Treatment with the Immunomodulator AIC649 in Combination with Entecavir Produces Antiviral Efficacy in the Woodchuck Model of Chronic Hepatitis B
Abstract
1. Introduction
2. Materials and Methods
2.1. Investigational Drugs
2.2. Animals
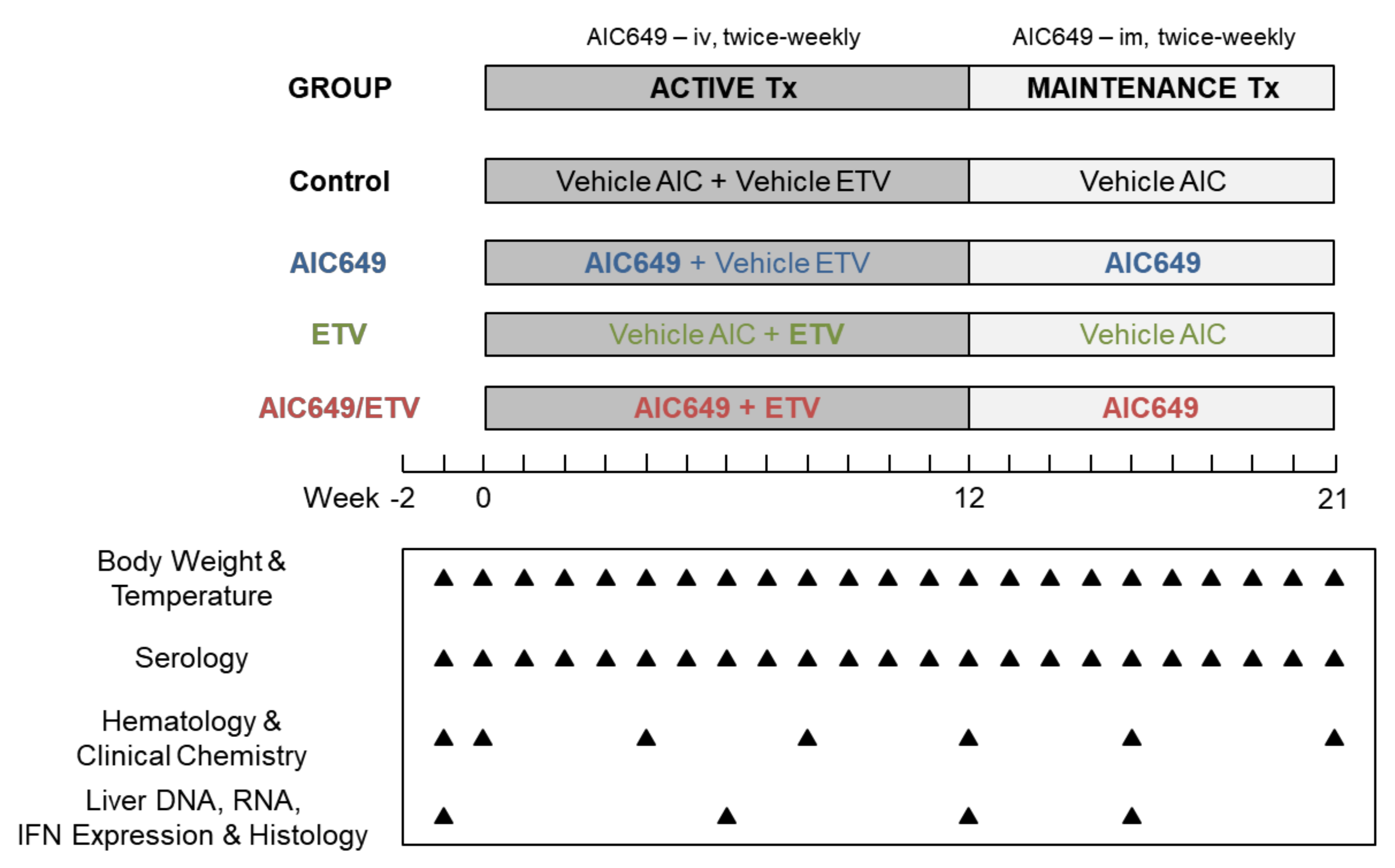
2.3. Study Design
2.4. Blood Collection
2.5. Liver Tissue Collection
2.6. Serum WHV Parameters
2.7. Liver WHV Parameters
2.8. Hematology and Clinical Chemistry Parameters
2.9. Liver IFN Expression
2.10. Liver Histology
2.11. Statistical Analysis
3. Results
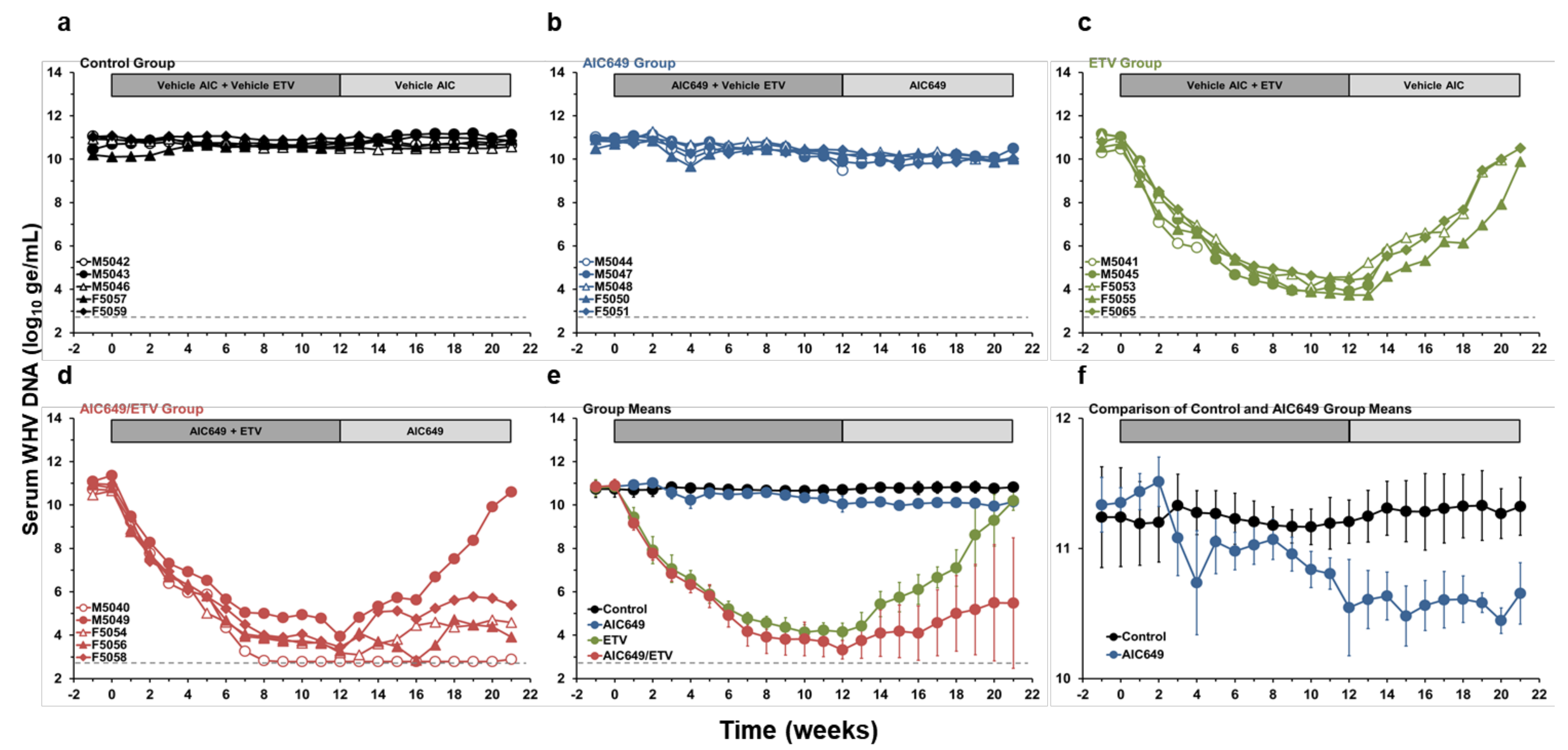
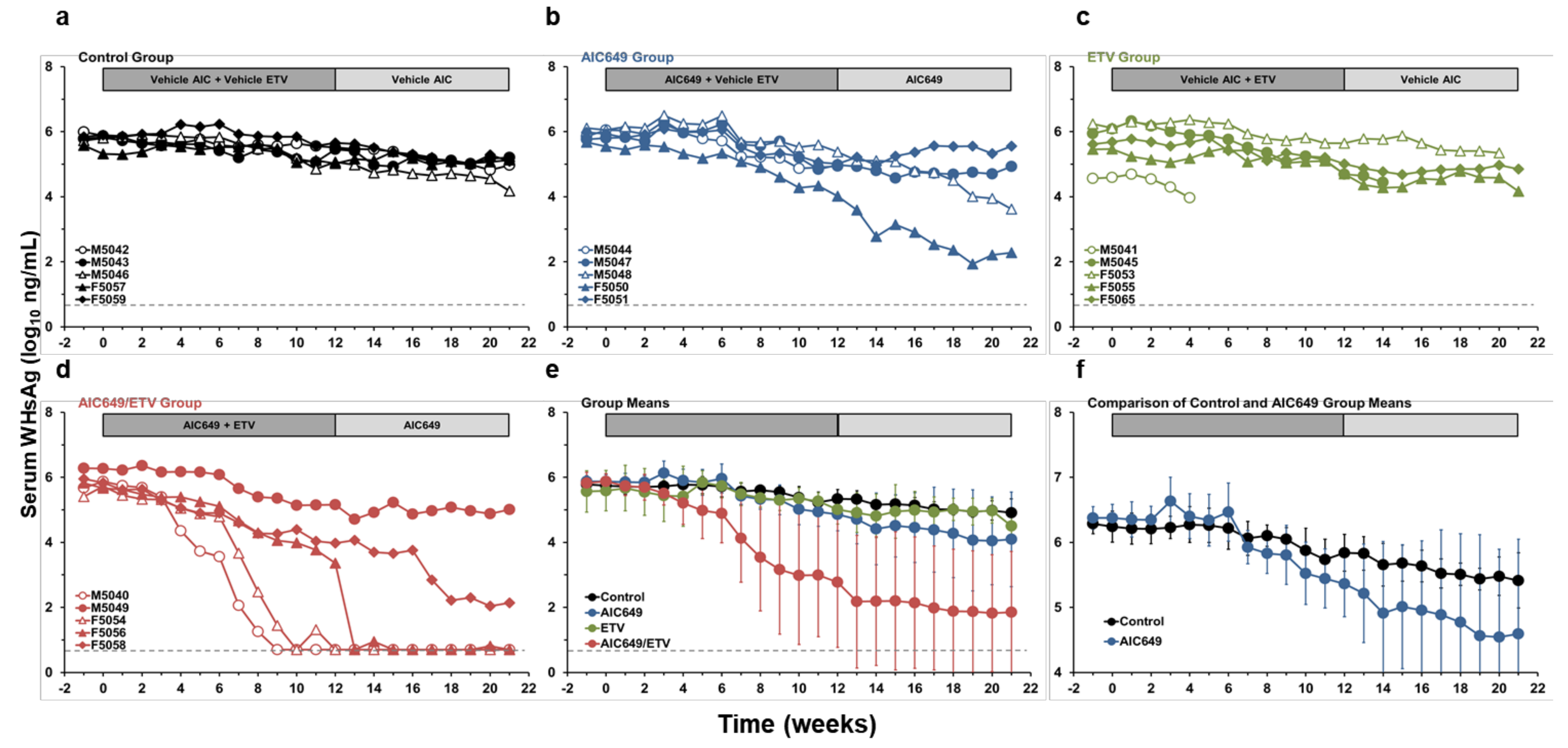
3.1. AIC649 Modestly Reduced Viremia and Antigenemia in the Periphery, but the Antiviral Efficacy was Enhanced in Combination with ETV
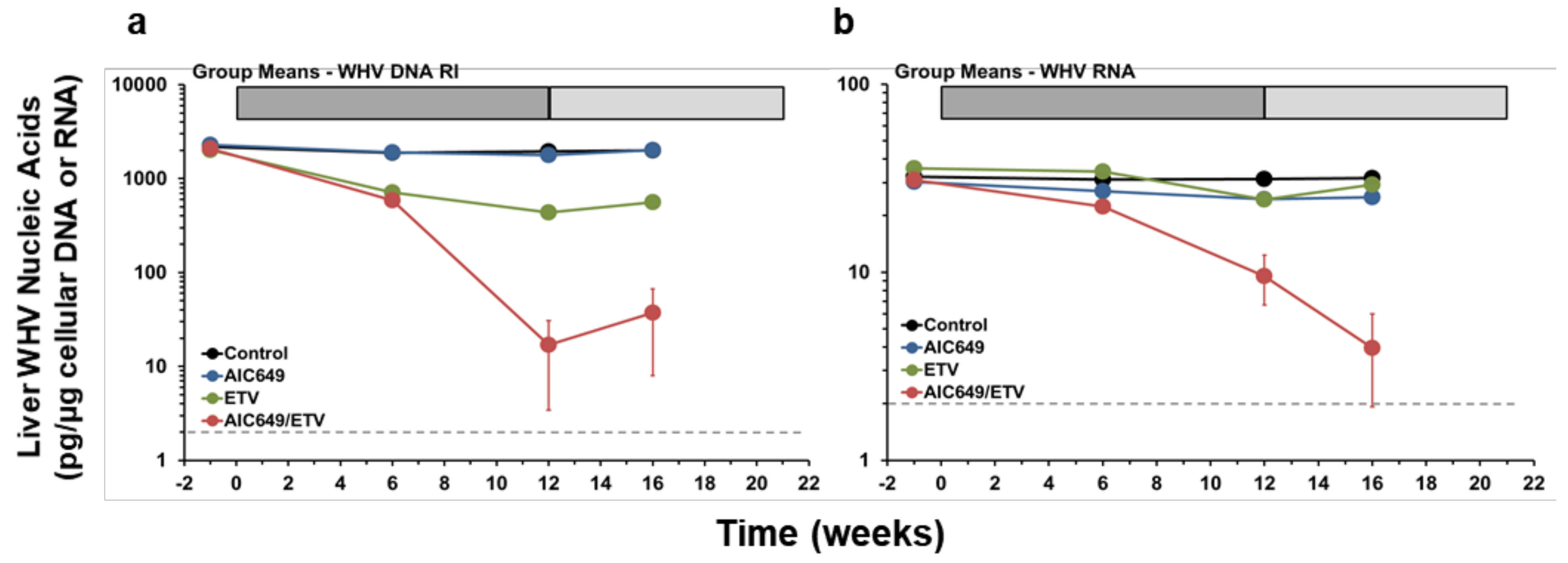
3.2. AIC649 Modestly Suppressed WHV Replication in the Liver, but the Antiviral Efficacy was Again Enhanced in Combination with ETV
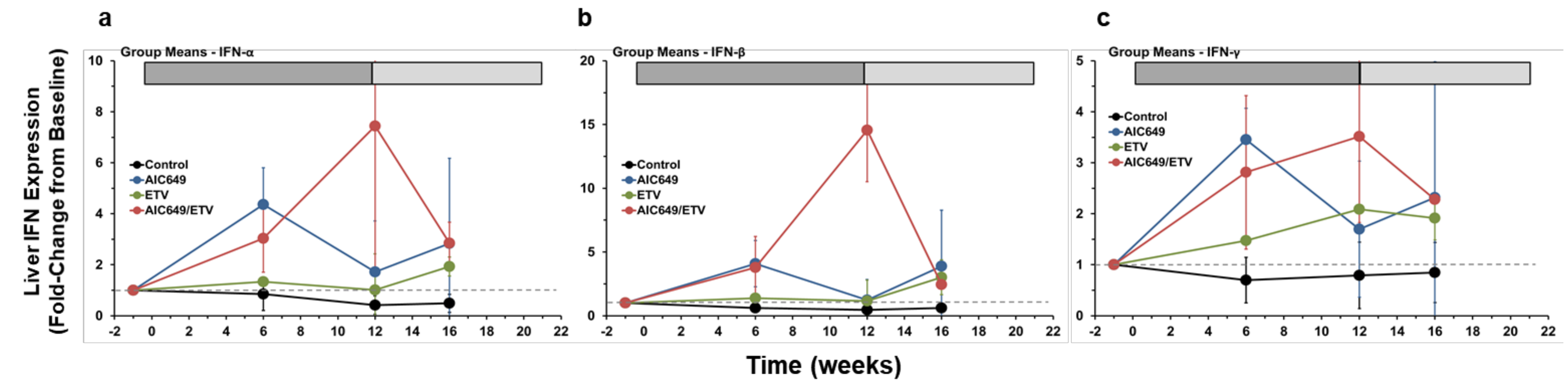
3.3. AIC649 Induced Type I and II IFNs in the Liver, but the Intrahepatic IFN Expression was Enhanced in Combination with ETV
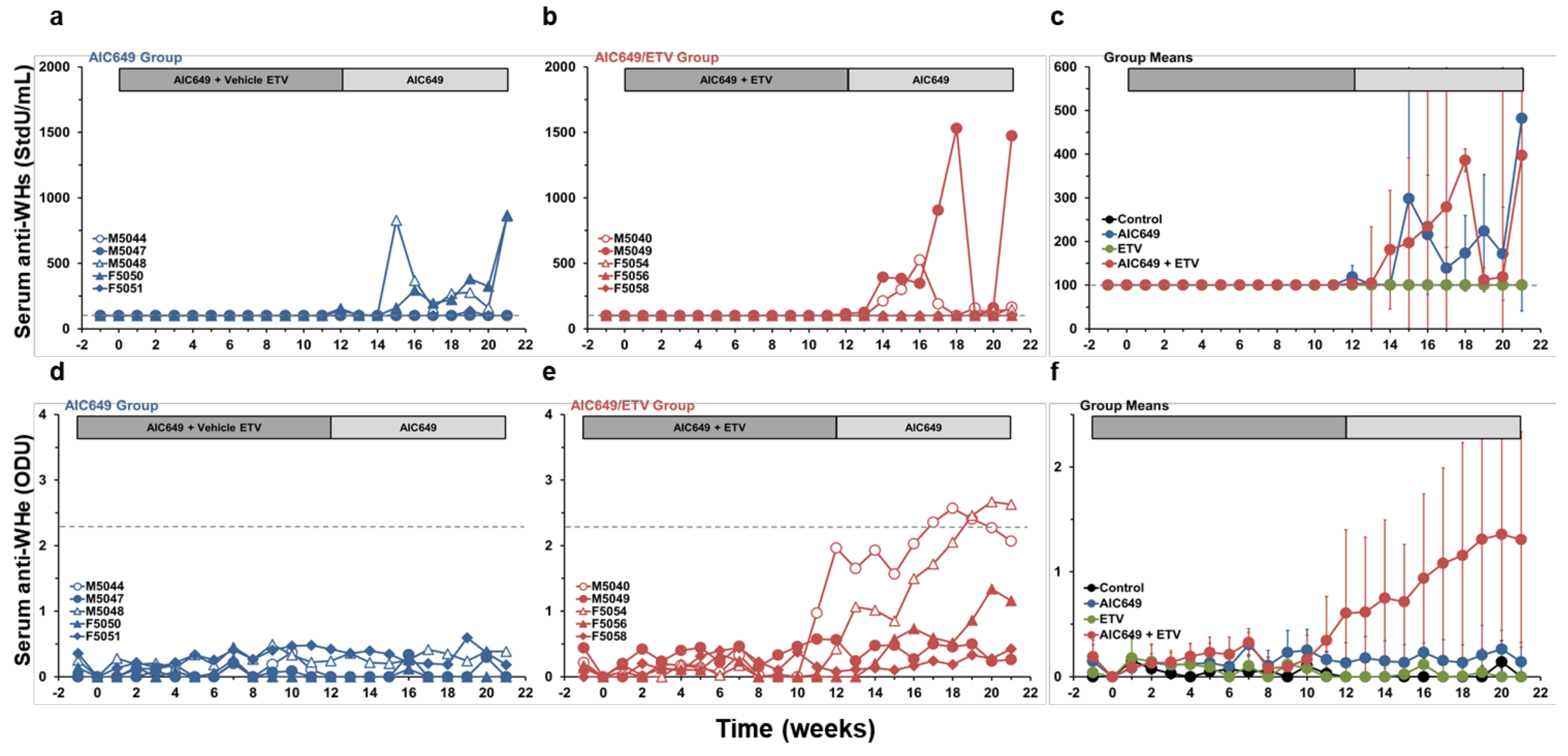
3.4. AIC649 Induced Anti-WHs Antibodies in the Periphery, but Anti-WHe Antibodies were Only Elicited in Combination with ETV
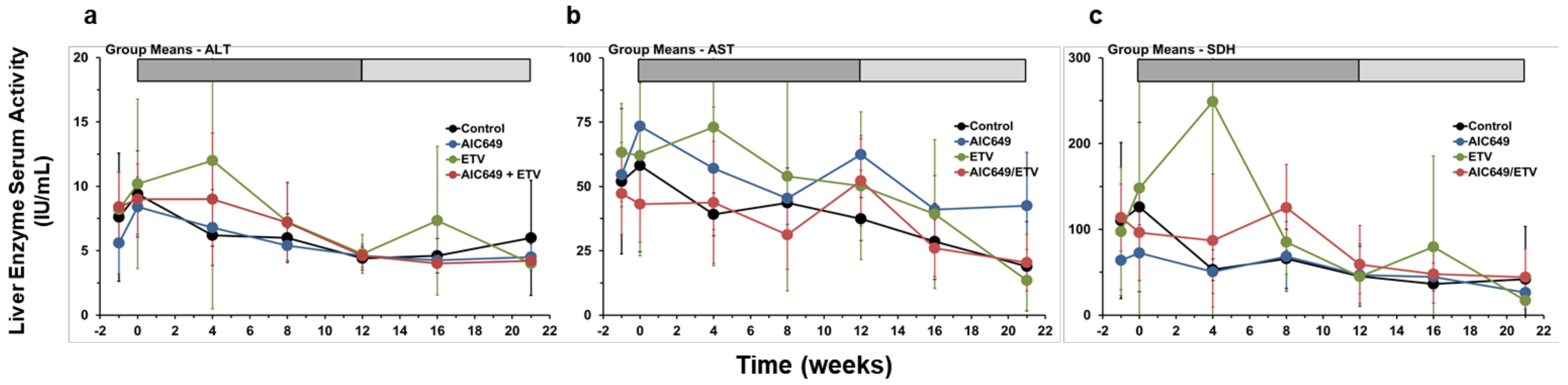
3.5. AIC649 Treatment, Alone and in Combination with ETV, was Well Tolerated in Woodchucks
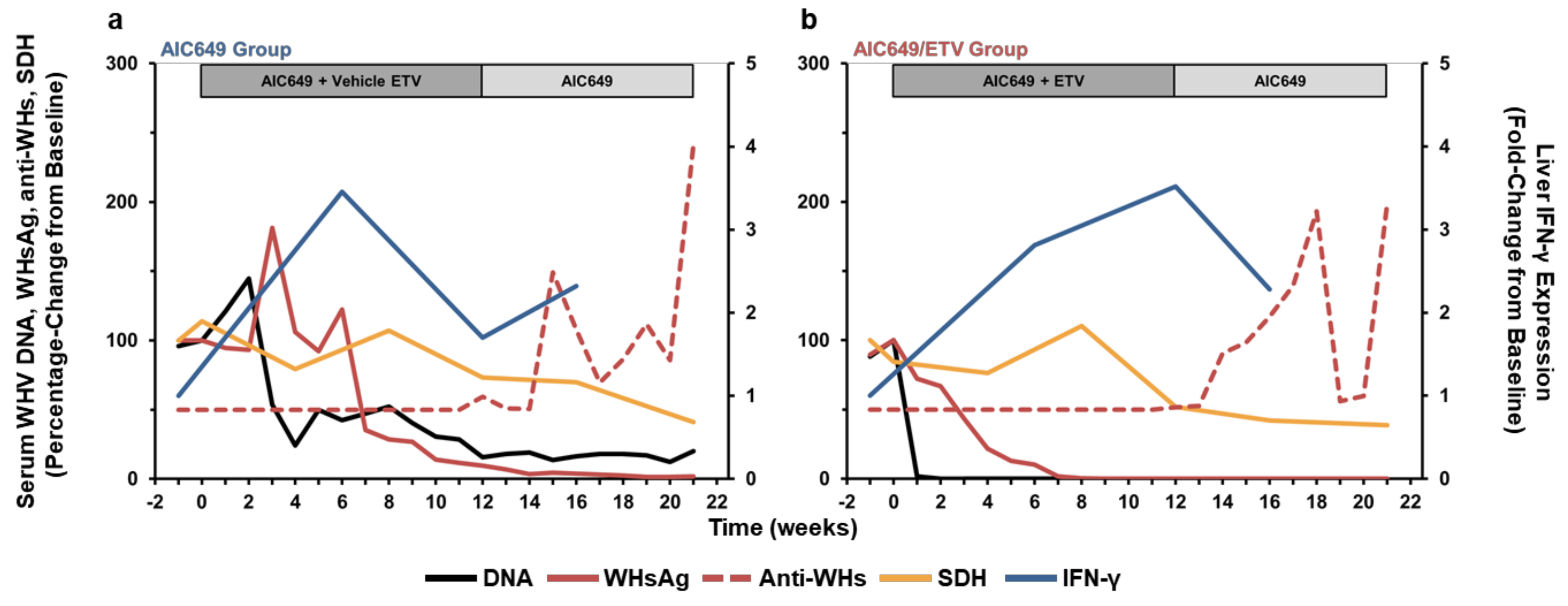
3.6. AIC649 Treatment, Alone and in Combination with ETV, Induced Tightly Regulated Immunological and Virological Responses During Active and Maintenance Treatment
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization Hepatitis B Fact Sheet. Available online: http://www.who.int/mediacentre/factsheets/fs204/en/ (accessed on 21 January 2021).
- Zoulim, F.; Testoni, B.; Lebosse, F. Kinetics of intrahepatic covalently closed circular DNA and serum hepatitis B surface antigen during antiviral therapy for chronic hepatitis B: Lessons from experimental and clinical studies. Clin. Gastroenterol. Hepatol. 2013, 11, 1011–1013. [Google Scholar] [CrossRef] [PubMed]
- Locarnini, S.; Zoulim, F. Molecular genetics of HBV infection. Antivir. Ther. 2010, 15 (Suppl. 3), 3–14. [Google Scholar] [CrossRef]
- Liang, T.J.; Block, T.M.; McMahon, B.J.; Ghany, M.G.; Urban, S.; Guo, J.T.; Locarnini, S.; Zoulim, F.; Chang, K.M.; Lok, A.S. Present and future therapies of hepatitis B: From discovery to cure. Hepatology 2015, 62, 1893–1908. [Google Scholar] [CrossRef]
- Lok, A.S.; Zoulim, F.; Dusheiko, G.; Ghany, M.G. Hepatitis B cure: From discovery to regulatory approval. Hepatology 2017, 66, 1296–1313. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.F.; Wang, C.; Lau, G. Treatment of chronic hepatitis B infection-2017. Liver Int. 2017, 37 (Suppl. 1), 59–66. [Google Scholar] [CrossRef]
- Likhitsup, A.; Lok, A.S. Understanding the Natural History of Hepatitis B Virus Infection and the New Definitions of Cure and the Endpoints of Clinical Trials. Clin. Liver Dis. 2019, 23, 401–416. [Google Scholar] [CrossRef] [PubMed]
- Bertoletti, A.; Ferrari, C. Innate and adaptive immune responses in chronic hepatitis B virus infections: Towards restoration of immune control of viral infection. Gut 2012, 61, 1754–1764. [Google Scholar] [CrossRef]
- Tan, A.; Koh, S.; Bertoletti, A. Immune Response in Hepatitis B Virus Infection. Cold Spring Harb. Perspect. Med. 2015, 5, a021428. [Google Scholar] [CrossRef]
- Zoulim, F.; Lebosse, F.; Levrero, M. Current treatments for chronic hepatitis B virus infections. Curr. Opin. Virol. 2016, 18, 109–116. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.; Lok, A.S. Hepatitis B therapy. Nat. Rev. Gastroenterol. Hepatol. 2011, 8, 275–284. [Google Scholar] [CrossRef]
- Fitzgerald, K.A.; Kagan, J.C. Toll-like Receptors and the Control of Immunity. Cell 2020, 180, 1044–1066. [Google Scholar] [CrossRef]
- Miyake, K.; Shibata, T.; Ohto, U.; Shimizu, T. Emerging roles of the processing of nucleic acids and Toll-like receptors in innate immune responses to nucleic acids. J. Leukoc. Biol. 2017, 101, 135–142. [Google Scholar] [CrossRef]
- Krieg, A.M. Therapeutic potential of Toll-like receptor 9 activation. Nat. Rev. Drug Discov. 2006, 5, 471–484. [Google Scholar] [CrossRef]
- Suslov, A.; Wieland, S.; Menne, S. Modulators of innate immunity as novel therapeutics for treatment of chronic hepatitis B. Curr. Opin Virol. 2018, 30, 9–17. [Google Scholar] [CrossRef]
- Gane, E.J.; Lim, Y.S.; Gordon, S.C.; Visvanathan, K.; Sicard, E.; Fedorak, R.N.; Roberts, S.; Massetto, B.; Ye, Z.; Pflanz, S.; et al. The oral toll-like receptor-7 agonist GS-9620 in patients with chronic hepatitis B virus infection. J. Hepatol. 2015, 63, 320–328. [Google Scholar] [CrossRef]
- Janssen, H.L.A.; Brunetto, M.R.; Kim, Y.J.; Ferrari, C.; Massetto, B.; Nguyen, A.H.; Joshi, A.; Woo, J.; Lau, A.H.; Gaggar, A.; et al. Safety, efficacy and pharmacodynamics of vesatolimod (GS-9620) in virally suppressed patients with chronic hepatitis B. J. Hepatol. 2018, 68, 431–440. [Google Scholar] [CrossRef]
- Boni, C.; Vecchi, A.; Rossi, M.; Laccabue, D.; Giuberti, T.; Alfieri, A.; Lampertico, P.; Grossi, G.; Facchetti, F.; Brunetto, M.R.; et al. TLR7 Agonist Increases Responses of Hepatitis B Virus-Specific T Cells and Natural Killer Cells in Patients With Chronic Hepatitis B Treated With Nucleos(T)Ide Analogues. Gastroenterology 2018, 154, 1764–1777. [Google Scholar] [CrossRef]
- Luk, A.; Jiang, Q.; Glavini, K.; Triyatni, M.; Zhao, N.; Racek, T.; Zhu, Y.; Grippo, J.F. A Single and Multiple Ascending Dose Study of Toll-Like Receptor 7 Agonist (RO7020531) in Chinese Healthy Volunteers. Clin. Transl. Sci. 2020, 13, 985–993. [Google Scholar] [CrossRef] [PubMed]
- Gane, E.; Dunba, P.R.; Brooks, A.; Zhao, Y.; Tan, S.; Lau, A.; Yang, J.; Gaggar, A.; Subramanian, M.; Kottilil, S.; et al. Efficacy and safety of 24 weeks treatment with oral TLR8 agonist, selgantolimod, in virally-suppressed adult patients with chronic hepatitis B: A phase 2 study. J. Hepatol. 2020, 73, S52. [Google Scholar] [CrossRef]
- Yuen, M.-F.; Chen, C.-Y.; Liu, C.-J.; Jeng, R.W.-J.; Elkhashab, M.; Coffin, C.; Kim, W.; Greenbloom, S.; Ramji, A.; Lim, Y.-S.; et al. Ascending dose cohort study of inarigivir-A novel RIG I agonist in chronic HBV patients: Final results of the ACHIEVE trial. J. Hepatol. 2019, 70, e47. [Google Scholar] [CrossRef]
- Agarwal, K.; Ahn, S.H.; Elkhashab, M.; Lau, A.H.; Gaggar, A.; Bulusu, A.; Tian, X.; Cathcart, A.L.; Woo, J.; Subramanian, G.M.; et al. Safety and efficacy of vesatolimod (GS-9620) in patients with chronic hepatitis B who are not currently on antiviral treatment. J. Viral Hepat. 2018, 25, 1331–1340. [Google Scholar] [CrossRef]
- Fosdick, A.; Zheng, J.; Pflanz, S.; Frey, C.R.; Hesselgesser, J.; Halcomb, R.L.; Wolfgang, G.; Tumas, D.B. Pharmacokinetic and pharmacodynamic properties of GS-9620, a novel Toll-like receptor 7 agonist, demonstrate interferon-stimulated gene induction without detectable serum interferon at low oral doses. J. Pharmacol. Exp. Ther. 2014, 348, 96–105. [Google Scholar] [CrossRef]
- Agarwal, K.; Afdhal, N.; Coffin, C.; Fung, S.; Dusheiko, G.; Foster, G.; Elkhashab, M.; Tam, E.; Ramji, A.; Iyer, R.; et al. Liver toxicity in the Phase 2 Catalyst 206 trial of Inarigivir 400 mg daily added to a nucleoside in HBV EAg negative patients. J. Hepatol. 2020, 73, S125. [Google Scholar] [CrossRef]
- Summers, J.; Smolec, J.M.; Snyder, R. A virus similar to human hepatitis B virus associated with hepatitis and hepatoma in woodchucks. Proc. Natl. Acad. Sci. USA 1978, 75, 4533–4537. [Google Scholar] [CrossRef]
- Galibert, F.; Chen, T.N.; Mandart, E. Nucleotide sequence of a cloned woodchuck hepatitis virus genome: Comparison with the hepatitis B virus sequence. J. Virol. 1982, 41, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Girones, R.; Cote, P.J.; Hornbuckle, W.E.; Tennant, B.C.; Gerin, J.L.; Purcell, R.H.; Miller, R.H. Complete nucleotide sequence of a molecular clone of woodchuck hepatitis virus that is infectious in the natural host. Proc. Natl. Acad. Sci. USA 1989, 86, 1846–1849. [Google Scholar] [CrossRef] [PubMed]
- Menne, S.; Cote, P.J. The woodchuck as an animal model for pathogenesis and therapy of chronic hepatitis B virus infection. World J. Gastroenterol. 2007, 13, 104–124. [Google Scholar] [CrossRef] [PubMed]
- Cote, P.J.; Korba, B.E.; Miller, R.H.; Jacob, J.R.; Baldwin, B.H.; Hornbuckle, W.E.; Purcell, R.H.; Tennant, B.C.; Gerin, J.L. Effects of age and viral determinants on chronicity as an outcome of experimental woodchuck hepatitis virus infection. Hepatology 2000, 31, 190–200. [Google Scholar] [CrossRef]
- Popper, H.; Roth, L.; Purcell, R.H.; Tennant, B.C.; Gerin, J.L. Hepatocarcinogenicity of the woodchuck hepatitis virus. Proc. Natl. Acad. Sci. USA 1987, 84, 866–870. [Google Scholar] [CrossRef] [PubMed]
- Tennant, B.C.; Toshkov, I.A.; Peek, S.F.; Jacob, J.R.; Menne, S.; Hornbuckle, W.E.; Schinazi, R.D.; Korba, B.E.; Cote, P.J.; Gerin, J.L. Hepatocellular carcinoma in the woodchuck model of hepatitis B virus infection. Gastroenterology 2004, 127, S283–S293. [Google Scholar] [CrossRef] [PubMed]
- Snyder, R.L.; Tyler, G.; Summers, J. Chronic hepatitis and hepatocellular carcinoma associated with woodchuck hepatitis virus. Am. J. Pathol. 1982, 107, 422–425. [Google Scholar]
- Roggendorf, M.; Kosinska, A.D.; Liu, J.; Lu, M. The Woodchuck, a Nonprimate Model for Immunopathogenesis and Therapeutic Immunomodulation in Chronic Hepatitis B Virus Infection. Cold Spring Harb. Perspect. Med. 2015, 5, a021451. [Google Scholar] [CrossRef]
- Kosinska, A.D.; Liu, J.; Lu, M.; Roggendorf, M. Therapeutic vaccination and immunomodulation in the treatment of chronic hepatitis B: Preclinical studies in the woodchuck. Med. Microbiol. Immunol. 2015, 204, 103–114. [Google Scholar] [CrossRef]
- Michalak, T.I. Diverse Virus and Host-Dependent Mechanisms Influence the Systemic and Intrahepatic Immune Responses in the Woodchuck Model of Hepatitis B. Front. Immunol. 2020, 11, 853. [Google Scholar] [CrossRef]
- Fletcher, S.P.; Chin, D.J.; Ji, Y.; Iniguez, A.L.; Taillon, B.; Swinney, D.C.; Ravindran, P.; Cheng, D.T.; Bitter, H.; Lopatin, U.; et al. Transcriptomic analysis of the woodchuck model of chronic hepatitis B. Hepatology 2012, 56, 820–830. [Google Scholar] [CrossRef]
- Alioto, T.S.; Cruz, F.; Gomez-Garrido, J.; Triyatni, M.; Gut, M.; Frias, L.; Esteve-Codina, A.; Menne, S.; Kiialainen, A.; Kumpesa, N.; et al. The Genome Sequence of the Eastern Woodchuck (Marmota monax)-A Preclinical Animal Model for Chronic Hepatitis B. G3 (Bethesda) 2019, 9, 3943–3952. [Google Scholar] [CrossRef]
- Menne, S.; Butler, S.D.; George, A.L.; Tochkov, I.A.; Zhu, Y.; Xiong, S.; Gerin, J.L.; Cote, P.J.; Tennant, B.C. Antiviral effects of lamivudine, emtricitabine, adefovir dipivoxil, and tenofovir disoproxil fumarate administered orally alone and in combination to woodchucks with chronic woodchuck hepatitis virus infection. Antimicrob. Agents Chemother. 2008, 52, 3617–3632. [Google Scholar] [CrossRef] [PubMed]
- Mason, W.S.; Cullen, J.; Moraleda, G.; Saputelli, J.; Aldrich, C.E.; Miller, D.S.; Tennant, B.; Frick, L.; Averett, D.; Condreay, L.D.; et al. Lamivudine therapy of WHV-infected woodchucks. Virology 1998, 245, 18–32. [Google Scholar] [CrossRef][Green Version]
- Colonno, R.J.; Genovesi, E.V.; Medina, I.; Lamb, L.; Durham, S.K.; Huang, M.L.; Corey, L.; Littlejohn, M.; Locarnini, S.; Tennant, B.C.; et al. Long-term entecavir treatment results in sustained antiviral efficacy and prolonged life span in the woodchuck model of chronic hepatitis infection. J. Infect. Dis. 2001, 184, 1236–1245. [Google Scholar] [CrossRef]
- Iyer, R.V.; Maguire, O.; Kim, M.; Curtin, L.I.; Sexton, S.; Fisher, D.T.; Schihl, S.A.; Fetterly, G.; Menne, S.; Minderman, H. Dose-Dependent Sorafenib-Induced Immunosuppression Is Associated with Aberrant NFAT Activation and Expression of PD-1 in T Cells. Cancers (Basel) 2019, 11, 681. [Google Scholar] [CrossRef]
- Fairman, J.; Liu, K.H.; Menne, S. Prevention of liver tumor formation in woodchucks with established hepatocellular carcinoma by treatment with cationic liposome-DNA complexes. BMC Cancer 2017, 17, 172. [Google Scholar] [CrossRef] [PubMed]
- Putzer, B.M.; Stiewe, T.; Rodicker, F.; Schildgen, O.; Ruhm, S.; Dirsch, O.; Fiedler, M.; Damen, U.; Tennant, B.; Scherer, C.; et al. Large nontransplanted hepatocellular carcinoma in woodchucks: Treatment with adenovirus-mediated delivery of interleukin 12/B7.1 genes. J. Natl. Cancer Inst. 2001, 93, 472–479. [Google Scholar] [CrossRef]
- Kosinska, A.D.; Zhang, E.; Johrden, L.; Liu, J.; Seiz, P.L.; Zhang, X.; Ma, Z.; Kemper, T.; Fiedler, M.; Glebe, D.; et al. Combination of DNA prime--adenovirus boost immunization with entecavir elicits sustained control of chronic hepatitis B in the woodchuck model. PLoS Pathog. 2013, 9, e1003391. [Google Scholar] [CrossRef]
- Rodriguez-Madoz, J.R.; Liu, K.H.; Quetglas, J.I.; Ruiz-Guillen, M.; Otano, I.; Crettaz, J.; Butler, S.D.; Bellezza, C.A.; Dykes, N.L.; Tennant, B.C.; et al. Semliki forest virus expressing interleukin-12 induces antiviral and antitumoral responses in woodchucks with chronic viral hepatitis and hepatocellular carcinoma. J. Virol. 2009, 83, 12266–12278. [Google Scholar] [CrossRef] [PubMed]
- Korba, B.E.; Cote, P.; Hornbuckle, W.; Tennant, B.C.; Gerin, J.L. Treatment of chronic woodchuck hepatitis virus infection in the Eastern woodchuck (Marmota monax) with nucleoside analogues is predictive of therapy for chronic hepatitis B virus infection in humans. Hepatology 2000, 31, 1165–1175. [Google Scholar] [CrossRef]
- Korolowicz, K.E.; Iyer, R.P.; Czerwinski, S.; Suresh, M.; Yang, J.; Padmanabhan, S.; Sheri, A.; Pandey, R.K.; Skell, J.; Marquis, J.K.; et al. Antiviral Efficacy and Host Innate Immunity Associated with SB 9200 Treatment in the Woodchuck Model of Chronic Hepatitis B. PLoS ONE 2016, 11, e0161313. [Google Scholar] [CrossRef] [PubMed]
- Daffis, S.; Balsitis, S.; Chamberlain, J.; Zheng, J.; Santos, R.; Rowe, W.; Ramakrishnan, D.; Pattabiraman, D.; Spurlock, S.; Chu, R.; et al. Toll-Like Receptor 8 Agonist GS-9688 Induces Sustained Efficacy in the Woodchuck Model of Chronic Hepatitis B. Hepatology 2021, 73, 53–67. [Google Scholar] [CrossRef]
- Lambotin, M.; Raghuraman, S.; Stoll-Keller, F.; Baumert, T.F.; Barth, H. A look behind closed doors: Interaction of persistent viruses with dendritic cells. Nat. Rev. Microbiol. 2010, 8, 350–360. [Google Scholar] [CrossRef]
- Xie, Q.; Shen, H.C.; Jia, N.N.; Wang, H.; Lin, L.Y.; An, B.Y.; Gui, H.L.; Guo, S.M.; Cai, W.; Yu, H.; et al. Patients with chronic hepatitis B infection display deficiency of plasmacytoid dendritic cells with reduced expression of TLR9. Microbes. Infect. 2009, 11, 515–523. [Google Scholar] [CrossRef]
- van der Molen, R.G.; Sprengers, D.; Binda, R.S.; de Jong, E.C.; Niesters, H.G.; Kusters, J.G.; Kwekkeboom, J.; Janssen, H.L. Functional impairment of myeloid and plasmacytoid dendritic cells of patients with chronic hepatitis B. Hepatology 2004, 40, 738–746. [Google Scholar] [CrossRef]
- Op den Brouw, M.L.; Binda, R.S.; van Roosmalen, M.H.; Protzer, U.; Janssen, H.L.; van der Molen, R.G.; Woltman, A.M. Hepatitis B virus surface antigen impairs myeloid dendritic cell function: A possible immune escape mechanism of hepatitis B virus. Immunology 2009, 126, 280–289. [Google Scholar] [CrossRef]
- Puig, M.; Tosh, K.W.; Schramm, L.M.; Grajkowska, L.T.; Kirschman, K.D.; Tami, C.; Beren, J.; Rabin, R.L.; Verthelyi, D. TLR9 and TLR7 agonists mediate distinct type I IFN responses in humans and nonhuman primates in vitro and in vivo. J. Leukoc. Biol. 2012, 91, 147–158. [Google Scholar] [CrossRef]
- Siegemund, S.; Hartl, A.; von Buttlar, H.; Dautel, F.; Raue, R.; Freudenberg, M.A.; Fejer, G.; Buttner, M.; Kohler, G.; Kirschning, C.J.; et al. Conventional bone marrow-derived dendritic cells contribute to toll-like receptor-independent production of alpha/beta interferon in response to inactivated parapoxvirus ovis. J. Virol. 2009, 83, 9411–9422. [Google Scholar] [CrossRef]
- von Buttlar, H.; Siegemund, S.; Buttner, M.; Alber, G. Identification of Toll-like receptor 9 as parapoxvirus ovis-sensing receptor in plasmacytoid dendritic cells. PLoS ONE 2014, 9, e106188. [Google Scholar] [CrossRef]
- Weber, O.; Mercer, A.A.; Friebe, A.; Knolle, P.; Volk, H.D. Therapeutic immunomodulation using a virus--the potential of inactivated orf virus. Eur. J. Clin. Microbiol. Infect. Dis. 2013, 32, 451–460. [Google Scholar] [CrossRef]
- Friebe, A.; Siegling, A.; Friederichs, S.; Volk, H.D.; Weber, O. Immunomodulatory effects of inactivated parapoxvirus ovis (ORF virus) on human peripheral immune cells: Induction of cytokine secretion in monocytes and Th1-like cells. J. Virol. 2004, 78, 9400–9411. [Google Scholar] [CrossRef]
- Weber, O.; Siegling, A.; Friebe, A.; Limmer, A.; Schlapp, T.; Knolle, P.; Mercer, A.; Schaller, H.; Volk, H.D. Inactivated parapoxvirus ovis (Orf virus) has antiviral activity against hepatitis B virus and herpes simplex virus. J. Gen. Virol. 2003, 84, 1843–1852. [Google Scholar] [CrossRef]
- Friebe, A.; Friederichs, S.; Scholz, K.; Janssen, U.; Scholz, C.; Schlapp, T.; Mercer, A.; Siegling, A.; Volk, H.D.; Weber, O. Characterization of immunostimulatory components of orf virus (parapoxvirus ovis). J. Gen. Virol. 2011, 92, 1571–1584. [Google Scholar] [CrossRef] [PubMed]
- Kruse, N.; Weber, O. Selective induction of apoptosis in antigen-presenting cells in mice by Parapoxvirus ovis. J. Virol. 2001, 75, 4699–4704. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Friebe, A.; Siegling, A.; Weber, O. Inactivated Orf-virus shows disease modifying antiviral activity in a guinea pig model of genital herpesvirus infection. J. Microbiol. Immunol. Infect. 2018, 51, 587–592. [Google Scholar] [CrossRef]
- Paulsen, D.; Urban, A.; Knorr, A.; Hirth-Dietrich, C.; Siegling, A.; Volk, H.D.; Mercer, A.A.; Limmer, A.; Schumak, B.; Knolle, P.; et al. Inactivated ORF virus shows antifibrotic activity and inhibits human hepatitis B virus (HBV) and hepatitis C virus (HCV) replication in preclinical models. PLoS ONE 2013, 8, e74605. [Google Scholar] [CrossRef]
- Paulsen, D.; Weber, O.; Ruebsamen-Schaeff, H.; Tennant, B.C.; Menne, S. AIC649 Induces a Bi-Phasic Treatment Response in the Woodchuck Model of Chronic Hepatitis B. PLoS ONE 2015, 10, e0144383. [Google Scholar] [CrossRef]
- Hornbuckle, W.E.; Graham, E.S.; Roth, L.; Baldwin, B.H.; Wickenden, C.; Tennant, B.C. Laboratory assessment of hepatic injury in the woodchuck (Marmota monax). Lab. Anim. Sci. 1985, 35, 376–381. [Google Scholar]
- Paulsen, D.; Korolowicz, K.E.; Li, B.; Huang, X.; Suresh, M.; Yon, C.; Leng, X.; Kallakury, B.V.; Tucker, R.D.; Urban, A.; et al. AIC649 in combination with entecavir leads to WHsAg loss in the woodchuck animal model of chronic hepatitis B. Hepatology 2017, 66, 1268A. [Google Scholar]
- Cote, P.J.; Roneker, C.; Cass, K.; Schodel, F.; Peterson, D.; Tennant, B.; De Noronha, F.; Gerin, J. New enzyme immunoassays for the serologic detection of woodchuck hepatitis virus infection. Viral Immunol. 1993, 6, 161–169. [Google Scholar] [CrossRef]
- Bellezza, C.A.; Concannon, P.W.; Hornbuckle, W.E.; Roth, L.; Tennant, B.C. Woodchucks as laboratory animals. In Laboratory Animal Medicine, 2nd ed.; Elsevier Science: Amsterdam, The Netherlands, 2002; pp. 309–328. [Google Scholar]
- Peek, S.F.; Cote, P.J.; Jacob, J.R.; Toshkov, I.A.; Hornbuckle, W.E.; Baldwin, B.H.; Wells, F.V.; Chu, C.K.; Gerin, J.L.; Tennant, B.C.; et al. Antiviral activity of clevudine [L-FMAU, (1-(2-fluoro-5-methyl-beta, L-arabinofuranosyl) uracil)] against woodchuck hepatitis virus replication and gene expression in chronically infected woodchucks (Marmota monax). Hepatology 2001, 33, 254–266. [Google Scholar] [CrossRef] [PubMed]
- Tennant, B.C.; Baldwin, B.H.; Graham, L.A.; Ascenzi, M.A.; Hornbuckle, W.E.; Rowland, P.H.; Tochkov, I.A.; Yeager, A.E.; Erb, H.N.; Colacino, J.M.; et al. Antiviral activity and toxicity of fialuridine in the woodchuck model of hepatitis B virus infection. Hepatology 1998, 28, 179–191. [Google Scholar] [CrossRef] [PubMed]
- Korolowizc, K.E.; Li, B.; Huang, X.; Yon, C.; Rodrigo, E.; Corpuz, M.; Plouffe, D.M.; Kallakury, B.V.; Suresh, M.; Wu, T.Y.; et al. Liver-Targeted Toll-Like Receptor 7 Agonist Combined With Entecavir Promotes a Functional Cure in the Woodchuck Model of Hepatitis B Virus. Hepatol. Commun. 2019, 3, 1296–1310. [Google Scholar] [CrossRef] [PubMed]
- Meng, Z.; Zhang, X.; Pei, R.; Zhang, E.; Kemper, T.; Vollmer, J.; Davis, H.L.; Glebe, D.; Gerlich, W.; Roggendorf, M.; et al. Combination therapy including CpG oligodeoxynucleotides and entecavir induces early viral response and enhanced inhibition of viral replication in a woodchuck model of chronic hepadnaviral infection. Antiviral Res. 2016, 125, 14–24. [Google Scholar] [CrossRef]
- Guidotti, L.G.; Morris, A.; Mendez, H.; Koch, R.; Silverman, R.H.; Williams, B.R.; Chisari, F.V. Interferon-regulated pathways that control hepatitis B virus replication in transgenic mice. J. Virol. 2002, 76, 2617–2621. [Google Scholar] [CrossRef]
- Isogawa, M.; Robek, M.D.; Furuichi, Y.; Chisari, F.V. Toll-like receptor signaling inhibits hepatitis B virus replication in vivo. J. Virol. 2005, 79, 7269–7272. [Google Scholar] [CrossRef] [PubMed]
- Genovesi, E.V.; Lamb, L.; Medina, I.; Taylor, D.; Seifer, M.; Innaimo, S.; Colonno, R.J.; Standring, D.N.; Clark, J.M. Efficacy of the carbocyclic 2′-deoxyguanosine nucleoside BMS-200475 in the woodchuck model of hepatitis B virus infection. Antimicrob Agents Chemother. 1998, 42, 3209–3217. [Google Scholar] [CrossRef] [PubMed]
- Menne, S.; Wildum, S.; Steiner, G.; Suresh, M.; Korolowicz, K.; Balarezo, M.; Yon, C.; Murreddu, M.; Hong, X.; Kallakury, B.V.; et al. Efficacy of an Inhibitor of Hepatitis B Virus Expression in Combination With Entecavir and Interferon-alpha in Woodchucks Chronically Infected With Woodchuck Hepatitis Virus. Hepatol. Commun. 2020, 4, 916–931. [Google Scholar] [CrossRef]
- Lampertico, P. Discontinuation of nucleoside analogues in hepatitis B virus infection. Gastroenterol. Hepatol. (N. Y.) 2013, 9, 656–658. [Google Scholar]
- Suresh, M.; Korolowicz, K.E.; Balarezo, M.; Iyer, R.P.; Padmanabhan, S.; Cleary, D.; Gimi, R.; Sheri, A.; Yon, C.; Kallakury, B.V.; et al. Antiviral Efficacy and Host Immune Response Induction during Sequential Treatment with SB 9200 Followed by Entecavir in Woodchucks. PLoS ONE 2017, 12, e0169631. [Google Scholar] [CrossRef] [PubMed]
- Berraondo, P.; Di Scala, M.; Korolowicz, K.; Thampi, L.M.; Otano, I.; Suarez, L.; Fioravanti, J.; Aranda, F.; Ardaiz, N.; Yang, J.; et al. Liver-directed gene therapy of chronic hepadnavirus infection using interferon alpha tethered to apolipoprotein A-I. J. Hepatol. 2015, 63, 329–336. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Zhang, E.; Ma, Z.; Wu, W.; Kosinska, A.; Zhang, X.; Moller, I.; Seiz, P.; Glebe, D.; Wang, B.; et al. Enhancing virus-specific immunity in vivo by combining therapeutic vaccination and PD-L1 blockade in chronic hepadnaviral infection. PLoS Pathog. 2014, 10, e1003856. [Google Scholar] [CrossRef]
- Fletcher, S.P.; Chin, D.J.; Gruenbaum, L.; Bitter, H.; Rasmussen, E.; Ravindran, P.; Swinney, D.C.; Birzele, F.; Schmucki, R.; Lorenz, S.H.; et al. Intrahepatic Transcriptional Signature Associated with Response to Interferon-alpha Treatment in the Woodchuck Model of Chronic Hepatitis B. PLoS Pathog. 2015, 11, e1005103. [Google Scholar] [CrossRef]
- Fletcher, S.P.; Chin, D.J.; Cheng, D.T.; Ravindran, P.; Bitter, H.; Gruenbaum, L.; Cote, P.J.; Ma, H.; Klumpp, K.; Menne, S. Identification of an intrahepatic transcriptional signature associated with self-limiting infection in the woodchuck model of hepatitis B. Hepatology 2013, 57, 13–22. [Google Scholar] [CrossRef]
- Wang, Y.; Menne, S.; Jacob, J.R.; Tennant, B.C.; Gerin, J.L.; Cote, P.J. Role of type 1 versus type 2 immune responses in liver during the onset of chronic woodchuck hepatitis virus infection. Hepatology 2003, 37, 771–780. [Google Scholar] [CrossRef]
- Burton, A.R.; Pallett, L.J.; McCoy, L.E.; Suveizdyte, K.; Amin, O.E.; Swadling, L.; Alberts, E.; Davidson, B.R.; Kennedy, P.T.; Gill, U.S.; et al. Circulating and intrahepatic antiviral B cells are defective in hepatitis B. J. Clin. Invest. 2018, 128, 4588–4603. [Google Scholar] [CrossRef] [PubMed]
- Boni, C.; Laccabue, D.; Lampertico, P.; Giuberti, T.; Vigano, M.; Schivazappa, S.; Alfieri, A.; Pesci, M.; Gaeta, G.B.; Brancaccio, G.; et al. Restored function of HBV-specific T cells after long-term effective therapy with nucleos(t)ide analogues. Gastroenterology 2012, 143, 963–973. [Google Scholar] [CrossRef]
- McHutchison, J.G.; Bacon, B.R.; Gordon, S.C.; Lawitz, E.; Shiffman, M.; Afdhal, N.H.; Jacobson, I.M.; Muir, A.; Al-Adhami, M.; Morris, M.L.; et al. Phase 1B, randomized, double-blind, dose-escalation trial of CPG 10101 in patients with chronic hepatitis C virus. Hepatology 2007, 46, 1341–1349. [Google Scholar] [CrossRef]
- Flink, H.J.; Sprengers, D.; Hansen, B.E.; van Zonneveld, M.; de Man, R.A.; Schalm, S.W.; Janssen, H.L.; Group, H.B.V.S. Flares in chronic hepatitis B patients induced by the host or the virus? Relation to treatment response during Peg-interferon {alpha}-2b therapy. Gut 2005, 54, 1604–1609. [Google Scholar] [CrossRef] [PubMed]
- Hadziyannis, S.J.; Sevastianos, V.; Rapti, I.; Vassilopoulos, D.; Hadziyannis, E. Sustained responses and loss of HBsAg in HBeAg-negative patients with chronic hepatitis B who stop long-term treatment with adefovir. Gastroenterology 2012, 143, 629–636. [Google Scholar] [CrossRef] [PubMed]
- Wieland, S.F.; Guidotti, L.G.; Chisari, F.V. Intrahepatic induction of alpha/beta interferon eliminates viral RNA-containing capsids in hepatitis B virus transgenic mice. J. Virol. 2000, 74, 4165–4173. [Google Scholar] [CrossRef] [PubMed]
- Lucifora, J.; Xia, Y.; Reisinger, F.; Zhang, K.; Stadler, D.; Cheng, X.; Sprinzl, M.F.; Koppensteiner, H.; Makowska, Z.; Volz, T.; et al. Specific and nonhepatotoxic degradation of nuclear hepatitis B virus cccDNA. Science 2014, 343, 1221–1228. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Nie, H.; Mao, R.; Mitra, B.; Cai, D.; Yan, R.; Guo, J.T.; Block, T.M.; Mechti, N.; Guo, H. Interferon-inducible ribonuclease ISG20 inhibits hepatitis B virus replication through directly binding to the epsilon stem-loop structure of viral RNA. PLoS Pathog. 2017, 13, e1006296. [Google Scholar] [CrossRef]
- Menne, S.; Tumas, D.B.; Liu, K.H.; Thampi, L.; AlDeghaither, D.; Baldwin, B.H.; Bellezza, C.A.; Cote, P.J.; Zheng, J.; Halcomb, R.; et al. Sustained efficacy and seroconversion with the Toll-like receptor 7 agonist GS-9620 in the Woodchuck model of chronic hepatitis B. J. Hepatol. 2015, 62, 1237–1245. [Google Scholar] [CrossRef]
- Jung, J.; Yi, A.K.; Zhang, X.; Choe, J.; Li, L.; Choi, Y.S. Distinct response of human B cell subpopulations in recognition of an innate immune signal, CpG DNA. J. Immunol. 2002, 169, 2368–2373. [Google Scholar] [CrossRef]
- AiCuris Company Website. Available online: https://www.aicuris.com/66n119 (accessed on 21 January 2021).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korolowicz, K.E.; Suresh, M.; Li, B.; Huang, X.; Yon, C.; Leng, X.; Kallakury, B.V.; Tucker, R.D.; Menne, S. Treatment with the Immunomodulator AIC649 in Combination with Entecavir Produces Antiviral Efficacy in the Woodchuck Model of Chronic Hepatitis B. Viruses 2021, 13, 648. https://doi.org/10.3390/v13040648
Korolowicz KE, Suresh M, Li B, Huang X, Yon C, Leng X, Kallakury BV, Tucker RD, Menne S. Treatment with the Immunomodulator AIC649 in Combination with Entecavir Produces Antiviral Efficacy in the Woodchuck Model of Chronic Hepatitis B. Viruses. 2021; 13(4):648. https://doi.org/10.3390/v13040648
Chicago/Turabian StyleKorolowicz, Kyle E., Manasa Suresh, Bin Li, Xu Huang, Changsuek Yon, Xuebing Leng, Bhaskar V. Kallakury, Robin D. Tucker, and Stephan Menne. 2021. "Treatment with the Immunomodulator AIC649 in Combination with Entecavir Produces Antiviral Efficacy in the Woodchuck Model of Chronic Hepatitis B" Viruses 13, no. 4: 648. https://doi.org/10.3390/v13040648
APA StyleKorolowicz, K. E., Suresh, M., Li, B., Huang, X., Yon, C., Leng, X., Kallakury, B. V., Tucker, R. D., & Menne, S. (2021). Treatment with the Immunomodulator AIC649 in Combination with Entecavir Produces Antiviral Efficacy in the Woodchuck Model of Chronic Hepatitis B. Viruses, 13(4), 648. https://doi.org/10.3390/v13040648





