya and Zika Virus Replication in Human Skin Fibroblasts. Int. J. Mol. Sci. 2019, 20, 1695. [Google Scholar] [CrossRef]
- Mahmoud, S.; Hasabelnaby, S.; Hammad, S.; Sakr, T. Antiviral Nucleoside and Nucleotide Analogs: A Review. J. Adv. Pharm. Res. 2018, 2, 73–88. [Google Scholar] [CrossRef]
- Amie, S.M.; Daly, M.B.; Noble, E.; Schinazi, R.F.; Bambara, R.A.; Kim, B. Anti-HIV host factor SAMHD1 regulates viral sensitivity to nucleoside reverse transcriptase inhibitors via modulation of cellular deoxyribonucleoside triphosphate (dNTP) levels. J. Biol. Chem. 2013, 288, 20683–20691. [Google Scholar] [CrossRef] [PubMed]
- Huber, A.D.; Michailidis, E.; Schultz, M.L.; Ong, Y.T.; Bloch, N.; Puray-Chavez, M.N.; Leslie, M.D.; Ji, J.; Lucas, A.D.; Kirby, K.A.; et al. SAMHD1 has differential impact on the efficacies of HIV nucleoside reverse transcriptase inhibitors. Antimicrob. Agents Chemother. 2014, 58, 4915–4919. [Google Scholar] [CrossRef]
- Ballana, E.; Badia, R.; Terradas, G.; Torres-Torronteras, J.; Ruiz, A.; Pauls, E.; Riveira-Muñoz, E.; Clotet, B.; Martí, R.; Esté, J.A. SAMHD1 Specifically Affects the Antiviral Potency of Thymidine Analog HIV Reverse Transcriptase Inhibitors. Antimicrob. Agents Chemother. 2014, 58, 4804–4813. [Google Scholar] [CrossRef][Green Version]
- Littler, E.; Stuart, A.D.; Chee, M.S. Human cytomegalovirus UL97 open reading frame encodes a protein that phosphorylates the antiviral nucleoside analogue ganciclovir. Nature 1992, 358, 160–162. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, V.; Talarico, C.L.; Stanat, S.C.; Davis, M.; Coen, D.M.; Biron, K.K. A protein kinase homologue controls phosphorylation of ganciclovir in human cytomegalovirus-infected cells. Nature 1992, 358, 162–164. [Google Scholar] [CrossRef] [PubMed]
- Topalis, D.; Gillemot, S.; Snoeck, R.; Andrei, G. Thymidine kinase and protein kinase in drug-resistant herpesviruses: Heads of a Lernaean Hydra. Drug Resist. Updates 2018, 37, 1–16. [Google Scholar] [CrossRef]
- Talarico, C.L.; Burnette, T.C.; Miller, W.H.; Smith, S.L.; Davis, M.G.; Stanat, S.C.; Ng, T.I.; He, Z.; Coen, D.M.; Roizman, B.; et al. Acyclovir Is Phosphorylated by the Human Cytomegalovirus UL97 Protein. Antimicrob. Agents Chemother. 1999, 43, 1941–1946. [Google Scholar] [CrossRef]
- Hakki, M. Moving Past Ganciclovir and Foscarnet: Advances in CMV Therapy. Curr. Hematol. Malig. Rep. 2020, 15, 90–102. [Google Scholar] [CrossRef] [PubMed]
- Prichard, M.N. Function of human cytomegalovirus UL97 kinase in viral infection and its inhibition by maribavir. Rev. Med. Virol. 2009, 19, 215–229. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).