Comparison of Enterococcus faecalis Biofilm Removal Efficiency among Bacteriophage PBEF129, Its Endolysin, and Cefotaxime
Abstract
:1. Introduction
2. Materials and Methods
2.1. Bacterial Strains and Bacteriophage
2.2. Bacteriophage Propagation and Purification
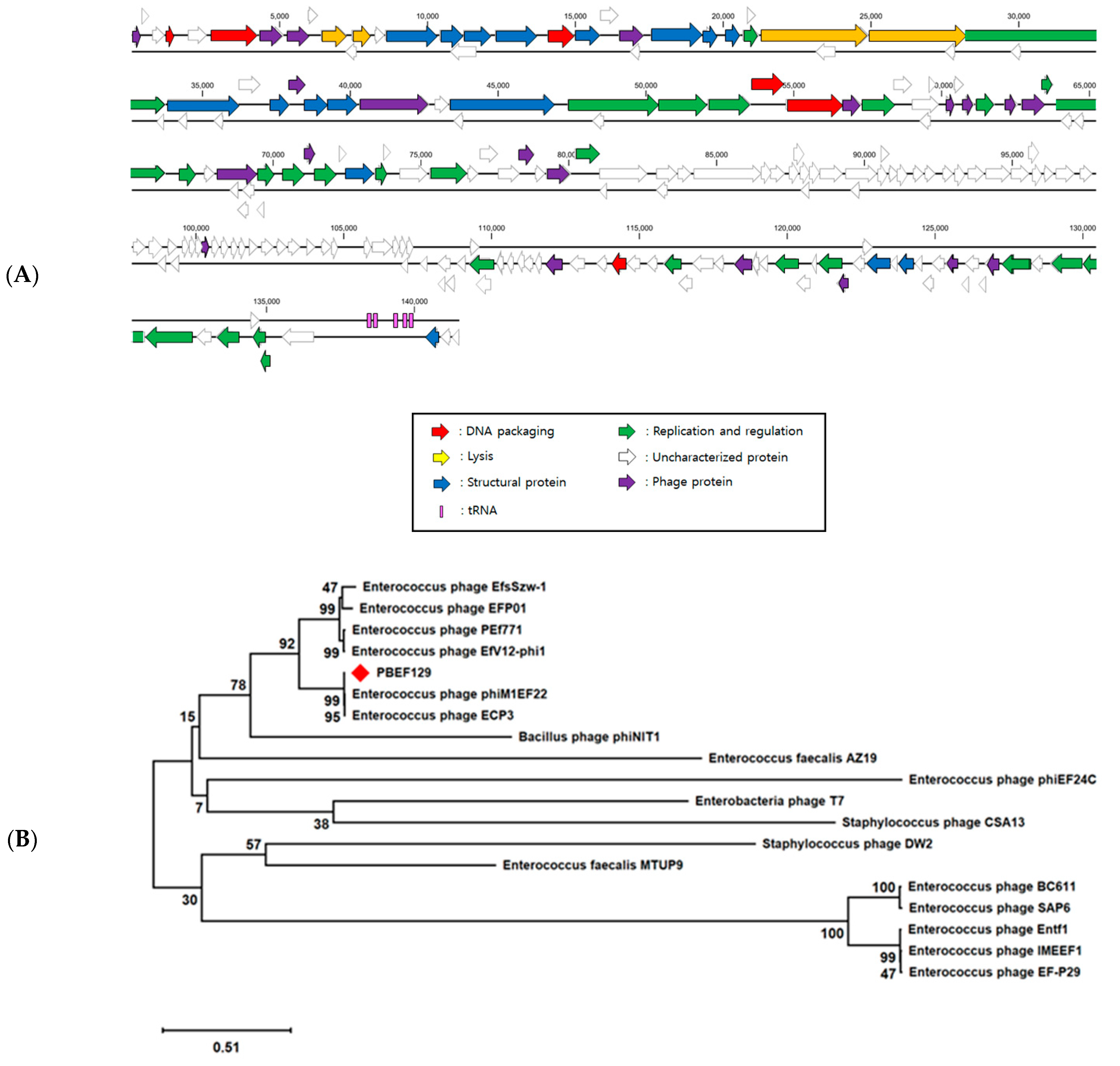
2.3. Genomic Analysis of Bacteriophage PBEF129
2.4. Cloning and Purification of Recombinant Phage Endolysin
2.5. Biochemical Activity (amidase) Test of Purified Endolysin
2.6. Antibacterial Activity Test of Purified Endolysin on Live Bacterial Cells
2.7. Human Cell Line and Formation of a Bacterial Biofilm In Vitro
2.8. Statistical Analysis
3. Results
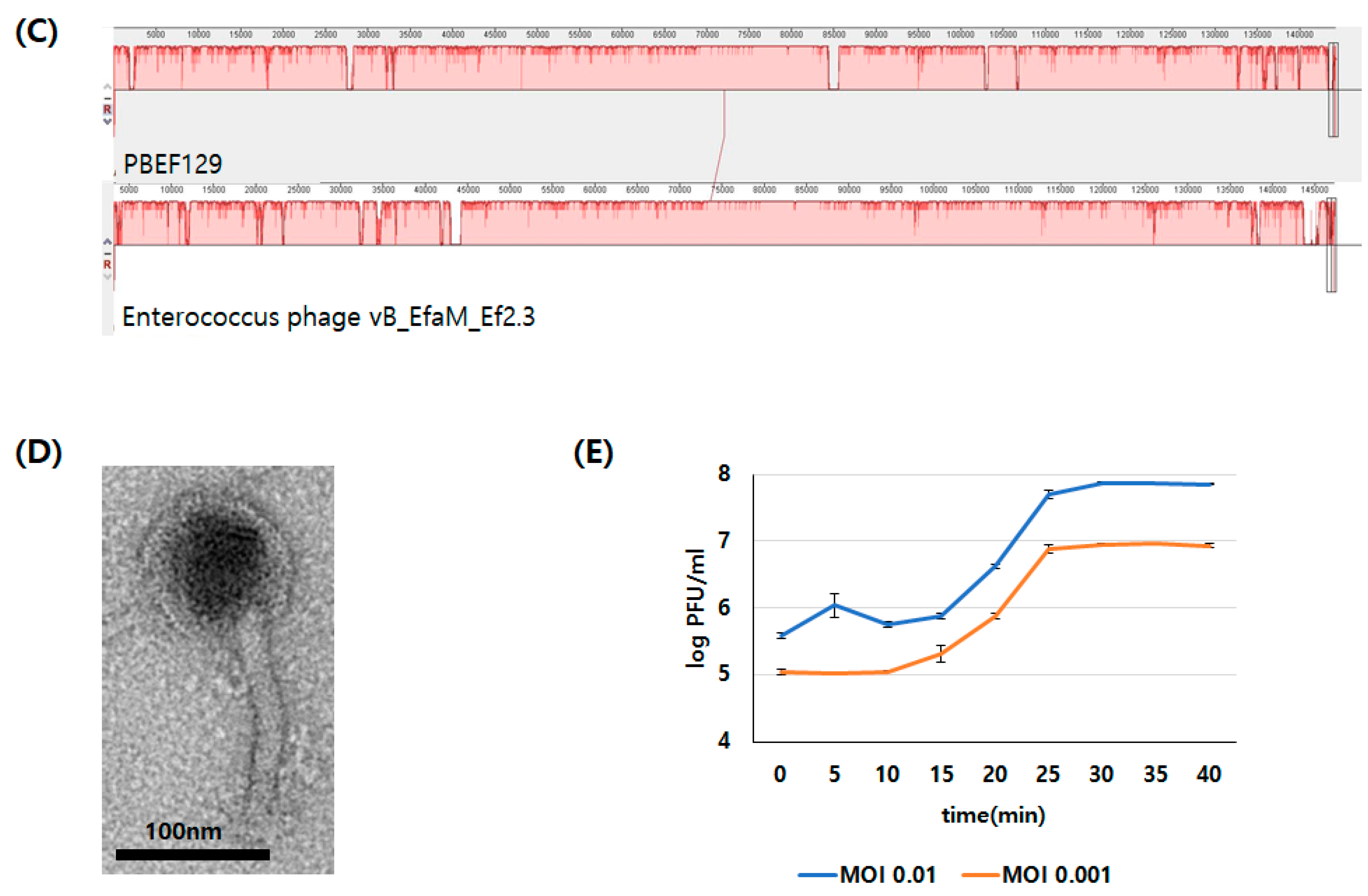
3.1. Genomic Analysis of Bacteriopahge PBEF129 Infecting E. feacalis
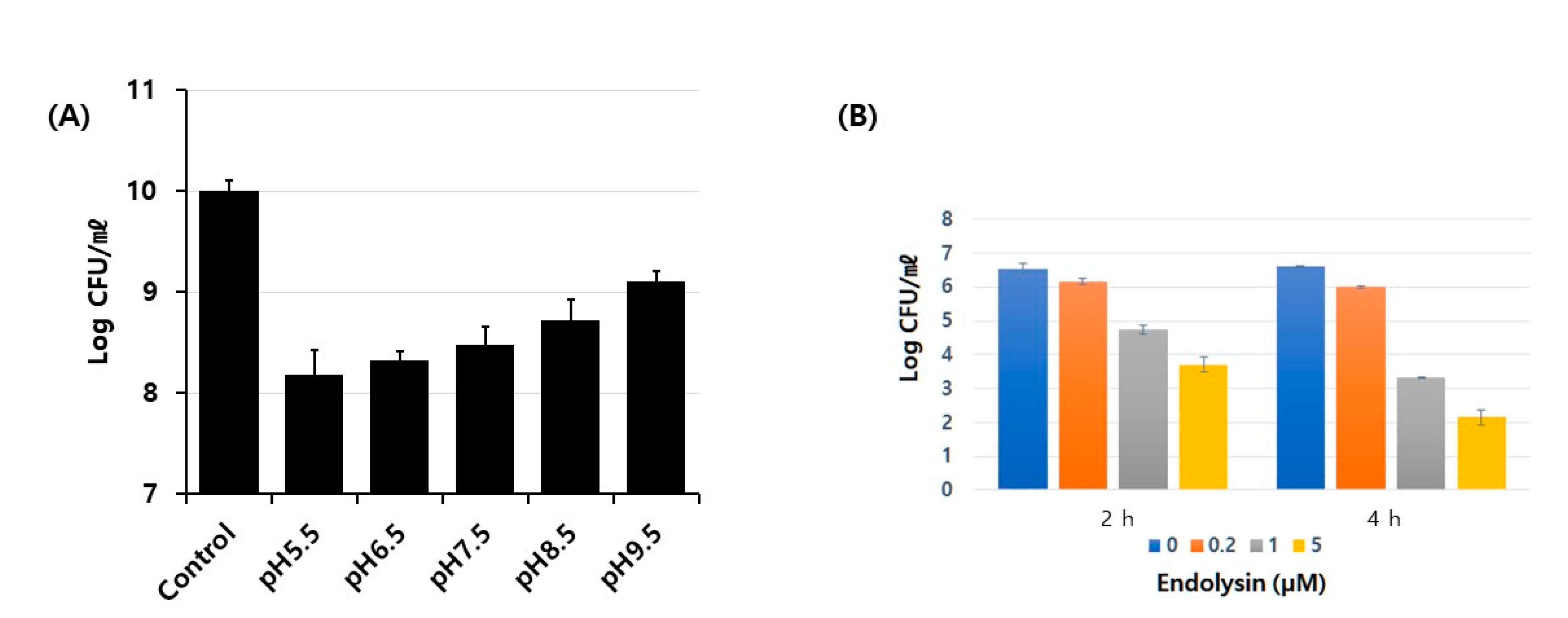
3.2. Biochemical Characterization of Enzymatic Activity of Putative Endolysin from Phage PBEF129
3.3. Characterization of Antibacterial Activity on Live Bacterial Cells
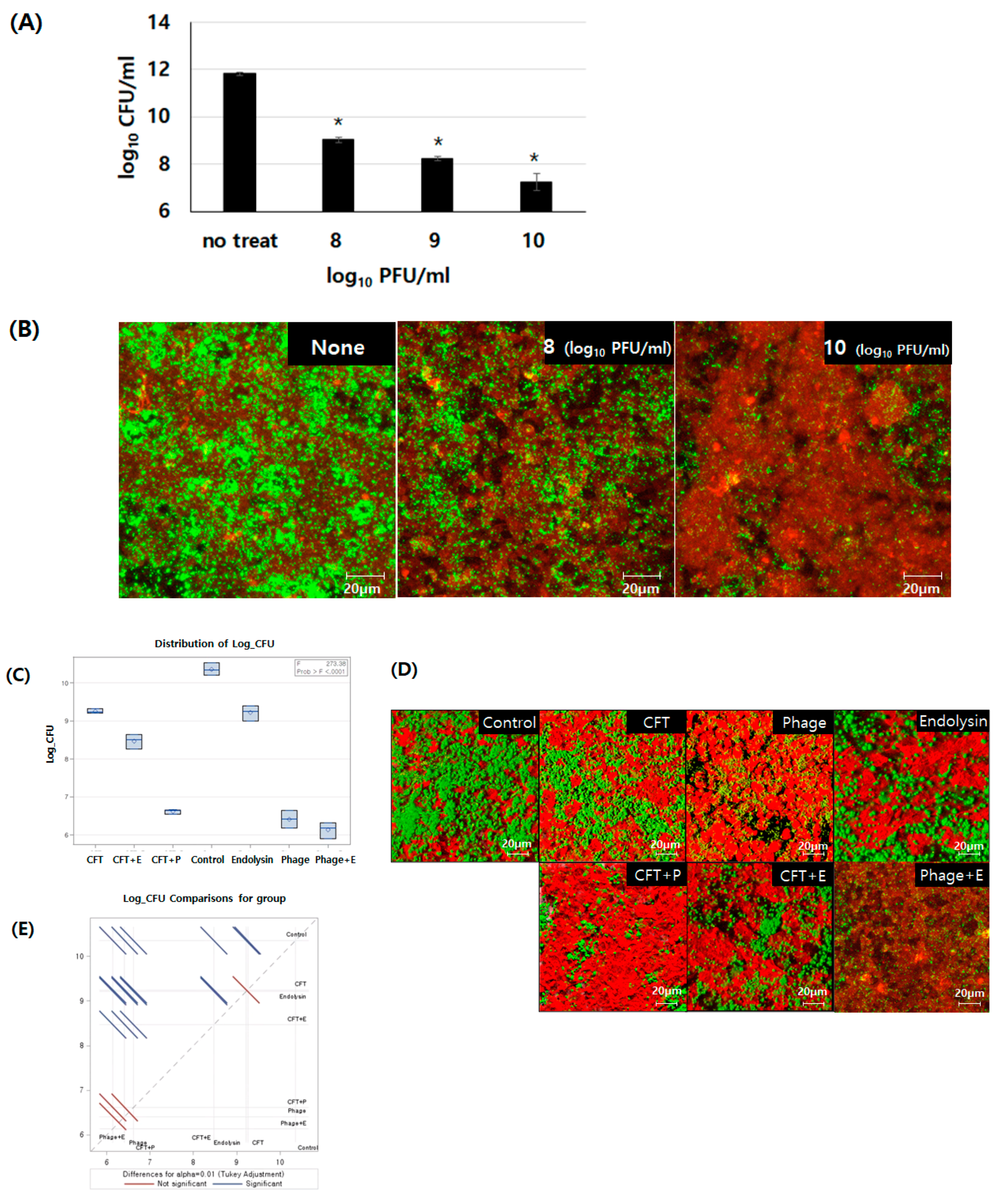
3.4. Comparison of Removal Efficiency Among Cefotaxime, Bacteriophage PBEF129, Its Endolysin, and their Combinations on E. faecalis Biofilm Formed on Human Intestinal Cells Grown In Vitro
4. Discussion
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shiadeh, S.M.J.; Pormohammad, A.; Hashemi, A.; Lak, P. Global prevalence of antibiotic resistance in blood-isolated Enterococcus faecalis and Enterococcus faecium: A systematic review and meta-analysis. Infect. Drug Resist. 2019, 12, 2713–2725. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beganovic, M.; Luther, M.K.; Rice, L.B.; Arias, C.A.; Rybak, M.J.; LaPlante, K.L. A Review of combination antimicrobial therapy for enterococcus faecalis bloodstream infections and infective endocarditis. Clin. Infect. Dis. 2018, 67, 303–309. [Google Scholar] [CrossRef] [Green Version]
- Gao, W.; Howden, B.P.; Stinear, T.P. Evolution of virulence in Enterococcus faecium, a hospital-adapted opportunistic pathogen. Curr. Opin. Microbiol. 2018, 41, 76–82. [Google Scholar] [CrossRef]
- Høiby, N.; Bjarnsholt, T.; Givskov, M.; Molin, S.; Ciofu, O. Antibiotic resistance of bacterial biofilms. Int. J. Antimicrob. Agents 2010, 35, 322–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, J.-X.; Bai, B.; Lin, Z.-W.; Pu, Z.-Y.; Yao, W.-M.; Chen, Z.; Li, D.-Y.; Deng, X.-B.; Deng, Q.-W.; Yu, Z.-J. Characterization of biofilm formation by Enterococcus faecalis isolates derived from urinary tract infections in China. J. Med. Microbiol. 2018, 67, 60–67. [Google Scholar] [CrossRef] [PubMed]
- Khalifa, L.; Brosh, Y.; Gelman, D.; Coppenhagen-Glazer, S.; Beyth, S.; Poradosu-Cohen, R.; Que, Y.-A.; Beyth, N.; Hazan, R. Targeting enterococcus faecalis biofilms with phage therapy. Appl. Environ. Microbiol. 2015, 81, 2696–2705. [Google Scholar] [CrossRef] [Green Version]
- Khatoon, Z.; McTiernan, C.D.; Suuronen, E.J.; Mah, T.-F.; Alarcon, E.I. Bacterial biofilm formation on implantable devices and approaches to its treatment and prevention. Heliyon 2018, 4, e01067. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, D.; Misba, L.; Khan, A.U. Antibiotics versus biofilm: An emerging battleground in microbial communities. Antimicrob. Resist. Infect. Control. 2019, 8, 76. [Google Scholar] [CrossRef] [PubMed]
- Bolocan, A.S.; Upadrasta, A.; de Almeida Bettio, P.H.; Clooney, A.G.; Draper, L.A.; Ross, R.P.; Hill, C. Evaluation of phage therapy in the context of enterococcus faecalis and its associated diseases. Viruses 2019, 11, 366. [Google Scholar] [CrossRef] [Green Version]
- Chatterjee, A.; Johnson, C.N.; Luong, P.; Hullahalli, K.; McBride, S.W.; Schubert, A.M.; Palmer, K.L.; Carlson, P.E.; Duerkop, B.A. Bacteriophage resistance alters antibiotic-mediated intestinal expansion of enterococci. Infect. Immun. 2019, 87, e00085-19. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khalifa, L.; Shlezinger, M.; Beyth, S.; Houri-Haddad, Y.; Coppenhagen-Glazer, S.; Beyth, N.; Hazan, R. Phage therapy against enterococcus faecalis in dental root canals. J. Oral Microbiol. 2016, 8, 32157. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tagliaferri, T.L.; Jansen, M.; Horz, H.-P. Fighting pathogenic bacteria on two fronts: Phages and antibiotics as combined strategy. Front. Cell. Infect. Microbiol. 2019, 9, 22. [Google Scholar] [CrossRef] [PubMed]
- Carmine, A.A.; Brogden, R.N.; Heel, R.C.; Speight, T.M.; Avery, G.S. Cefotaxime A review of its antibacterial activity, pharmacological properties and therapeutic use. Drugs 1983, 25, 223–289. [Google Scholar] [CrossRef]
- Moellering, R.C., Jr.; Eliopoulos, G.M. Activity of cefotaxime against Enterococci. Diagn. Microbiol. Infect. Dis. 1984, 2, 85S–90S. [Google Scholar]
- Gondil, V.S.; Harjai, K.; Chhibber, S. Endolysins as emerging alternative therapeutic agents to counter drug-resistant infections. Int. J. Antimicrob. Agents 2020, 55, 105844. [Google Scholar] [CrossRef]
- Abdelkader, K.; Gerstmans, H.; Saafan, A.; Dishisha, T.; Briers, Y. The preclinical and clinical progress of bacteriophages and their lytic enzymes: The parts are easier than the whole. Viruses 2019, 11, 96. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliveira, H.; São-José, C.; Azeredo, J. Phage-derived peptidoglycan degrading enzymes: Challenges and future prospects for in vivo therapy. Viruses 2018, 10, 292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, M.; Zhang, Y.; Li, X.; Liang, J.; Hu, L.; Gong, P.; Zhang, L.; Cai, R.; Zhang, H.; Ge, J. Endolysin LysEF-P10 shows potential as an alternative treatment strategy for multidrug-resistant enterococcus faecalis infections. Sci. Rep. 2017, 7, 10164. [Google Scholar] [CrossRef] [Green Version]
- Proença, D.; Fernandes, S.; Leandro, C.; Silva, F.A.; Santos, S.; Lopes, F.; Mato, R.; Cavaco-Silva, P.; Pimentel, M.; São-José, C. Phage endolysins with broad antimicrobial activity against enterococcus faecalis clinical strains. Microb. Drug Resist. 2012, 18, 322–332. [Google Scholar] [CrossRef]
- Serrano-Maldonado, C.E.; García-Cano, I.; González-Canto, A.; Ruiz-May, E.; Elizalde-Contreras, J.M.; Quirasco, M. Cloning and characterization of a novel N-acetylglucosaminidase (AtlD) from Enterococcus faecalis. J. Mol. Microbiol. Biotechnol. 2018, 28, 14–27. [Google Scholar] [CrossRef]
- Stevens, R.H.; Zhang, H.; Sedgley, C.; Bergman, A.; Manda, A.R. The prevalence and impact of lysogeny among oral isolates of Enterococcus faecalis. J. Oral Microbiol. 2019, 11, 1643207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gong, P.; Cheng, M.; Li, X.; Jiang, H.; Yu, C.; Kahaer, N.; Li, J.; Zhang, L.; Xia, F.; Hu, L.; et al. Characterization of Enterococcus faecium bacteriophage IME-EFm5 and its endolysin LysEFm5. Virology 2016, 492, 11–20. [Google Scholar] [CrossRef]
- Lu, N.; Sun, Y.; Wang, Q.; Qiu, Y.; Chen, Z.; Wen, Y.; Wang, S.; Song, Y. Cloning and characterization of endolysin and holin from Streptomyces avermitilis bacteriophage phiSASD1 as potential novel antibiotic candidates. Int. J. Biol. Macromol. 2020, 147, 980–989. [Google Scholar] [CrossRef]
- Kim, N.-H.; Park, W.B.; Cho, J.E.; Choi, Y.J.; Choi, S.J.; Jun, S.Y.; Kang, C.K.; Song, K.-H.; Choe, P.G.; Bang, J.-H.; et al. Effects of phage endolysin SAL200 combined with antibiotics on staphylococcus aureus infection. Antimicrob. Agents Chemother. 2018, 62, e00731-18. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y. Bacteriophage-derived endolysins applied as potent biocontrol agents to enhance food safety. Microorganisms 2020, 8, 724. [Google Scholar] [CrossRef] [PubMed]
- Korea National Research Resources Center. Available online: http://knrrc.swu.ac.kr/sale/sale.jsp (accessed on 3 March 2021).
- Lee, K.; Lee, K.-M.; Kim, D.; Yoon, S.S. Molecular determinants of the thickened matrix in a dual-species pseudomonas aeruginosa and enterococcus faecalis biofilm. Appl. Environ. Microbiol. 2017, 83, e01182-17. [Google Scholar] [CrossRef] [Green Version]
- Kropinski, A.M.; Mazzocco, A.; Waddell, T.E.; Lingohr, E.; Johnson, R.P. Enumeration of bacteriophages by double agar overlay plaque assay. In NMR-Based Metabolomics; Springer: Berlin/Heidelberg, Germany, 2009; Volume 501, pp. 69–76. [Google Scholar]
- Kim, M.S.; Kim, Y.D.; Hong, S.S.; Park, K.; Ko, K.S.; Myung, H. Phage-encoded colanic acid-degrading enzyme permits lytic phage infection of a capsule-forming resistant mutant escherichia coli strain. Appl. Environ. Microbiol. 2015, 81, 900–909. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, H.; Lei, R.; Ding, S.-W.; Zhu, S. Skewer: A fast and accurate adapter trimmer for next-generation sequencing paired-end reads. BMC Bioinform. 2014, 15, 182. [Google Scholar] [CrossRef] [PubMed]
- Allam, A.; Kalnis, P.; Solovyev, V. Karect: Accurate correction of substitution, insertion and deletion errors for next-generation sequencing data. Bioinformatics 2015, 31, 3421–3428. Available online: https://github.com/aminallam/karect (accessed on 3 March 2021). [CrossRef] [Green Version]
- Baaijens, J.A.; El Aabidine, A.Z.; Rivals, E.; Schönhuth, A. De novo assembly of viral quasispecies using overlap graphs. Genome Res. 2017, 27, 835–848. [Google Scholar] [CrossRef] [Green Version]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Pathosystems Resource Integration Center. Available online: https://www.patricbrc.org/ (accessed on 3 March 2021).
- Enzymatic Assay of Amidase (EC3.5.1.4). Available online: https://www.sigmaaldrich.com/content/dam/sigma-aldrich/docs/Sigma/General_Information/2/amidase.pdf (accessed on 3 March 2021).
- CLSI. M07: Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically, 11th ed.; CLSI: Wayne, PA, USA, 2018. [Google Scholar]
- Lowe, T.M.; Chan, P.P. tRNAscan-SE On-line: Search and contextual analysis of transfer RNA genes. Nucl. Acids Res. 2016, 44, W54–W57. [Google Scholar] [CrossRef]
- Hatfull, G.F.; Hendrix, R.W. Bacteriophages and their genomes. Curr. Opin. Virol. 2011, 1, 298–303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McKenney, P.T.; Yan, J.; Vaubourgeix, J.; Becattini, S.; Lampen, N.; Motzer, A.; Larson, P.J.; Dannaoui, D.; Fujisawa, S.; Xavier, J.B.; et al. Intestinal bile acids induce a morphotype switch in vancomycin-resistant enterococcus that facilitates intestinal colonization. Cell Host Microbe 2019, 25, 695–705.e5. [Google Scholar] [CrossRef]
- Ghosh, A.; Borst, L.; Stauffer, S.H.; Suyemoto, M.; Moisan, P.; Zurek, L.; Gookin, J.L. Mortality in kittens is associated with a shift in ileum mucosa-associated enterococci from enterococcus hirae to biofilm-forming enterococcus faecalis and adherent escherichia coli. J. Clin. Microbiol. 2013, 51, 3567–3578. [Google Scholar] [CrossRef] [Green Version]
- Shridhar, S.; Dhanashree, B. Antibiotic susceptibility pattern and biofilm formation in clinical isolates of enterococcus spp. Interdiscip. Perspect. Infect. Dis. 2019, 2019, 7854968. [Google Scholar] [CrossRef] [Green Version]
- Duan, Y.; Llorente, C.; Lang, S.; Brandl, K.; Chu, H.; Jiang, L.; White, R.C.; Clarke, T.H.; Nguyen, K.; Torralba, M.; et al. Bacteriophage targeting of gut bacterium attenuates alcoholic liver disease. Nature 2019, 575, 505–511. [Google Scholar] [CrossRef] [PubMed]
- São-José, C. Engineering of phage-derived lytic enzymes: Improving their potential as antimicrobials. Antibiotics 2018, 7, 29. [Google Scholar] [CrossRef] [Green Version]
- Schmelcher, M.; Donovan, D.M.; Loessner, M.J. Bacteriophage endolysins as novel antimicrobials. Futur. Microbiol. 2012, 7, 1147–1171. [Google Scholar] [CrossRef] [Green Version]
- Ding, Y.; Zhang, Y.; Huang, C.; Wang, J.; Wang, X. An endolysin LysSE24 by bacteriophage LPSE1 confers specific bactericidal activity against multidrug-resistant salmonella strains. Microorganisms 2020, 8, 737. [Google Scholar] [CrossRef]
- Swift, S.M.; Rowley, D.T.; Young, C.; Franks, A.; Hyman, P.; Donovan, D.M. The endolysin from the Enterococcus faecalis bacteriophage VD13 and conditions stimulating its lytic activity. FEMS Microbiol. Lett. 2016, 363, fnw216. [Google Scholar] [CrossRef] [Green Version]
- Lu, S.-H.; Bischoff, K.M.; Rich, J.O.; Liu, S.; Skory, C.D. Recombinant bacteriophage LysKB317 endolysin mitigates Lactobacillus infection of corn mash fermentations. Biotechnol. Biofuels 2020, 13, 157. [Google Scholar] [CrossRef]
- Grygorcewicz, B.; Roszak, M.; Golec, P.; Śleboda-Taront, D.; Łubowska, N.; Górska, M.; Jursa-Kulesza, J.; Rakoczy, R.; Wojciuk, B.; Dołęgowska, B. Antibiotics act with vB_AbaP_AGC01 phage against acinetobacter baumannii in human heat-inactivated plasma blood and galleria mellonella models. Int. J. Mol. Sci. 2020, 21, 4390. [Google Scholar] [CrossRef]
- Letrado, P.; Corsini, B.; Díez-Martínez, R.; Bustamante, N.; Yuste, J.E.; García, P. Bactericidal synergism between antibiotics and phage endolysin Cpl-711 to kill multidrug-resistant pneumococcus. Futur. Microbiol. 2018, 13, 1215–1223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cha, Y.; Son, B.; Ryu, S. Effective removal of staphylococcal biofilms on various food contact surfaces by Staphylococcus aureus phage endolysin LysCSA13. Food Microbiol. 2019, 84, 103245. [Google Scholar] [CrossRef] [PubMed]
- Pennone, V.; Sanz-Gaitero, M.; O’Connor, P.; Coffey, A.; Jordan, K.; Van Raaij, M.J.; McAuliffe, O.; Gaitero, S.-; Connor, O.; Raaij, V. Inhibition of L. monocytogenes biofilm formation by the amidase domain of the phage vB_LmoS_293 endolysin. Viruses 2019, 11, 722. [Google Scholar] [CrossRef] [Green Version]
- Zhang, H.; Buttaro, B.A.; Fouts, D.E.; Sanjari, S.; Evans, B.S.; Stevens, R.H. Bacteriophage φEf11 ORF28 endolysin, a multifunctional lytic enzyme with properties distinct from all other identified enterococcus faecalis phage endolysins. Appl. Environ. Microbiol. 2019, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melo, L.D.R.; Brandão, A.; Akturk, E.; Santos, S.B.; Azeredo, J. Characterization of a new staphylococcus aureus kayvirus harboring a lysin active against biofilms. Viruses 2018, 10, 182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Idelevich, E.A.; Knaack, D.; Nugroho, N.T.; Peters, G.; Bisdas, T.; Molinaro, S.; Torsello, G.B.; Becker, K.; Herten, M. Comparative in vitro activity of bacteriophage endolysin HY-133 against Staphylococcus aureus attached to vascular graft surface. Med. Microbiol. Immunol. 2020, 209, 51–57. [Google Scholar] [CrossRef]
- Olsen, N.M.C.; Thiran, E.; Hasler, T.; Vanzieleghem, T.; Belibasakis, G.N.; Mahillon, J.; Loessner, M.J.; Schmelcher, M. Synergistic removal of static and dynamic staphylococcus aureus biofilms by combined treatment with a bacteriophage endolysin and a polysaccharide depolymerase. Viruses 2018, 10, 438. [Google Scholar] [CrossRef] [Green Version]
- Yan, Z.; Huang, M.; Melander, C.; Kjellerup, B.V. Dispersal and inhibition of biofilms associated with infections. J. Appl. Microbiol. 2019, 128, 1279–1288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abedon, S.T. Phage-antibiotic combination treatments: Antagonistic impacts of antibiotics on the pharmacodynamics of phage therapy? Antibiotics 2019, 8, 182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.; Im, J.; Na, H.; Ryu, S.; Yun, C.-H.; Han, S.H. The novel enterococcus phage vB_EfaS_HEf13 has broad lytic activity against clinical isolates of enterococcus faecalis. Front. Microbiol. 2019, 10, 2877. [Google Scholar] [CrossRef] [PubMed]

| Antibiotic (μg/mL) | Enterococcus faecalis Strains | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| CCARM 5511 * | CCARM 5518 | CCARM 5520 | CCARM 5526 | CCARM 5537 | CCARM 5539 | CCARM 5548 | CCARM 5568 | CCARM 5569 | |
| Ampicillin | 4 | 4 | 4 | 4 | 4 | 4 | 4 | ||
| Ciprofloxacin | ≥2 | ≤1 | ≥2 | ≥2 | ≤1 | ≥2 | ≥2 | 1 | ≥2 |
| Erythromycin | ≥4 | ≥4 | ≥4 | ≥4 | ≥4 | ||||
| Gentamycin | ≥500 | ≥500 | ≥500 | ≥500 | ≥500 | ≥500 | ≥500 | ≥500 | ≥500 |
| Levofloxacin | ≥4 | 2 | ≥4 | ≥4 | ≤1 | ≥4 | ≥4 | ≥2 | ≥4 |
| Linezolid | ≤2 | ≤2 | ≤2 | ≤2 | ≤2 | ≤2 | ≤2 | ≤1 | ≤1 |
| Penicillin | ≥8 | 8 | ≥8 | 8 | 8 | ≥8 | ≥8 | 8 | ≥8 |
| Rifampin | ≥2 | ≤1 | ≤1 | ≤1 | ≥2 | ≥2 | ≤1 | ≤1 | ≤1 |
| Streptomycin | ≥1000 | ≤1000 | ≤1000 | ≤1000 | ≥1000 | ≥1000 | ≤1000 | ≤1000 | ≤1000 |
| Quinupristin- dalfopristin | ≥2 | ≥2 | ≥2 | ≥2 | ≥2 | ≥2 | ≥2 | ≥2 | ≥2 |
| Teicoplanin | ≤4 | ≤4 | ≤4 | ≤4 | ≤4 | ≤4 | ≤4 | ≤1 | ≤1 |
| Tetracycline | ≥8 | ≥8 | ≥8 | ≥8 | ≥8 | ≥8 | ≥8 | ≥8 | ≥8 |
| Vancomycin | 2 | 1 | 2 | 2 | 2 | 2 | 2 | 2 | 2 |
| E. faecalis Strain | Phage PBEF129 | Endolysin |
|---|---|---|
| CCARM5511 | − | + |
| CCARM5518 | + | + |
| CCARM5520 | + | + |
| CCARM5526 | + | + |
| CCARM5537 | − | + |
| CCARM5539 | + | + |
| CCARM5548 | + | + |
| CCARM5568 | + | + |
| CCARM5569 | + | + |
| CCARM5571 | + | + |
| ATCC19433 | + | + |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oh, H.K.; Hwang, Y.J.; Hong, H.W.; Myung, H. Comparison of Enterococcus faecalis Biofilm Removal Efficiency among Bacteriophage PBEF129, Its Endolysin, and Cefotaxime. Viruses 2021, 13, 426. https://doi.org/10.3390/v13030426
Oh HK, Hwang YJ, Hong HW, Myung H. Comparison of Enterococcus faecalis Biofilm Removal Efficiency among Bacteriophage PBEF129, Its Endolysin, and Cefotaxime. Viruses. 2021; 13(3):426. https://doi.org/10.3390/v13030426
Chicago/Turabian StyleOh, Hyun Keun, Yoon Jung Hwang, Hye Won Hong, and Heejoon Myung. 2021. "Comparison of Enterococcus faecalis Biofilm Removal Efficiency among Bacteriophage PBEF129, Its Endolysin, and Cefotaxime" Viruses 13, no. 3: 426. https://doi.org/10.3390/v13030426
APA StyleOh, H. K., Hwang, Y. J., Hong, H. W., & Myung, H. (2021). Comparison of Enterococcus faecalis Biofilm Removal Efficiency among Bacteriophage PBEF129, Its Endolysin, and Cefotaxime. Viruses, 13(3), 426. https://doi.org/10.3390/v13030426






