The Successful Elimination of Sylvatic Rabies Using Oral Vaccination of Foxes in Slovenia
Abstract
1. Introduction
2. Materials and Methods
3. Results
3.1. The Incidence of Rabies in Slovenia
3.2. Bait Consumption and Immunization Rate
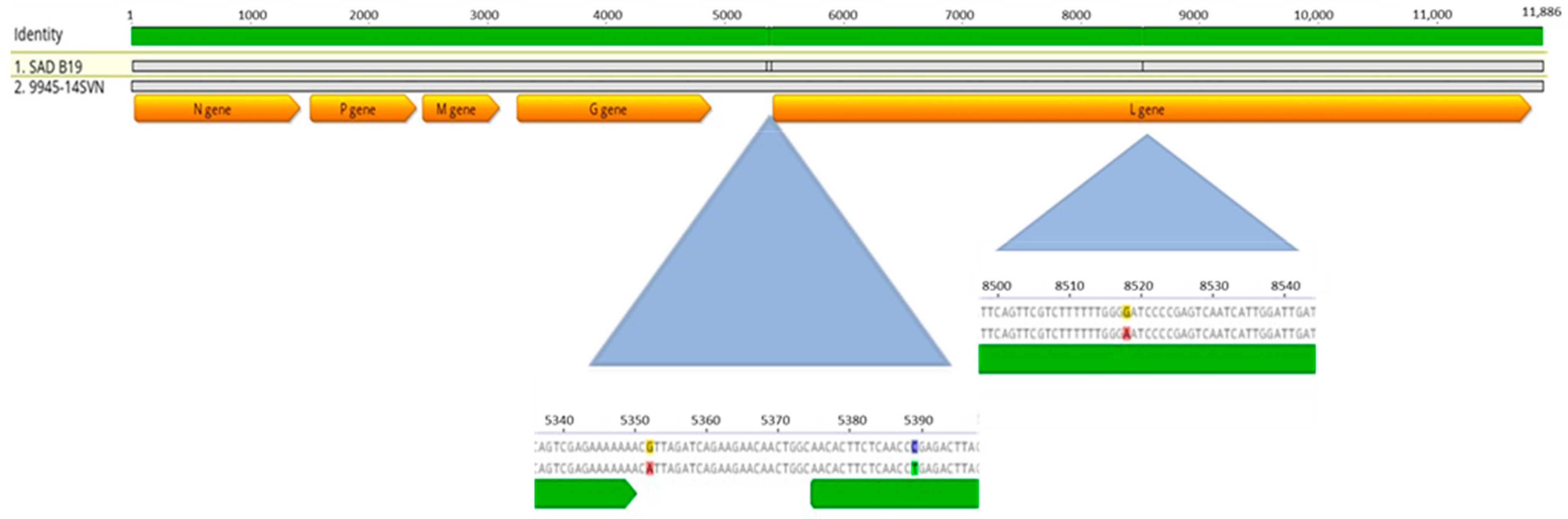
3.3. Genetic Characterization of Rabies-Positive Samples
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Rabies Vaccines and Immunoglobulins. In WHO Position, Weekly Epidemiological Record; WHO: Geneva, Switzerland, 2018; Volume 16, pp. 201–220. [Google Scholar]
- Nel, L.H. Vaccines for lyssaviruses other than rabies. Expert Rev. Vaccines 2005, 4, 533–540. [Google Scholar] [CrossRef] [PubMed]
- ICTV Code. Available online: https://talk.ictvonline.org/ictv-reports/ictv_online_report/negative-sense-rna-viruses/w/rhabdoviridae/795/genus-lyssavirus (accessed on 11 January 2021).
- Pastoret, P.-P.; Kappeler, A.; Aubert, A. European rabies control and its history. In Historical Perspective of Rabies in Europe and the Mediterranean Basin; King, A.A., Fooks, A.R., Aubert, M., Wandeler, A.I., Eds.; OIE: Paris, France, 2004; pp. 337–350. [Google Scholar]
- Pastoret, P.; Brochier, B. Epidemiology and control of fox rabies in Europe. Vaccine 1999, 17, 1750–1754. [Google Scholar] [CrossRef]
- Mūller, T.; Schluter, H. Oral immunization of red foxes (Vulpes, L.) in Europe—A review. J. Eltik. Vet. Microbiol. 1998, 9, 35–59. [Google Scholar]
- Steck, F.; Wandeler, A.; Bichsel, P.; Capt, S.; Häfliger, U.; Schneider, L. Oral immunization of foxes against rabies laboratory and field studies. Comp. Immunol. Microbiol. Infect. Dis. 1982, 5, 165–171. [Google Scholar] [CrossRef]
- Robardet, E.; Bosnjak, D.; Englund, L.; Demetriou, P.; Martín, P.R.; Cliquet, F. Zero Endemic Cases of Wildlife Rabies (Classical Rabies Virus, RABV) in the European Union by 2020: An Achievable Goal. Trop. Med. Infect. Dis. 2019, 4, 124. [Google Scholar] [CrossRef]
- Mulatti, P.; Bonfanti, L.; Patregnani, T.; Lorenzetto, M.; Ferrè, N.; Gagliazzo, L.; Casarotto, C.; Ponti, A.M.; Ferri, G.; Marangon, S. 2008–2011 sylvatic rabies epidemic in Italy: Challenges and experiences. Pathog. Glob. Health 2013, 107, 346–353. [Google Scholar] [CrossRef][Green Version]
- Tasioudi, K.E.; Iliadou, P.; Agianniotaki, E.I.; Robardet, E.; Liandris, E.; Doudounakis, S.; Tzani, M.; Tsaroucha, P.; Picard-Meyer, E.; Cliquet, F.; et al. Recurrence of Animal Rabies, Greece, 2012. Emerg. Infect. Dis. 2014, 20, 326–328. [Google Scholar] [CrossRef]
- Cliquet, F.; Picard-Meyer, E.; Robardet, E. Rabies in Europe: What are the risks? Expert Rev. Antiinfect. Ther. 2014, 12, 905–908. [Google Scholar] [CrossRef]
- Železnik, Z.; Grom, J.; Valenčak, Z. Steklina v Sloveniji od 1971 do 1981. Zbornik ob 25. Letnici Ustanovitve Jugoslovanskega Mikrobiološkega Društva; Slovensko Mikrobiološko Društvo: Ljubljana, Slovenija, 1982; pp. 35–36. [Google Scholar]
- Hostnik, P.; Rihtarič, D.; Maurer Wernig, J.; Mlinarič, E.; Toplak, I. Zaključek programa peroralnega cepljenja lisic v Sloveniji. Vestnik VZbSi 2020, 15, 95–102. [Google Scholar]
- Statistical Office of the Republic of Slovenia. Data on Forestry and Hunting—Hunting. Available online: https://www.stat.si/doc/letopis/2013/17_13/17-12-13.html (accessed on 11 January 2021).
- Hostnik, P.; Toplak, I.; Barlič-Maganja, D.; Grom, J.; Bidovec, A. Control of Rabies in Slovenia. J. Wildl. Dis. 2006, 42, 459–465. [Google Scholar] [CrossRef] [PubMed]
- Rihtarič, D. Proučevanje Molekularno-Epidemioloških Značilnosti Virusa Stekline pri Divjih in Domačih Živalih v Sloveniji. Ph.D. Thesis, University of Ljubljana, Ljubljana, Slovenia, 7 May 2013. [Google Scholar]
- Kodrnja, E. Rabies movement and eradication in Croatia. Vet. Arhiv 1970, 100, 563–564. [Google Scholar]
- Bedeković, T.; Janković, I.L.; Šimić, I.; Krešić, N.; Lojkić, I.; Sučec, I.; Robardet, E.; Cliquet, F. Control and elimination of rabies in Croatia. PLoS ONE 2018, 13, e0204115. [Google Scholar] [CrossRef]
- Vitásek, J. A review of rabies elimination in Europe. Veterinární Med. 2012, 49, 171–185. [Google Scholar] [CrossRef]
- Nagy, A.; Kerekes, B. History and epizootiology of rabies. Hung. Vet. J. 1995, 50, 70–77. [Google Scholar]
- Balint, K. Rabies—Current situation. Hung. Vet. J. 2000, 122, 195–200. [Google Scholar]
- Hornyák, Á.; Juhász, T.; Forró, B.; Kecskeméti, S.; Bányai, K. Resurgence of rabies in Hungary during 2013–2014: An attempt to track the origin of identified strains. Transbound. Emerg. Dis. 2017, 65, e14–e24. [Google Scholar] [CrossRef]
- Vogl, D. The reinfection of rabies in Karnten (Carinthia) in Austria—The run of events. Rabies Bull. Eur. 2002, 26, 10–11. [Google Scholar]
- Müller, T.; Bätza, H.-J.; Beckert, A.; Bunzenthal, C.; Cox, J.H.; Freuling, C.M.; Fooks, A.R.; Frost, J.; Geue, L.; Hoeflechner, A.; et al. Analysis of vaccine-virus-associated rabies cases in red foxes (Vulpes vulpes) after oral rabies vaccination campaigns in Germany and Austria. Arch. Virol. 2009, 154, 1081–1091. [Google Scholar] [CrossRef] [PubMed]
- Bellani, L.; Gagliardi, G.; Irsara, A.; Mantovani, A.; Prosperi, S. Situation and control of rabies in Italy. Bull. Off. Int. Epizoot. 1976, 86, 243–252. [Google Scholar]
- De Benedictis, P.; Capua, I.; Mutinelli, F.; Wernig, J.M.; Arič, T.; Hostnik, P. Update 331 on fox rabies in Italy and Slovenia. Rabies Bull. Eur. 2009, 1, 5–7. [Google Scholar]
- Curk, A.; Carpenter, T. Efficacy of the first oral vaccination against fox rabies in Slovenia. Rev. Sci. Tech. OIE 1994, 13, 763–775. [Google Scholar] [CrossRef]
- Dean, D.J.; Abelseth, M.K. Laboratory techniques in rabies: The fluorescent antibody test. Monogr. Ser. World Heal. Organ. 1973, 23, 73–84. [Google Scholar]
- Cliquet, F.; Freuling, C.; Smreczak, M.; van der Poel, W.H.M.; Horton, D.; Fooks, A.R.; Robardet, E.; Picard-Meyer, E.; Müller, T. Development of Harmonized Schemes for Monitoring and Reporting of Rabies in Animals in the European Union. EFSA J. 2010, 7, 60. [Google Scholar]
- Wasniewski, M.; Labbe, A.; Tribout, L.; Rieder, J.; Labadie, A.; Schereffer, J.; Cliquet, F. Evaluation of a rabies ELISA as an alternative method to seroneutralisation tests in the context of international trade of domestic carnivores. J. Virol. Methods 2014, 195, 211–220. [Google Scholar] [CrossRef]
- Bourhy, H.; Kissi, B.; Audry, L.; Smreczak, M.; Sadkowska-Todys, M.; Kulonen, K.; Tordo, N.; Zmudzinski, J.F.; Holmes, E.C. Ecology and evolution of rabies virus in Europe. J. Gen. Virol. 1999, 80, 2545–2557. [Google Scholar] [CrossRef]
- Felsenstein, J. PHYLIP-Phylogeny inference package (Version 3.2). Cladistics 1989, 5, 164–166. [Google Scholar]
- De Benedictis, P.; Gallo, T.; Iob, A.; Coassin, R.; Squecco, G.; Ferri, G.; d’Ancona, F.; Marangon, S.; Capua, I.; Mutinelli, F. Emergence of fox rabies in north-eastern Italy. Eurosurveillance 2008, 13, 19033. [Google Scholar]
- Lojkič, I.; Galić, M.; Čač, Ž.; Jelić, I.; Bedeković, T.; Lojkić, M.; Cvetnić, Ž. Bites of a rabid wolf in 67-old man in north-eastern part of Croatia. Rabies Bull. Eur. 2009, 33, 5–7. [Google Scholar]
- McElhinney, L.M.; Marston, D.A.; Freuling, C.M.; Cragg, W.; Stankov, S.; Lalosević, D.; Müller, T.; Fooks, A.R. Molecular diversity and evolutionary history of rabies virus strains circulating in the Balkans. J. Gen. Virol. 2011, 92, 2171–2180. [Google Scholar] [CrossRef][Green Version]
- Hostnik, P.; Picard-Meyer, E.; Rihtaric, D.; Toplak, I.; Cliquet, F. Vaccine-induced Rabies in a Red Fox (Vulpes vulpes): Isolation of Vaccine Virus in Brain Tissue and Salivary Glands. J. Wildl. Dis. 2014, 50, 397–401. [Google Scholar] [CrossRef]
- Smith, G. The role of the Badger (Meles meles) in rabies epizootiology and the implications for Great Britain. Mammal Rev. 2002, 32, 12–25. [Google Scholar] [CrossRef]
- Müller, T.; Freuling, C.M.; Wysocki, P.; Roumiantzeff, M.; Freney, J.; Mettenleiter, T.C.; Vos, A. Terrestrial rabies control in the European Union: Historical achievements and challenges ahead. Vet. J. 2015, 203, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Arnold, J.; Humer, A.; Heltai, M.; Murariu, D.; Spassov, N.; Hackländer, K. Current status and distribution of golden jackals Canis aureus in Europe. Mammal Rev. 2011, 42, 1–11. [Google Scholar] [CrossRef]
- Kovač, Z. Epizootiologija Silvatične Stekline v Republiki Sloveniji v Letih 1970–1990. Master’s Thesis, Univestity of Ljubljana, Ljubljana, Slovenia, 1993. [Google Scholar]
- Cliquet, F.; Robardet, E.; Must, K.; Laine, M.; Peik, K.; Picard-Meyer, E.; Guiot, A.-L.; Niin, E. Eliminating Rabies in Estonia. PLoS Negl. Trop. Dis. 2012, 6, e1535. [Google Scholar] [CrossRef]
- Müller, T.F.; Schröder, R.; Wysocki, P.; Mettenleiter, T.C.; Freuling, C.M. Spatio-temporal Use of Oral Rabies Vaccines in Fox Rabies Elimination Programmes in Europe. PLoS Negl. Trop. Dis. 2015, 9, e0003953. [Google Scholar] [CrossRef] [PubMed]
- Masson, E.; Aubert, M.F.; Barrat, J.; Vuillaume, P. Comparison of the efficacy of the antirabies vaccines used for foxes in France. Vet. Res. 1996, 27, 255–266. [Google Scholar]
- World Health Organization. WHO Expert Consultation on Rabies: Second Report WHO Technical Report. Series 982. 2013. Available online: http://apps.who.int/iris/bitstream/10665/85346/1/9789240690943_eng.pdf (accessed on 3 January 2019).
- Neumann, E.J.; Bonistalli, K.N. Effect of blood sample handling post-collection on Erysipelothrix rhusiopathiae antibody titres. Vet. J. 2009, 180, 325–329. [Google Scholar] [CrossRef] [PubMed]
- Robardet, E.; Picard-Meyer, E.; Dobroštana, M.; Jaceviciene, I.; Mähar, K.; Muižniece, Z.; Pridotkas, G.; Masiulis, M.; Niin, E.; Olševskis, E.; et al. Rabies in the Baltic States: Decoding a Process of Control and Elimination. PLoS Negl. Trop. Dis. 2016, 10, e0004432. [Google Scholar] [CrossRef] [PubMed]
- Shiraishi, R.; Nishimura, M.; Nakashima, R.; Enta, C.; Hirayama, N. Determination of critical factors causing cytotoxicity in the virus neutralization test. J. Virol. Methods 2014, 199, 46–52. [Google Scholar] [CrossRef] [PubMed]
- Vuillaume, P.; Bruyere, V.; Aubert, M. Comparison of the effectiveness of two protocols of antirabies bait distribution for foxes (Vulpes vulpes). Vet. Res. 1998, 29, 537–546. [Google Scholar] [PubMed]
- Dietzschold, B.; Wunner, W.H.; Wiktor, T.J.; Lopes, A.D.; Lafon, M.; Smith, C.L.; Koprowski, H. Characterization of an antigenic determinant of the glycoprotein that correlates with pathogenicity of rabies virus. Proc. Natl. Acad. Sci. USA 1983, 80, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Seif, I.; Coulon, P.; Rollin, P.E.; Flamand, A. Rabies virulence: Effect on pathogenicity and sequence characterization of rabies virus mutations affecting antigenic site III of the glycoprotein. J. Virol. 1985, 53, 926–934. [Google Scholar] [CrossRef] [PubMed]
- Tuffereau, C.; Leblois, H.; Benejean, J.; Coulon, P.; Lafay, F.; Flamand, A. Arginine or lysine in position 333 of ERA and CVS glycoprotein is necessary for rabies virulence in adult mice. Virology 1989, 172, 206–212. [Google Scholar] [CrossRef]
- Košir, M.; Kraigher, A. Postopki preprečevanja stekline v Sloveniji. Zdr. Vest 2012, 81, 363–371. [Google Scholar]
- Posedi, J. Self-declaration by Slovenia of freedom from Rabies. OIE Bull. 2016, 2, 73–81. [Google Scholar]




| Name of Vaccine-Induced Rabies Virus | Animal Species | Detection Period | FAT | Cell Culture Virus Isolation | RT-PCR | Sequencing | NGS |
|---|---|---|---|---|---|---|---|
| 537-08SVN | red fox | February 2008 | positive | positive | positive | 1092 nt of N gene | nd |
| 3511-12SVN | red fox | May 2012 | positive | positive | positive | 4351 nt of N-G gene | nd |
| 9945-14SVN | marten | November 2014 | positive | positive | positive | 1092 nt of N gene | 11.886 nt |
| 21082-18SVN | red fox | June 2018 | positive | positive | positive | 1092 nt of N gene | nd |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Černe, D.; Hostnik, P.; Toplak, I. The Successful Elimination of Sylvatic Rabies Using Oral Vaccination of Foxes in Slovenia. Viruses 2021, 13, 405. https://doi.org/10.3390/v13030405
Černe D, Hostnik P, Toplak I. The Successful Elimination of Sylvatic Rabies Using Oral Vaccination of Foxes in Slovenia. Viruses. 2021; 13(3):405. https://doi.org/10.3390/v13030405
Chicago/Turabian StyleČerne, Danijela, Peter Hostnik, and Ivan Toplak. 2021. "The Successful Elimination of Sylvatic Rabies Using Oral Vaccination of Foxes in Slovenia" Viruses 13, no. 3: 405. https://doi.org/10.3390/v13030405
APA StyleČerne, D., Hostnik, P., & Toplak, I. (2021). The Successful Elimination of Sylvatic Rabies Using Oral Vaccination of Foxes in Slovenia. Viruses, 13(3), 405. https://doi.org/10.3390/v13030405





