A Retrospective Cohort Study of Young Women Spontaneously Choosing to Be Vaccinated against HPV: Outcomes from Their First Cervical Cancer Screening Test
Abstract
1. Introduction
2. Methods
2.1. Setting
2.2. Study Design
2.3. Statistical Analysis
2.4. Ethics
3. Results
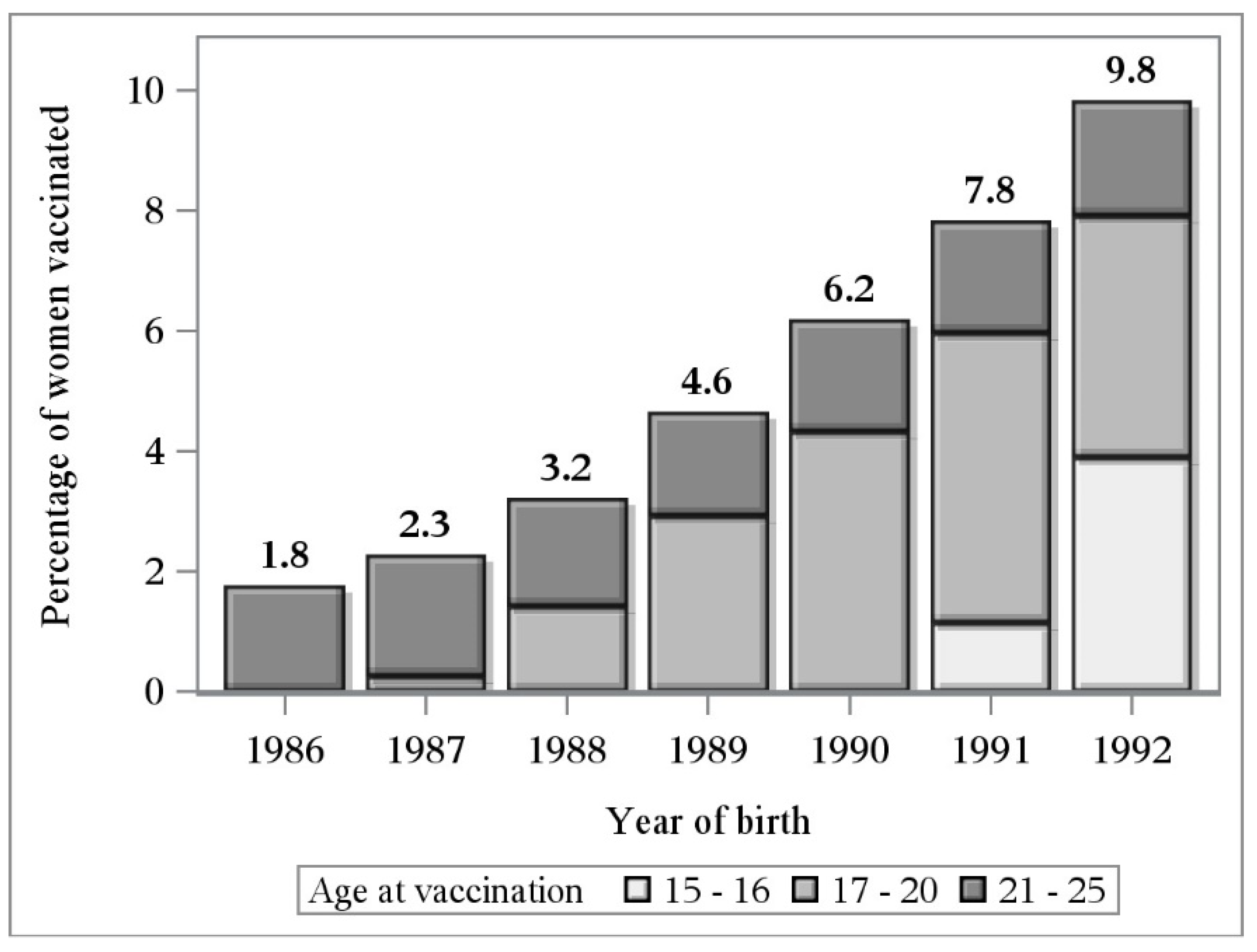
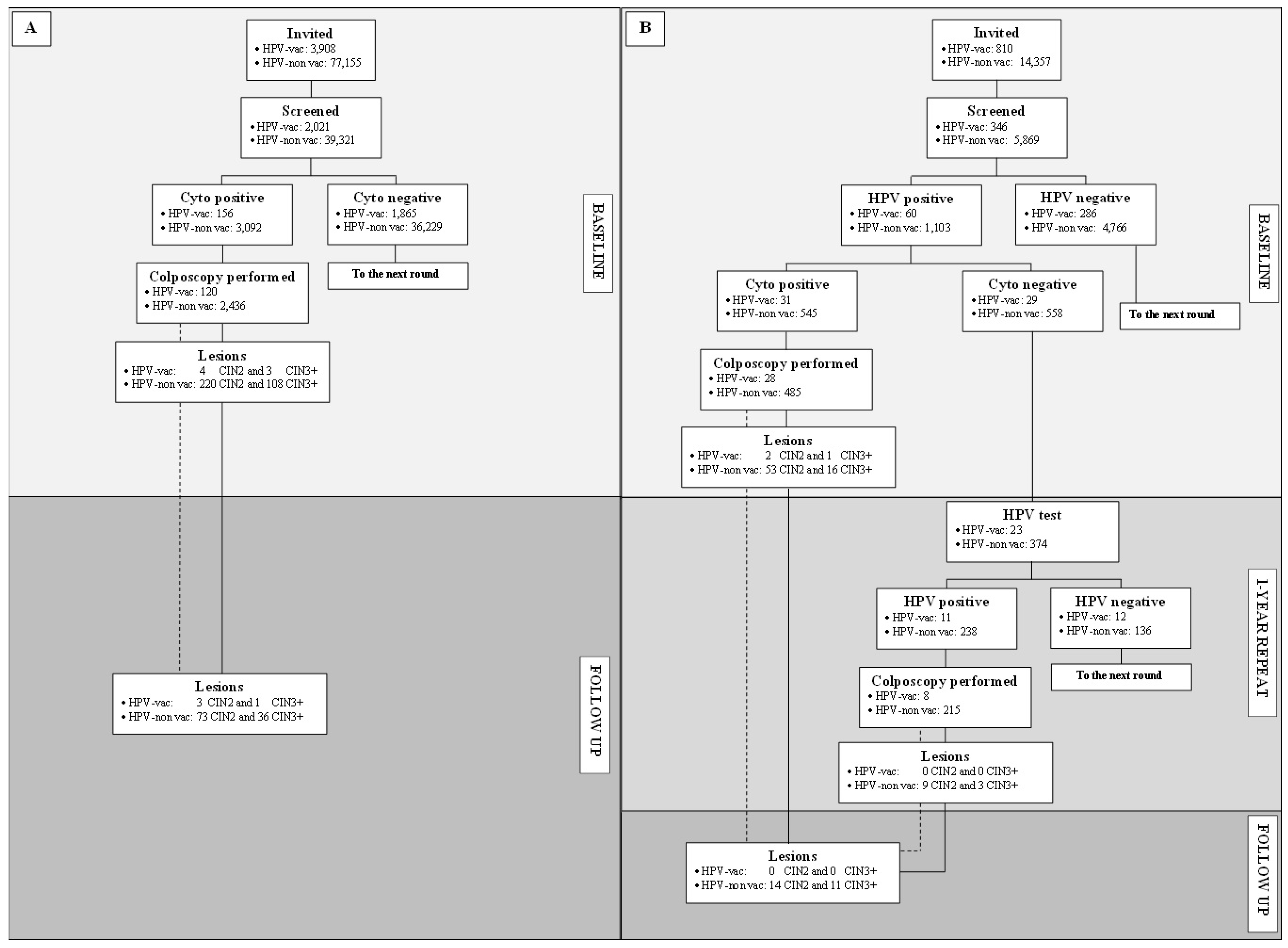
3.1. Study Cohorts
3.2. Compliance with Invitation
3.3. Overall Detection of CIN3+, CIN2+, and Cancer
3.4. Results of Cytology-Based Screening
3.5. Results of HPV-Based Screening
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gravitt, P.E.; Winer, R.L. Natural history of HPV infection across the lifespan: Role of latency. Viruses 2017, 9, 267. [Google Scholar] [CrossRef] [PubMed]
- Serraino, D.; Gini, A.; Taborelli, M.; Ronco, G.; Giorgi-Rossi, P.; Zappa, M.; Crocetti, E.; Franzo, E.; Falcini, F.; Visioli, C.B.; et al. Changes in cervical cancer incidence followiong the introduction of organized screening in Italy. Prev. Med. 2015, 75, 56–63. [Google Scholar] [CrossRef] [PubMed]
- Ronco, G.; Dillner, J.; Elfström, K.M.; Tunesi, S.; Snijders, P.J.; Arbyn, M.; Kitchener, H.; Segnan, N.; Gilham, C.; Giorgi-Rossi, P.; et al. Efficacy of HPV-based screening for prevention of invasive cervical cancer: Follow-up of four European randomised controlled trials. Lancet 2014, 383, 524–532. [Google Scholar] [CrossRef]
- Castle, P.E.; Maza, M. Prophylactic HPV vaccination: Past, present, and future. Epidemiol. Infect. 2016, 144, 449–468. [Google Scholar] [CrossRef]
- Herwijer, E.; Sundstrom, K.; Ploner, A.; Uhnoo, I.; Sparen, P.; Arnheim-Dahlstrom, L. Quadrivalent HPV vaccine effectiveness against high-grade cervical lesions by age at vaccination: A population-based study. Int. J. Cancer. 2016, 138, 2867–2874. [Google Scholar] [CrossRef]
- World Health Organization. WHO Report Human papillomavirus vaccines: WHO position paper, May 2017–Recommendations. Vaccine 2017, 35, 5753–5755. [Google Scholar] [CrossRef]
- Arbyn, M.; Xu, L. Efficacy and safety of prophylactic HPV vaccines. A Cochrane review of randomized trials. Expert Rev. Vaccines 2018, 17, 1085–1091. [Google Scholar] [CrossRef] [PubMed]
- Drolet, M.; Benard, E.; Perez, N.; Brisson, M. HPV Vaccination Impact Study Group. Population-level impact and herd effects following the introduction of human papillomavirus vaccination programmes: Updated systematic review and meta-analysis. Lancet 2019, 394, 497–509. [Google Scholar] [CrossRef]
- Lei, J.L.; Ploner, A.; Elfstrom, K.M.; Wang, J.; Roth, A.; Fang, F.; Sundstrom, K.; Dillner, J.; Sparen, P. HPV vaccination and the risk of invasive cervical cancer. N. Engl. J. Med. 2020, 383, 1340–1348. [Google Scholar] [CrossRef]
- Giorgi Rossi, P.; Carozzi, F.; Federici, A.; Ronco, G.; Zappa, M.; Franceschi, S. The Italian Screening in HPV vaccinated girls Consensus Conference group. Cervical cancer screening in women vaccinated against human papillomavirus infection: Recommendations from a consensus conference. Prev. Med. 2017, 98, 21–30. [Google Scholar] [CrossRef]
- Ronco, G.; Biggeri, A.; Confortini, M.; Giorgi Rossi, P.; Naldoni, C.; Segnan, N.; Sideri, M.; Zappa, M.; Zorzi, M.; Calvia, M.; et al. Ricerca del DNA di papillomavirus umano (HPV) come test primario per lo screening dei precursori del cancro del collo uterino. HTA report. Epidemiol. Prev. 2012, 36, e1–e72. [Google Scholar]
- Zorzi, M.; Del Mistro, A.; Farruggio, A.; de’Bartolomeis, L.; Frayle-Salamanca, H.; Baboci, L.; Bertazzo, A.; Cocco, P.; Fedato, C.; Gennaro, M.; et al. Use of a high-risk human papillomavirus DNA test as the primary test in a cervical cancer screening programme: A population-based cohort study. Br. J. Obstet. Gynecol. 2013, 120, 1260–1268. [Google Scholar] [CrossRef]
- Maggino, T.; Sciarrone, R.; Murer, B.; Dei Rossi, M.R.; Fedato, C.; Maran, M.; Lorio, M.; Soldà, M.; Zago, F.; Giorgi Rossi, P.; et al. Screening women for cervical cancer carcinoma with a HPV mRNA test: First results from the Venice pilot program. Br. J. Cancer 2016, 115, 125–132. [Google Scholar] [CrossRef]
- Direzione Prevenzione, Sicurezza alimentare, Veterinaria—U.O. Prevenzione e Sanità Pubblica, Regione Veneto. Rapporto Attività Vaccinale. 2018. Available online: https://rdv.app.box.com/s/fddysxtt7yhoa95y76edo10dxxj2oa5 (accessed on 28 January 2021).
- Acuti Martellucci, C.; Nomura, S.; Yoneoka, D.; Ueda, P.; Brotherton, J.M.L.; Canfell, K.; Palmer, M.; Manzoli, L.; Giorgi Rossi, P.; De Togni, A.; et al. Human papillomavirus vaccine effectiveness within a cervical cancer screening programme: Cohort study. Br. J. Obstet. Gynecol. 2021, 128, 532–539. [Google Scholar] [CrossRef] [PubMed]
- Palmer, T.J.; McFadden, M.; Pollock, K.G.J.; Kavanagh, K.; Cuschieri, K.; Cruickshank, M.; Nicoll, S.; Robertson, C. HPV immunization and increased uptake of cervical screening in Scottish women; observational study of routinely collected national data. Br. J. Cancer 2016, 114, 576–581. [Google Scholar] [CrossRef]
- Kreush, T.; Wang, J.; Sparen, P.; Sundstrom, K. Opportunistic HPV vaccination at age 16–23 and cervical screening attendance in Sweden: A national register-based cohort study. BMJ Open 2018, 8, e024477. [Google Scholar] [CrossRef]
- Kann, H.; Hortlund, M.; Eklund, C.; Dillner, J.; Faust, H. Human papillomavirus types in cervical dysplasia among young HPV-vaccinated women: Population-based nested case-control study. Int. J. Cancer 2020, 146, 2539–2546. [Google Scholar] [CrossRef]
- Acampora, A.; Grossi, A.; Barbara, A.; Colamesta, V.; Causio, F.A.; Calabrò, G.E.; Boccia, S.; de Waure, C. Increasing HPV vaccination uptake among adolescents: A systematic review. Int. J. Environ. Res. Public Health 2020, 17, 7997. [Google Scholar] [CrossRef]
- Mariani, L.; Preti, M.; Cristoforoni, P.; Stigliano, C.M.; Perino, A. Overview of the benefits and potential issues of the nonavalent HPV vaccine. Int. J. Gynecol. Obstet. 2017, 136, 258–265. [Google Scholar] [CrossRef]
- Sundstrom, K.; Herweijer, E.; Wang, J. Cervical screening in high-income countries: The need for quality assurance, adjunct biomarkers and rational adaptation to HPV vaccination. Prev. Med. 2020, 106382. [Google Scholar] [CrossRef]
- Lew, J.B.; Simms, K.T.; Smith, M.A.; Hall, M.; Kang, Y.J.; Xu, X.M.; Caruana, M.; Velentzis, L.S.; Bessell, T.; Saville, M.; et al. Primary HPV testing versus cytology-based cervical screening in women in Australia vaccinated for HPV and unvaccinated: Effectiveness and economic assessment for the National Cervical Screening Program. Lancet Public Health 2017, 2, e96–e107. [Google Scholar] [CrossRef]
- Lei, J.; Ploner, A.; Lehtinen, M.; Sparen, P.; Dillner, J.; Elfstrom, K.M. Impact of HPV vaccination on cervical screening performance: A population-based cohort study. Br. J. Cancer 2020, 123, 155–160. [Google Scholar] [CrossRef]
| HPV Vaccinated | HPV Non-Vaccinated | All | p-Value 1 | |
|---|---|---|---|---|
| Women invited | 4718 | 91,512 | 96,230 | |
| Screening test proposed, n (%) Cytology HPV | 3908 (82.8) 810 (17.2) | 77,155 (84.3) 14,357 (15.7) | 81,063 (84.2) 15,167 (15.8) | |
| Screened women, n (%) | 2367 (50.2) | 45,190 (49.4) | 47,557 (49.4) | 0.3 |
| Undelivered invitations, n (%) | 31 (0.7) | 1248 (1.4) | 1279 (1.3) | <0.0001 |
| Not screened: referred a recent cytology, n (%) | 661 (14) | 8092 (8.8) | 8753 (9.1) | <0.0001 |
| Crude 2 compliance, % | 50.5 | 50.1 | 50.1 | 0.57 |
| Adjusted 3 compliance, % Cytology HPV | 60.8 62.3 53.4 | 56.6 58.2 48 | 56.8 58.4 48.3 | <0.0001 <0.0001 0.01 |
| Age at Vaccination (Years) | 15–16 | 17–20 | 21–25 | p-Value 1 |
|---|---|---|---|---|
| Number of screened women | 302 | 1210 | 855 | |
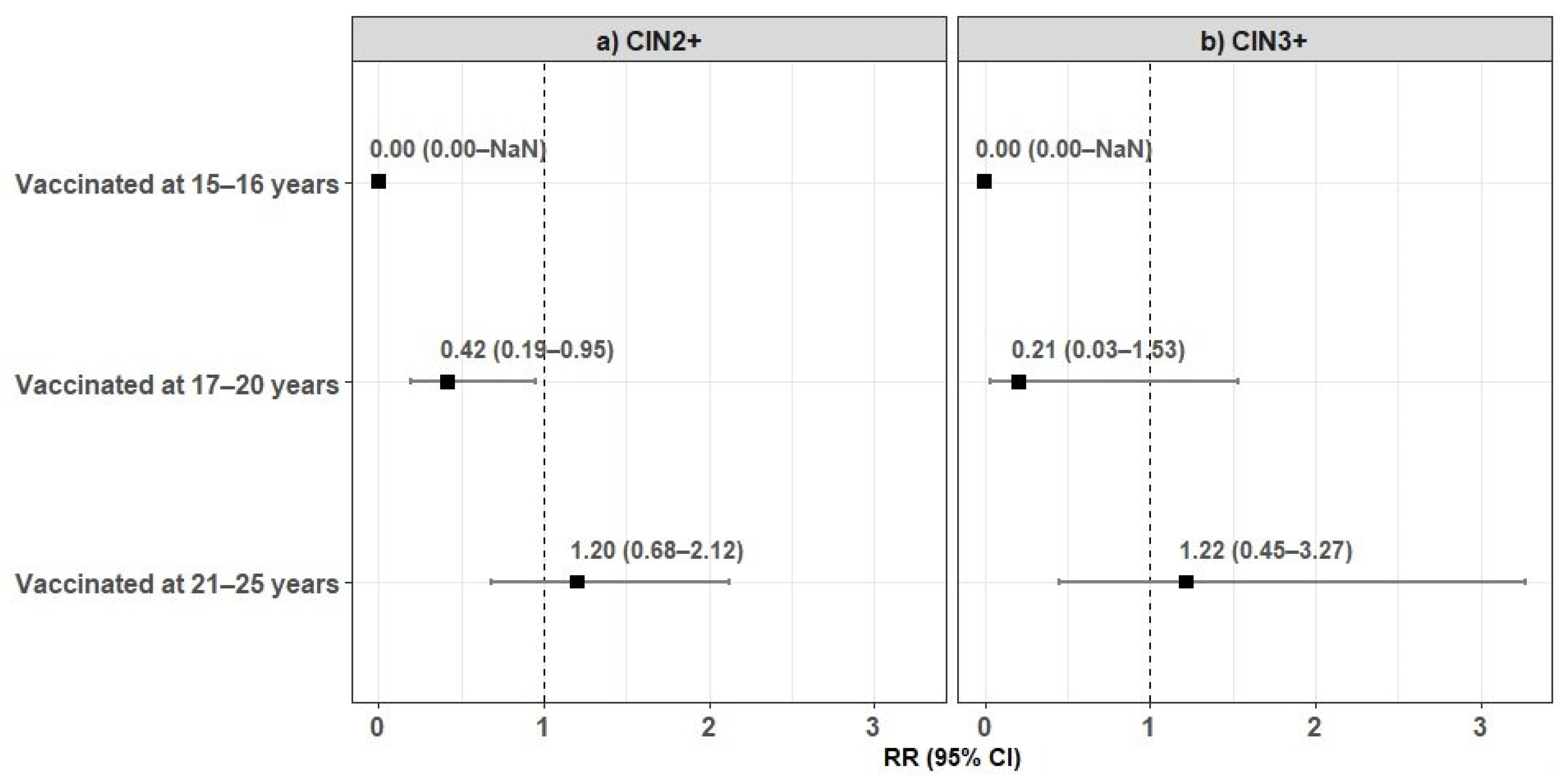
| CIN2 × 1000 screened women (n) | 0 (0) | 4.13 (5) | 9.36 (8) | 0.12 |
| p-value 2 | 0.18 | 0.18 | 0.56 | |
| CIN3+ × 1000 screened women (n) | 0 (0) | 0.83 (1) | 4.68 (4) | 0.17 |
| p-value 2 | 0.63 | 0.1 | 0.58 | |
| CIN2+ × 1000 screened women (n) | 0 (0) | 4.96 (6) | 14.04 (12) | 0.02 |
| p-value 2 | 0.05 | 0.03 | 0.52 |
| HPV Vaccinated | HPV Non-Vaccinated | All | Relative Risk (95% CI) 1 | p-Value 2 | |
|---|---|---|---|---|---|
| Cytology-Based Screening | |||||
| Women screened | 2021 | 39,321 | 41,342 | ||
| Positive cytology % (n) | 7.7 (156) | 7.9 (3092) | 7.9 (3248) | 1.0 (0.8–1.1) | 0.86 |
| Low grade cytology % (n) | 7.1 (143) | 7.2 (2819) | 7.2 (2962) | 1 (0.8–1.2) | 0.91 |
| High grade cytology % (n) | 0.6 (13) | 0.7 (273) | 0.7 (286) | 0.9 (0.5–1.6) | 0.89 |
| CIN3+ detection ‰ at baseline * (n) | 1.48 (3) | 2.75 (108) | 2.68 (111) | 0.5 (0.2–1.7) | 0.4 |
| CIN2+ detection ‰ at baseline * (n) | 5.44 (11) | 8.34 (328) | 8.2 (339) | 0.7 (0.4–1.2) | 0.2 |
| PPV for CIN3+ % (CIN3+ / colposcopies) | 2.5 (3/120) | 4.4 (108/2436) | 4.3 (111/2556) | 0.6 (0.2–1.7) | 0.49 |
| CIN3+ detection ‰ overall ° (n) | 1.98 (4) | 3.66 (144) | 3.58 (148) | 0.5 (0.2–1.5) | 0.33 |
| HPV Test-Based Screening | |||||
| Women screened | 346 | 5869 | 6215 | ||
| Positive HPV % (n) | 17.3 (60) | 18.8 (1103) | 18.7 (1163) | 0.9 (0.7–1.2) | 0.54 |
| Positive triage cytology among HPV+ % (n) | 9 (31) | 9.3 (545) | 9.3 (576) | 1 (0.7–1.4) | 0.91 |
| CIN3+ detection ‰ at baseline * (n) | 2.89 (1) | 2.73 (16) | 2.74 (17) | 1.1 (0.1–8.0) | 1 |
| CIN2+ detection ‰ at baseline * (n) | 8.67 (3) | 11.76 (69) | 11.58 (72) | 0.7 (0.2–2.3) | 0.8 |
| PPV for CIN3+ % (CIN3+ / colposcopies) | 3.6 (1/28) | 3.3 (16/485) | 3.3 (17/513) | 1.1 (0.1–7.9) | 1 |
| CIN3+ detection ‰ overall ° (n) | 2.89 (1) | 5.11 (30) | 4.98 (31) | 0.6 (0.1–4.1) | 1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Del Mistro, A.; Battagello, J.; Weis, L.; Bressan, V.; Selle, V.; Ramigni, M.; Dal Zotto, A.; Maggiolo, A.; Gori, S.; Frayle, H.; et al. A Retrospective Cohort Study of Young Women Spontaneously Choosing to Be Vaccinated against HPV: Outcomes from Their First Cervical Cancer Screening Test. Viruses 2021, 13, 486. https://doi.org/10.3390/v13030486
Del Mistro A, Battagello J, Weis L, Bressan V, Selle V, Ramigni M, Dal Zotto A, Maggiolo A, Gori S, Frayle H, et al. A Retrospective Cohort Study of Young Women Spontaneously Choosing to Be Vaccinated against HPV: Outcomes from Their First Cervical Cancer Screening Test. Viruses. 2021; 13(3):486. https://doi.org/10.3390/v13030486
Chicago/Turabian StyleDel Mistro, Annarosa, Jessica Battagello, Luca Weis, Vittoria Bressan, Vittorio Selle, Mauro Ramigni, Alessandra Dal Zotto, Antonio Maggiolo, Silvia Gori, Helena Frayle, and et al. 2021. "A Retrospective Cohort Study of Young Women Spontaneously Choosing to Be Vaccinated against HPV: Outcomes from Their First Cervical Cancer Screening Test" Viruses 13, no. 3: 486. https://doi.org/10.3390/v13030486
APA StyleDel Mistro, A., Battagello, J., Weis, L., Bressan, V., Selle, V., Ramigni, M., Dal Zotto, A., Maggiolo, A., Gori, S., Frayle, H., Zappa, M., Zorzi, M., & the Consensus Study Veneto Working Group. (2021). A Retrospective Cohort Study of Young Women Spontaneously Choosing to Be Vaccinated against HPV: Outcomes from Their First Cervical Cancer Screening Test. Viruses, 13(3), 486. https://doi.org/10.3390/v13030486






