Atypical Porcine Pestiviruses: Relationships and Conserved Structural Features
Abstract
:1. Introduction
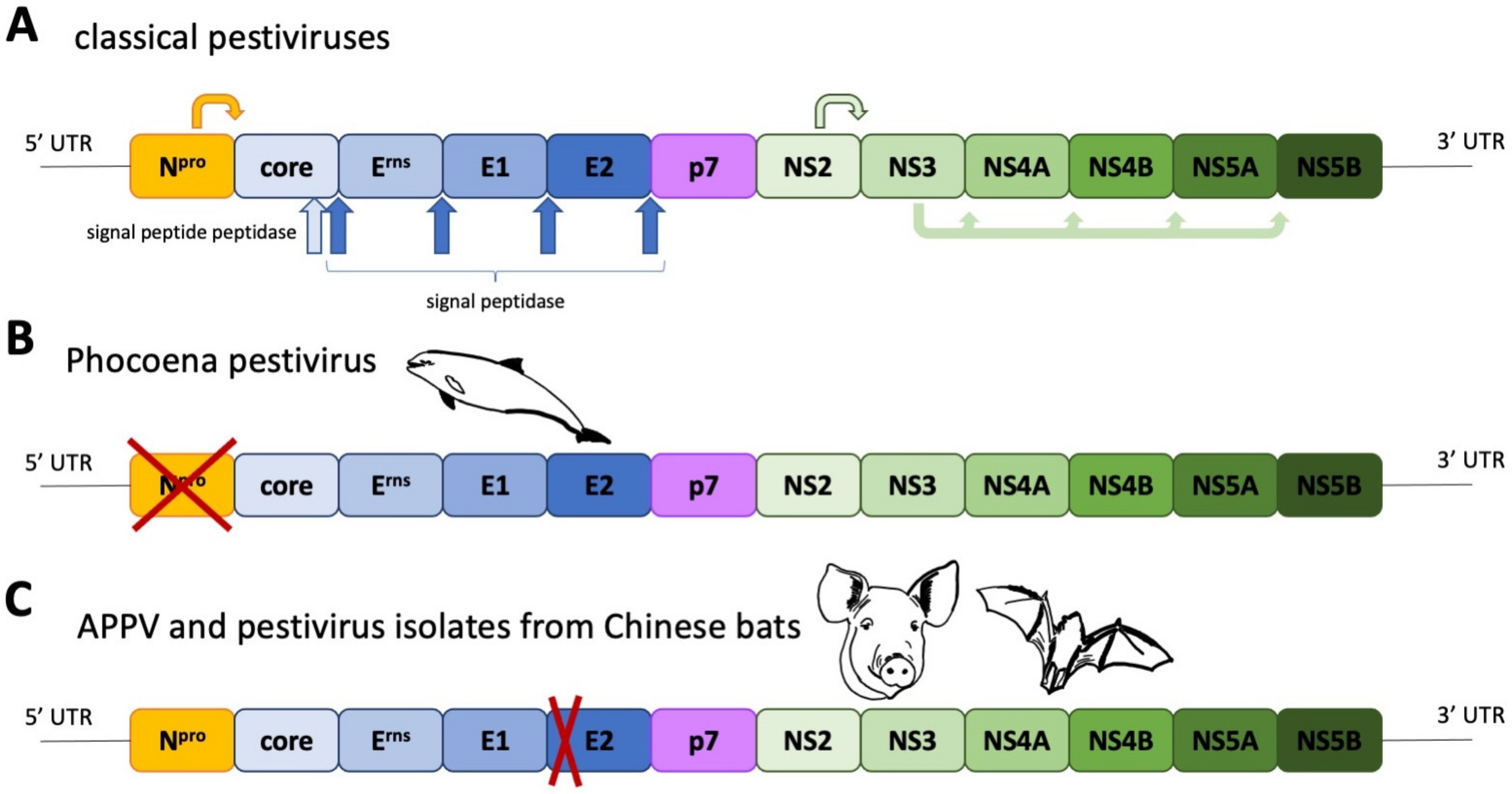
2. Phylogeny and Geographic Distribution of Atypical Porcine Pestiviruses
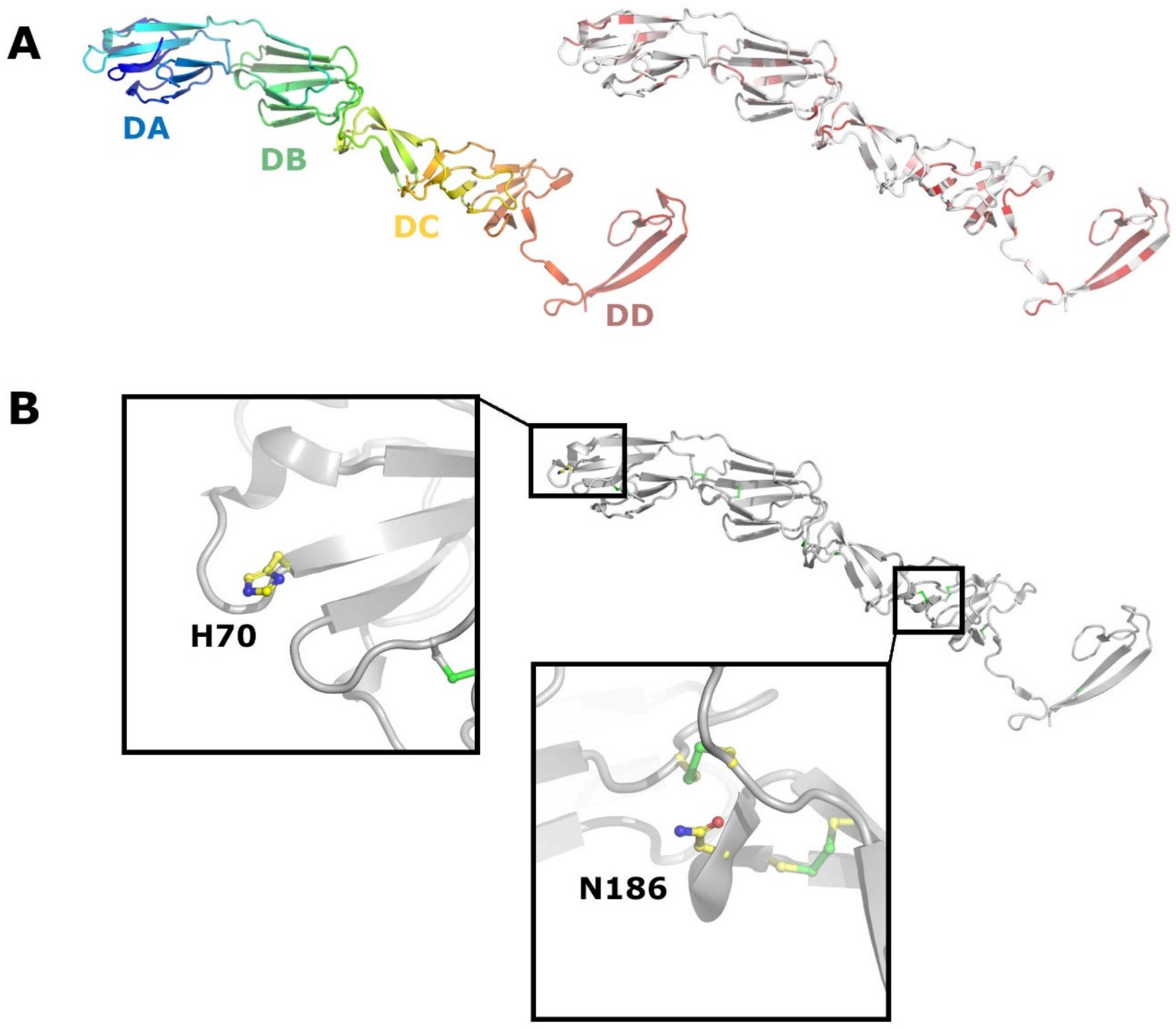
3. Special Traits of Atypical Porcine Pestiviruses
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Kirkland, P.D.; Frost, M.J.; Finlaison, D.S.; King, K.R.; Ridpath, J.F.; Gu, X. Identification of a novel virus in pigs—Bungowannah virus: A possible new species of pestivirus. Virus Res. 2007, 129, 26–34. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Ren, X.; Yang, L.; Hu, Y.; Yang, J.; He, G.; Zhang, J.; Dong, J.; Sun, L.; Du, J.; et al. Virome analysis for identification of novel mammalian viruses in bat species from Chinese provinces. J. Virol. 2012, 86, 10999–11012. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, Z.; Liu, B.; Du, J.; Zhang, J.; Lu, L.; Zhu, G.; Han, Y.; Su, H.; Yang, L.; Zhang, S.; et al. Discovery of Diverse Rodent and Bat Pestiviruses with Distinct Genomic and Phylogenetic Characteristics in Several Chinese Provinces. Front. Microbiol. 2018, 9, 2562. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Firth, C.; Bhat, M.; Firth, M.A.; Williams, S.H.; Frye, M.J.; Simmonds, P.; Conte, J.M.; Ng, J.; Garcia, J.; Bhuva, N.P.; et al. Detection of zoonotic pathogens and characterization of novel viruses carried by commensal Rattus norvegicus in New York City. mBio 2014, 5, e01933-14. [Google Scholar] [CrossRef] [Green Version]
- Jo, W.K.; van Elk, C.; van de Bildt, M.; van Run, P.; Petry, M.; Jesse, S.T.; Jung, K.; Ludlow, M.; Kuiken, T.; Osterhaus, A. An evolutionary divergent pestivirus lacking the Npro gene systemically infects a whale species. Emerg. Microbes Infect. 2019, 8, 1383–1392. [Google Scholar] [CrossRef] [Green Version]
- Lamp, B.; Schwarz, L.; Högler, S.; Riedel, C.; Sinn, L.; Rebel-Bauder, B.; Weissenböck, H.; Ladinig, A.; Rümenapf, T. Novel pestivirus species in pigs, Austria, 2015. Emerg. Infect. Dis. 2017, 23, 1176. [Google Scholar] [CrossRef]
- Arruda, B.L.; Arruda, P.H.; Magstadt, D.R.; Schwartz, K.J.; Dohlman, T.; Schleining, J.A.; Patterson, A.R.; Visek, C.A.; Victoria, J.G. Identification of a Divergent Lineage Porcine Pestivirus in Nursing Piglets with Congenital Tremors and Reproduction of Disease following Experimental Inoculation. PLoS ONE 2016, 11, e0150104. [Google Scholar] [CrossRef] [Green Version]
- Hause, B.M.; Collin, E.A.; Peddireddi, L.; Yuan, F.; Chen, Z.; Hesse, R.A.; Gauger, P.C.; Clement, T.; Fang, Y.; Anderson, G. Discovery of a novel putative atypical porcine pestivirus in pigs in the USA. J. Gen. Virol. 2015, 96, 2994–2998. [Google Scholar] [CrossRef]
- Smith, D.B.; Meyers, G.; Bukh, J.; Gould, E.A.; Monath, T.; Scott Muerhoff, A.; Pletnev, A.; Rico-Hesse, R.; Stapleton, J.T.; Simmonds, P.; et al. Proposed revision to the taxonomy of the genus Pestivirus, family Flaviviridae. J. Gen. Virol. 2017, 98, 2106–2112. [Google Scholar] [CrossRef]
- De Groof, A.; Deijs, M.; Guelen, L.; Van Grinsven, L.; Van Os-Galdos, L.; Vogels, W.; Derks, C.; Cruijsen, T.; Geurts, V.; Vrijenhoek, M.; et al. Atypical porcine pestivirus: A possible cause of congenital tremor type A-II in newborn piglets. Viruses 2016, 8, 271. [Google Scholar] [CrossRef]
- Postel, A.; Hansmann, F.; Baechlein, C.; Fischer, N.; Alawi, M.; Grundhoff, A.; Derking, S.; Tenhündfeld, J.; Pfankuche, V.M.; Herder, V.; et al. Presence of atypical porcine pestivirus (APPV) genomes in newborn piglets correlates with congenital tremor. Sci. Rep. 2016, 6, 27735. [Google Scholar] [CrossRef] [Green Version]
- Wu, Z.; Lu, L.; Du, J.; Yang, L.; Ren, X.; Liu, B.; Jiang, J.; Yang, J.; Dong, J.; Sun, L.; et al. Comparative analysis of rodent and small mammal viromes to better understand the wildlife origin of emerging infectious diseases. Microbiome 2018, 6, 178. [Google Scholar] [CrossRef]
- Schwarz, L.; Riedel, C.; Högler, S.; Sinn, L.J.; Voglmayr, T.; Wöchtl, B.; Dinhopl, N.; Rebel-Bauder, B.; Weissenböck, H.; Ladinig, A.; et al. Congenital infection with atypical porcine pestivirus (APPV) is associated with disease and viral persistence. Vet. Res. 2017, 48, 1. [Google Scholar] [CrossRef] [Green Version]
- Postel, A.; Meyer, D.; Cagatay, G.N.; Feliziani, F.; De Mia, G.M.; Fischer, N.; Grundhoff, A.; Milićević, V.; Deng, M.C.; Chang, C.Y.; et al. High abundance and genetic variability of atypical porcine pestivirus in pigs from Europe and Asia. Emerg. Infect. Dis. 2017, 23, 2104–2107. [Google Scholar] [CrossRef] [Green Version]
- Beer, M.; Wernike, K.; Dräger, C.; Höper, D.; Pohlmann, A.; Bergermann, C.; Schröder, C.; Klinkhammer, S.; Blome, S.; Hoffmann, B. High Prevalence of Highly Variable Atypical Porcine Pestiviruses Found in Germany. Transbound. Emerg. Dis. 2017, 64, e22–e26. [Google Scholar] [CrossRef]
- Dénes, L.; Biksi, I.; Albert, M.; Szeredi, L.; Knapp, D.G.; Szilasi, A.; Bálint, Á.; Balka, G. Detection and phylogenetic characterization of atypical porcine pestivirus strains in Hungary. Transbound. Emerg. Dis. 2018, 65, 2039–2042. [Google Scholar] [CrossRef]
- Sozzi, E.; Salogni, C.; Lelli, D.; Barbieri, I.; Moreno, A.; Alborali, G.L.; Lavazza, A. Molecular Survey and Phylogenetic Analysis of Atypical Porcine Pestivirus (APPV) Identified in Swine and Wild Boar from Northern Italy. Viruses 2019, 11, 1142. [Google Scholar] [CrossRef] [Green Version]
- Muñoz-González, S.; Canturri, A.; Pérez-Simó, M.; Bohórquez, J.A.; Rosell, R.; Cabezón, O.; Segalés, J.; Domingo, M.; Ganges, L. First report of the novel atypical porcine pestivirus in Spain and a retrospective study. Transbound. Emerg. Dis. 2017, 64, 1645–1649. [Google Scholar] [CrossRef]
- Blomström, A.-L.; Fossum, C.; Wallgren, P.; Berg, M. Viral Metagenomic Analysis Displays the Co-Infection Situation in Healthy and PMWS Affected Pigs. PLoS ONE 2016, 11, e0166863. [Google Scholar] [CrossRef]
- Stenberg, H.; Jacobson, M.; Malmberg, M. Detection of atypical porcine pestivirus in Swedish piglets with congenital tremor type A-II. BMC Vet. Res. 2020, 16, 260. [Google Scholar] [CrossRef]
- Kaufmann, C.; Stalder, H.; Sidler, X.; Renzullo, S.; Gurtner, C.; Grahofer, A.; Schweizer, S.; Schweizer, M. Long-term circulation of atypical porcine pestivirus (APPV) within switzerland. Viruses 2019, 11, 653. [Google Scholar] [CrossRef] [Green Version]
- Congenital tremor associated with atypical porcine pestivirus. Vet. Rec. 2017, 180, 42–43. [CrossRef]
- Cagatay, G.N.; Antos, A.; Meyer, D.; Maistrelli, C.; Keuling, O.; Becher, P.; Postel, A. Frequent infection of wild boar with atypical porcine pestivirus (APPV). Transbound. Emerg. Dis. 2018, 65, 1087–1093. [Google Scholar] [CrossRef]
- Colom-Cadena, A.; Ganges, L.; Muñoz-González, S.; Castillo-Contreras, R.; Bohórquez, J.A.; Rosell, R.; Segalés, J.; Marco, I.; Cabezon, O. Atypical porcine pestivirus in wild boar (Sus scrofa), Spain. Vet. Rec. 2018, 183, 569. [Google Scholar] [CrossRef]
- Mósena, A.C.S.; Weber, M.N.; da Cruz, R.A.S.; Cibulski, S.P.; da Silva, M.S.; Puhl, D.E.; Hammerschmitt, M.E.; Takeuti, K.L.; Driemeier, D.; de Barcellos, D.E.S.N.; et al. Presence of atypical porcine pestivirus (APPV) in Brazilian pigs. Transbound. Emerg. Dis. 2018, 65, 22–26. [Google Scholar] [CrossRef]
- Gatto, I.R.H.; Harmon, K.; Bradner, L.; Silva, P.; Linhares, D.C.L.; Arruda, P.H.; de Oliveira, L.G.; Arruda, B.L. Detection of atypical porcine pestivirus in Brazil in the central nervous system of suckling piglets with congenital tremor. Transbound. Emerg. Dis. 2018, 65, 375–380. [Google Scholar] [CrossRef]
- Dessureault, F.G.; Choinière, M.; Provost, C.; Gagnon, C.A. First report of atypical porcine pestivirus in piglets with congenital tremor in Canada. Can. Vet. J. Rev. Vet. Can. 2018, 59, 429–432. [Google Scholar]
- Gatto, I.R.H.; Arruda, P.H.; Visek, C.A.; Victoria, J.G.; Patterson, A.R.; Krull, A.C.; Schwartz, K.J.; de Oliveira, L.G.; Arruda, B.L. Detection of atypical porcine pestivirus in semen from commercial boar studs in the United States. Transbound. Emerg. Dis. 2018, 65, e339–e343. [Google Scholar] [CrossRef] [PubMed]
- Shen, H.; Liu, X.; Zhang, P.; Wang, L.; Liu, Y.; Zhang, L.; Liang, P.; Song, C. Identification and characterization of atypical porcine pestivirus genomes in newborn piglets with congenital tremor in China. J. Vet. Sci. 2018, 19, 468–471. [Google Scholar] [CrossRef]
- Zhang, X.; Dai, R.; Li, Q.; Zhou, Q.; Luo, Y.; Lin, L.; Bi, Y.; Chen, F. Detection of three novel atypical porcine pestivirus strains in newborn piglets with congenital tremor in southern China. Infect. Genet. Evol. 2019, 68, 54–57. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.L.; Li, Y.Y.; He, L.L.; Wu, J.L.; Tang, X.Y.; Chen, G.H.; Mai, K.J.; Wu, R.T.; Li, Q.N.; Chen, Y.H.; et al. 12 novel atypical porcine pestivirus genomes from neonatal piglets with congenital tremors: A newly emerging branch and high prevalence in China. Virology 2019, 533, 50–58. [Google Scholar] [CrossRef]
- Yin, Y.; Shi, K.; Sun, W.; Mo, S. Complete Genome Sequence of an Atypical Porcine Pestivirus Strain, GX01-2018, from Guangxi Province, China. Microbiol. Resour. Announc. 2019, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, J.; Han, Z.; Li, J.; Huang, Y.; Yang, J.; Ding, H.; Zhang, J.; Zhu, M.; Zhang, Y.; Liao, J.; et al. Atypical porcine pestivirus as a novel type of pestivirus in pigs in China. Front. Microbiol. 2017, 8, 862. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Wen, W.; Hao, G.; Hu, Y.; Chen, H.; Qian, P.; Li, X. Phylogenetic and genomic characterization of a novel atypical porcine pestivirus in China. Transbound. Emerg. Dis. 2018, 65, e202–e204. [Google Scholar] [CrossRef]
- Wu, S.S.; Wang, Z.; Zhang, W.B.; Deng, S.Z. Complete genome sequence of an atypical porcine pestivirus isolated from Jiangxi Province, China. Genome Announc. 2018, 6. [Google Scholar] [CrossRef] [Green Version]
- Zhou, K.; Yue, H.; Tang, C.; Ruan, W.; Zhou, Q.; Zhang, B. Prevalence and genome characteristics of atypical porcine pestivirus in southwest China. J. Gen. Virol. 2019, 100, 84–88. [Google Scholar] [CrossRef]
- Pan, S.; Yan, Y.; Shi, K.; Wang, M.; Mou, C.; Chen, Z. Molecular characterization of two novel atypical porcine pestivirus (APPV) strains from piglets with congenital tremor in China. Transbound. Emerg. Dis. 2019, 66, 35–42. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, X.; Su, D.; Feng, J.; Wei, L.; Cai, W.; Li, J.; Lin, S.; Yan, H.; He, D. Detection and genetic characterization of atypical porcine pestivirus in piglets with congenital tremors in Southern China. Front. Microbiol. 2019, 10, 1406. [Google Scholar] [CrossRef] [Green Version]
- Choe, S.E.; Park, G.N.; Cha, R.M.; Park, B.K.; Hyun, B.H.; An, D.J. Prevalence and Genetic Diversity of Atypical Porcine Pestivirus (APPV) Detected in South Korean Wild Boars. Viruses 2020, 12, 680. [Google Scholar] [CrossRef]
- Postel, A.; Schmeiser, S.; Bernau, J.; Meindl-Boehmer, A.; Pridotkas, G.; Dirbakova, Z.; Mojzis, M.; Becher, P. Improved strategy for phylogenetic analysis of classical swine fever virus based on full-length E2 encoding sequences. Vet. Res. 2012, 43, 50. [Google Scholar] [CrossRef] [Green Version]
- Folgueiras-González, A.; van den Braak, R.; Simmelink, B.; Deijs, M.; van der Hoek, L.; de Groof, A. Atypical porcine pestivirus circulation and molecular evolution within an affected swine herd. Viruses 2020, 12, 1080. [Google Scholar] [CrossRef]
- Postel, A.; Meyer, D.; Petrov, A.; Becher, P. Recent emergence of a novel porcine pestivirus: Interference with classical swine fever diagnosis? Emerg. Microbes Infect. 2017, 6, 1–2. [Google Scholar] [CrossRef] [Green Version]
- Cagatay, G.N.; Meyer, D.; Wendt, M.; Becher, P.; Postel, A. Characterization of the humoral immune response induced after infection with atypical porcine pestivirus (APPV). Viruses 2019, 11, 880. [Google Scholar] [CrossRef]
- Chen, F.; Knutson, T.P.; Braun, E.; Jiang, Y.; Rossow, S.; Marthaler, D.G. Semi-quantitative duplex RT-PCR reveals the low occurrence of Porcine Pegivirus and Atypical Porcine Pestivirus in diagnostic samples from the United States. Transbound. Emerg. Dis. 2019, 66, 1420–1425. [Google Scholar] [CrossRef] [Green Version]
- Bauhofer, O.; Summerfield, A.; Sakoda, Y.; Tratschin, J.-D.; Hofmann, M.A.; Ruggli, N. Classical swine fever virus Npro interacts with interferon regulatory factor 3 and induces its proteasomal degradation. J. Virol. 2007, 81, 3087–3096. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Rijnbrand, R.; Jangra, R.K.; Devaraj, S.G.; Qu, L.; Ma, Y.; Lemon, S.M.; Li, K. Ubiquitination and proteasomal degradation of interferon regulatory factor-3 induced by Npro from a cytopathic bovine viral diarrhea virus. Virology 2007, 366, 277–292. [Google Scholar] [CrossRef] [Green Version]
- Hilton, L.; Moganeradj, K.; Zhang, G.; Chen, Y.-H.; Randall, R.E.; McCauley, J.W.; Goodbourn, S. The NPro Product of Bovine Viral Diarrhea Virus Inhibits DNA Binding by Interferon Regulatory Factor 3 and Targets It for Proteasomal Degradation. J. Virol. 2006, 80, 11723–11732. [Google Scholar] [CrossRef] [Green Version]
- Ruggli, N.; Summerfield, A.; Fiebach, A.R.; Guzylack-Piriou, L.; Bauhofer, O.; Lamm, C.G.; Waltersperger, S.; Matsuno, K.; Liu, L.; Gerber, M.; et al. Classical Swine Fever Virus Can Remain Virulent after Specific Elimination of the Interferon Regulatory Factor 3-Degrading Function of Npro. J. Virol. 2009, 83, 817–829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szymanski, M.R.; Fiebach, A.R.; Tratschin, J.D.; Gut, M.; Ramanujam, V.M.S.; Gottipati, K.; Patel, P.; Ye, M.; Ruggli, N.; Choi, K.H. Zinc Binding in Pestivirus Npro Is Required for Interferon Regulatory Factor 3 Interaction and Degradation. J. Mol. Biol. 2009, 391, 438–449. [Google Scholar] [CrossRef] [PubMed]
- Mou, C.; Pan, S.; Wu, H.; Chen, Z. Disruption of interferon-β production by the Npro of atypical porcine pestivirus. Virulence 2021, 12, 654–665. [Google Scholar] [CrossRef] [PubMed]
- Ivanyi-Nagy, R.; Lavergne, J.-P.; Gabus, C.; Ficheux, D.; Darlix, J.-L. RNA chaperoning and intrinsic disorder in the core proteins of Flaviviridae. Nucleic Acids Res. 2008, 36, 712–725. [Google Scholar] [CrossRef] [PubMed]
- Murray, C.L.; Marcotrigiano, J.; Rice, C.M. Bovine viral diarrhea virus core is an intrinsically disordered protein that binds RNA. J. Virol. 2008, 82, 1294–1304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krey, T.; Bontems, F.; Vonrhein, C.; Vaney, M.-C.; Bricogne, G.; Rümenapf, T.; Rey, F.A. Crystal structure of the pestivirus envelope glycoprotein Erns and mechanistic analysis of its ribonuclease activity. Structure 2012, 20, 862–873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schneider, R.; Unger, G.; Stark, R.; Schneider-Scherzer, E.; Thiel, H.J. Identification of a structural glycoprotein of an RNA virus as a ribonuclease. Science 1993, 261, 1169–1171. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, M.; Poole, E.; Goodbourn, S.; McCauley, J.W. Role for Bovine Viral Diarrhea Virus Erns Glycoprotein in the Control of Activation of Beta Interferon by Double-Stranded RNA. J. Virol. 2003, 78, 136–145. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reimann, I.; Semmler, I.; Beer, M. Packaged replicons of bovine viral diarrhea virus are capable of inducing a protective immune response. Virology 2007, 366, 377–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Widjojoatmodjo, M.N.; van Gennip, H.G.P.; Bouma, A.; van Rijn, P.A.; Moormann, R.J.M. Classical Swine Fever Virus Erns Deletion Mutants: Trans-Complementation and Potential Use as Nontransmissible, Modified, Live-Attenuated Marker Vaccines. J. Virol. 2000, 74, 2973–2980. [Google Scholar] [CrossRef] [Green Version]
- Dalmann, A.; Reimann, I.; Wernike, K.; Beer, M. Autonomously Replicating RNAs of Bungowannah Pestivirus: ERNS Is Not Essential for the Generation of Infectious Particle. J. Virol. 2020, 94, JVI.00436-20. [Google Scholar] [CrossRef]
- Iqbal, M.; McCauley, J.W.; Flick-Smith, H. Interactions of bovine viral diarrhoea virus glycoprotein Erns with cell surface glycosaminoglycans. J. Gen. Virol. 2000, 81, 451–459. [Google Scholar] [CrossRef]
- Magkouras, I.; Mätzener, P.; Rümenapf, T.; Peterhans, E.; Schweizer, M. RNase-dependent inhibition of extracellular, but not intracellular, dsRNA-induced interferon synthesis by Erns of pestiviruses. J. Gen. Virol. 2008, 89, 2501–2506. [Google Scholar] [CrossRef]
- Mätzener, P.; Magkouras, I.; Rümenapf, T.; Peterhans, E.; Schweizer, M. The viral RNase Erns prevents IFN type-I triggering by pestiviral single- and double-stranded RNAs. Virus Res. 2009, 140, 15–23. [Google Scholar] [CrossRef]
- Zürcher, C.; Sauter, K.-S.; Mathys, V.; Wyss, F.; Schweizer, M. Prolonged activity of the pestiviral RNase Erns as an interferon antagonist after uptake by clathrin-mediated endocytosis. J. Virol. 2014, 88, 7235–7243. [Google Scholar] [CrossRef] [Green Version]
- Python, S.; Gerber, M.; Suter, R.; Ruggli, N.; Summerfield, A. Efficient sensing of infected cells in absence of virus particles by plasmacytoid dendritic cells is blocked by the viral ribonuclease Erns. PLoS Pathog. 2013, 9, e1003412. [Google Scholar] [CrossRef]
- Iqbal, M.; McCauley, J.W. Identification of the glycosaminoglycan-binding site on the glycoprotein Erns of bovine viral diarrhoea virus by site-directed mutagenesis. J. Gen. Virol. 2002, 83, 2153–2159. [Google Scholar] [CrossRef]
- Burrack, S.; Aberle, D.; Bürck, J.; Ulrich, A.S.; Meyers, G. A new type of intracellular retention signal identified in a pestivirus structural glycoprotein. FASEB J. 2012, 26, 3292–3305. [Google Scholar] [CrossRef]
- Thiel, H.J.; Stark, R.; Weiland, E.; Rümenapf, T.; Meyers, G. Hog cholera virus: Molecular composition of virions from a pestivirus. J. Virol. 1991, 65, 4705–4712. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Nie, Y.; Wang, P.; Ding, M.; Deng, H. Characterization of classical swine fever virus entry by using pseudotyped viruses: E1 and E2 are sufficient to mediate viral entry. Virology 2004, 330, 332–341. [Google Scholar] [CrossRef] [Green Version]
- Ronecker, S.; Zimmer, G.; Herrler, G.; Greiser-Wilke, I.; Grummer, B. Formation of bovine viral diarrhea virus E1-E2 heterodimers is essential for virus entry and depends on charged residues in the transmembrane domains. J. Gen. Virol. 2008, 89, 2114–2121. [Google Scholar] [CrossRef]
- Wang, J.; Li, Y.; Modis, Y. Structural models of the membrane anchors of envelope glycoproteins E1 and E2 from pestiviruses. Virology 2014, 454–455, 93–101. [Google Scholar] [CrossRef] [Green Version]
- El Omari, K.; Iourin, O.; Harlos, K.; Grimes, J.M.; Stuart, D.I. Structure of a Pestivirus Envelope Glycoprotein E2 Clarifies Its Role in Cell Entry. Cell Rep. 2013, 3, 30–35. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Wang, J.; Kanai, R.; Modis, Y. Crystal structure of glycoprotein E2 from bovine viral diarrhea virus. Proc. Natl. Acad. Sci. USA 2013, 110, 6805–6810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Jose, J.; Xiang, Y.; Kuhn, R.J.; Rossmann, M.G. Structural changes of envelope proteins during alphavirus fusion. Nature 2010, 468, 705–708. [Google Scholar] [CrossRef] [PubMed]
- Krey, T.; Himmelreich, A.; Heimann, M.; Menge, C.; Thiel, H.-J.; Maurer, K.; Rümenapf, T. Function of bovine CD46 as a cellular receptor for bovine viral diarrhea virus is determined by complement control protein 1. J. Virol. 2006, 80, 3912–3922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maurer, K.; Krey, T.; Moennig, V.; Thiel, H.-J.; Rümenapf, T. CD46 is a cellular receptor for bovine viral diarrhea virus. J. Virol. 2004, 78, 1792–1799. [Google Scholar] [CrossRef] [Green Version]
- Dräger, C.; Beer, M.; Blome, S. Porcine complement regulatory protein CD46 and heparan sulfates are the major factors for classical swine fever virus attachment in vitro. Arch. Virol. 2015, 160, 739–746. [Google Scholar] [CrossRef]
- Cagatay, G.N.; Antos, A.; Suckstorff, O.; Isken, O.; Tautz, N.; Becher, P.; Postel, A. Porcine complement regulatory protein CD46 is a major receptor for atypical porcine pestivirus but not for classical swine fever virus. J. Virol. 2021, 95, JVI.02186-20. [Google Scholar] [CrossRef]
- Gladue, D.P.; Holinka, L.G.; Largo, E.; Fernandez Sainz, I.; Carrillo, C.; O’Donnell, V.; Baker-Branstetter, R.; Lu, Z.; Ambroggio, X.; Risatti, G.R.; et al. Classical swine fever virus p7 protein is a viroporin involved in virulence in swine. J. Virol. 2012, 86, 6778–6791. [Google Scholar] [CrossRef] [Green Version]
- Largo, E.; Gladue, D.P.; Huarte, N.; Borca, M.V.; Nieva, J.L. Pore-forming activity of pestivirus p7 in a minimal model system supports genus-specific viroporin function. Antivir. Res. 2014, 101, 30–36. [Google Scholar] [CrossRef]
- Guo, H.C.; Sun, S.Q.; Sun, D.H.; Wei, Y.Q.; Xu, J.; Huang, M.; Liu, X.T.; Liu, Z.X.; Luo, J.X.; Yin, H.; et al. Viroporin activity and membrane topology of classic swine fever virus p7 protein. Int. J. Biochem. Cell Biol. 2013, 45, 1186–1194. [Google Scholar] [CrossRef]
- Largo, E.; Verdiá-Báguena, C.; Aguilella, V.M.; Nieva, J.L.; Alcaraz, A. Ion channel activity of the CSFV p7 viroporin in surrogates of the ER lipid bilayer. Biochim. Biophys. Acta Biomembr. 2016, 1858, 30–37. [Google Scholar] [CrossRef] [Green Version]
- Madan, V.; Bartenschlager, R. Structural and functional properties of the hepatitis C virus p7 viroporin. Viruses 2015, 7, 4461–4481. [Google Scholar] [CrossRef] [Green Version]
- Lackner, T.; Müller, A.; Pankraz, A.; Becher, P.; Thiel, H.-J.; Gorbalenya, A.E.; Tautz, N. Temporal modulation of an autoprotease is crucial for replication and pathogenicity of an RNA virus. J. Virol. 2004, 78, 10765–10775. [Google Scholar] [CrossRef] [Green Version]
- Lackner, T.; Müller, A.; König, M.; Thiel, H.-J.; Tautz, N. Persistence of bovine viral diarrhea virus is determined by a cellular cofactor of a viral autoprotease. J. Virol. 2005, 79, 9746–9755. [Google Scholar] [CrossRef] [Green Version]
- Moulin, H.R.; Seuberlich, T.; Bauhofer, O.; Bennett, L.C.; Tratschin, J.-D.; Hofmann, M.A.; Ruggli, N. Nonstructural proteins NS2-3 and NS4A of classical swine fever virus: Essential features for infectious particle formation. Virology 2007, 365, 376–389. [Google Scholar] [CrossRef] [Green Version]
- Isken, O.; Postel, A.; Bruhn, B.; Lattwein, E.; Becher, P.; Tautz, N. CRISPR/Cas9-Mediated Knockout of DNAJC14 Verifies This Chaperone as a Pivotal Host Factor for RNA Replication of Pestiviruses. J. Virol. 2018, 93. [Google Scholar] [CrossRef] [Green Version]
- Grassmann, C.W.; Isken, O.; Behrens, S.E. Assignment of the multifunctional NS3 protein of bovine viral diarrhea virus during RNA replication: An in vivo and in vitro study. J. Virol. 1999, 73, 9196–9205. [Google Scholar] [CrossRef] [Green Version]
- Warrener, P.; Collett, M.S. Pestivirus NS3 (p80) protein possesses RNA helicase activity. J. Virol. 1995, 69, 1720–1726. [Google Scholar] [CrossRef] [Green Version]
- Tamura, J.K.; Warrener, P.; Collett, M.S. RNA-stimulated ntpase activity associated with the p80 protein of the pestivirus bovine viral diarrhea virus. Virology 1993, 193, 1–10. [Google Scholar] [CrossRef]
- Tautz, N.; Kaiser, A.; Thiel, H.J. NS3 serine protease of bovine viral diarrhea virus: Characterization of active site residues, NS4A cofactor domain, and protease-cofactor interactions. Virology 2000, 273, 351–363. [Google Scholar] [CrossRef] [Green Version]
- Wiskerchen, M.; Collett, M.S. Pestivirus gene expression: Protein p80 of bovine viral diarrhea virus is a proteinase involved in polyprotein processing. Virology 1991, 184, 341–350. [Google Scholar] [CrossRef]
- Xu, J.; Mendez, E.; Caron, P.R.; Lin, C.; Murcko, M.A.; Collett, M.S.; Rice, C.M. Bovine viral diarrhea virus NS3 serine proteinase: Polyprotein cleavage sites, cofactor requirements, and molecular model of an enzyme essential for pestivirus replication. J. Virol. 1997, 71, 5312–5322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becher, P.; Orlich, M.; Thiel, H.J. Complete genomic sequence of border disease virus, a pestivirus from sheep. J. Virol. 1998, 72, 5165–5173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tautz, N.; Elbers, K.; Stoll, D.; Meyers, G.; Thiel, H.J. Serine protease of pestiviruses: Determination of cleavage sites. J. Virol. 1997, 71, 5415–5422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamp, B.; Riedel, C.; Wentz, E.; Tortorici, M.-A.; Rümenapf, T. Autocatalytic cleavage within classical swine fever virus NS3 leads to a functional separation of protease and helicase. J. Virol. 2013, 87, 11872–11883. [Google Scholar] [CrossRef] [Green Version]
- Weiskircher, E.; Aligo, J.; Ning, G.; Konan, K.V. Bovine viral diarrhea virus NS4B protein is an integral membrane protein associated with Golgi markers and rearranged host membranes. Virol. J. 2009, 6, 185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tautz, N.; Tews, B.A.; Meyers, G. The Molecular Biology of Pestiviruses. Adv. Virus Res. 2015, 93, 47–160. [Google Scholar]
- Reed, K.E.; Gorbalenya, A.E.; Rice, C.M. The NS5A/NS5 proteins of viruses from three genera of the family flaviviridae are phosphorylated by associated serine/threonine kinases. J. Virol. 1998, 72, 6199–6206. [Google Scholar] [CrossRef] [Green Version]
- Tellinghuisen, T.L.; Paulson, M.S.; Rice, C.M. The NS5A Protein of Bovine Viral Diarrhea Virus Contains an Essential Zinc-Binding Site Similar to That of the Hepatitis C Virus NS5A Protein. J. Virol. 2006, 80, 7450–7458. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isken, O.; Langerwisch, U.; Schonherr, R.; Lamp, B.; Schroder, K.; Duden, R.; Rumenapf, T.H.; Tautz, N. Functional Characterization of Bovine Viral Diarrhea Virus Nonstructural Protein 5A by Reverse Genetic Analysis and Live Cell Imaging. J. Virol. 2014, 88, 82–98. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Xiao, J.; Xiao, J.; Sheng, C.; Wang, J.; Jia, L.; Zhi, Y.; Li, G.; Chen, J.; Xiao, M. Classical swine fever virus NS5A regulates viral RNA replication through binding to NS5B and 3′UTR. Virology 2012, 432, 376–388. [Google Scholar] [CrossRef] [Green Version]
- Grassmann, C.W.; Isken, O.; Tautz, N.; Behrens, S.-E. Genetic Analysis of the Pestivirus Nonstructural Coding Region: Defects in the NS5A Unit Can Be Complemented in trans. J. Virol. 2001, 75, 7791–7802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheng, C.; Chen, Y.; Xiao, J.; Xiao, J.; Wang, J.; Li, G.; Chen, J.; Xiao, M. Classical swine fever virus NS5A protein interacts with 3′-untranslated region and regulates viral RNA synthesis. Virus Res. 2012, 163, 636–643. [Google Scholar] [CrossRef] [PubMed]
- Xiao, M.; Wang, Y.; Zhu, Z.; Yu, J.; Wan, L.; Chen, J. Influence of NS5A protein of classical swine fever virus (CSFV) on CSFV internal ribosome entry site-dependent translation. J. Gen. Virol. 2009, 90, 2923–2928. [Google Scholar] [CrossRef] [PubMed]
- Sheng, C.; Wang, J.; Xiao, J.; Xiao, J.; Chen, Y.; Jia, L.; Zhi, Y.; Li, G.; Xiao, M. Classical swine fever virus NS5B protein suppresses the inhibitory effect of NS5A on viral translation by binding to NS5A. J. Gen. Virol. 2012, 93, 939–950. [Google Scholar] [CrossRef]
- Choi, K.H.; Groarke, J.M.; Young, D.C.; Kuhn, R.J.; Smith, J.L.; Pevear, D.C.; Rossmann, M.G. The structure of the RNA-dependent RNA polymerase from bovine viral diarrhea virus establishes the role of GTP in de novo initiation. Proc. Natl. Acad. Sci. USA 2004, 101, 4425–4430. [Google Scholar] [CrossRef] [Green Version]
- Choi, K.H.; Gallei, A.; Becher, P.; Rossmann, M.G. The Structure of Bovine Viral Diarrhea Virus RNA-Dependent RNA Polymerase and Its Amino-Terminal Domain. Structure 2006, 14, 1107–1113. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Wu, B.; Soca, W.A.; An, L. Crystal Structure of Classical Swine Fever Virus NS5B Reveals a Novel N-Terminal Domain. J. Virol. 2018, 92. [Google Scholar] [CrossRef] [Green Version]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riedel, C.; Aitkenhead, H.; El Omari, K.; Rümenapf, T. Atypical Porcine Pestiviruses: Relationships and Conserved Structural Features. Viruses 2021, 13, 760. https://doi.org/10.3390/v13050760
Riedel C, Aitkenhead H, El Omari K, Rümenapf T. Atypical Porcine Pestiviruses: Relationships and Conserved Structural Features. Viruses. 2021; 13(5):760. https://doi.org/10.3390/v13050760
Chicago/Turabian StyleRiedel, Christiane, Hazel Aitkenhead, Kamel El Omari, and Till Rümenapf. 2021. "Atypical Porcine Pestiviruses: Relationships and Conserved Structural Features" Viruses 13, no. 5: 760. https://doi.org/10.3390/v13050760
APA StyleRiedel, C., Aitkenhead, H., El Omari, K., & Rümenapf, T. (2021). Atypical Porcine Pestiviruses: Relationships and Conserved Structural Features. Viruses, 13(5), 760. https://doi.org/10.3390/v13050760





